J Clin Aesthet Dermatol. 2025;18(10):59–65.
by Elizabeth V. Seiverling, MD; Asghar Shah, BA; Martin A. Weinstock, MD, PhD; Jane M. Grant-Kels, MD; Nathan P. Falk, MD, MBA; and Daniel M. Siegel, MD
Dr. Seiverling is with the Department of Dermatology at the University of Pittsburgh in Pittsburgh, Pennsylvania. Mr. Shah is with the Warren Alpert Medical School of Brown University in Providence, Rhode Island. Dr. Weinstock is with Brown University and the Providence VA Medical Center in Providence, Rhode Island. Dr. Grant-Kels is with the Department of Dermatology at the University of Connecticut School of Medicine in Farmington, Connecticut and the Department of Dermatology at the University of Florida College of Medicine in Gainesville, Florida. Dr. Falk is with the Department of Family Medicine and Rural Health at Florida State University College of Medicine in Tallahassee, Florida. Dr. Siegel is with SUNY Downstate Health Sciences University in Brooklyn, New York.
FUNDING: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
DISCLOSURES: Dr. Seiverling is a consultant for Melatech and was compensated by DermaSensor, Inc. for clinical research activities for this study. Drs. Grant-Kels and Siegel are advisors to DermaSensor. The remaining authors have no conflicts of interest to disclose.
Abstract: Background: Skin cancer is the most common cancer faced by adults in the United States. Melanoma, while a less common subtype of skin cancer compared to basal cell and squamous cell carcinomas, is associated with greater rates of metastases, mortality, and morbidity, and its rate of incidence is projected to increase. Primary care physicians (PCPs) can play an important role in skin cancer detection and in the decision to refer a patient to a dermatologist. Technologies such as the elastic scattering spectroscopy (ESS) device (DermaSensor, Inc.), a handheld, noninvasive assistive tool, may help in the evaluation of a skin growth and improve appropriate referral decision making. Methods: A total of 50 malignant and 50 benign lesions were assessed by each of the 118 physicians (board-certified internal and family medicine physicians), yielding 5,900 malignant and benign lesion assessments without the device and 5,900 with the ESS device. Physicians were also surveyed regarding their confidence in their management decision. Results: The study met the primary endpoint; the area under the receiver operating characteristic (AUROC) of the PCPs aided with the device was 0.671 (95% confidence interval [CI]: 0.611-0.732) compared with the AUROC unaided by the device of 0.630 (95% CI: 0.582-0.678), a significant increase (p=0.036). When asked whether the device would provide value to their decision making, 91.5% of respondents either agreed or strongly agreed. Conclusion: The ESS device improved PCP accuracy in managing lesions suggestive of melanoma and increased their sensitivity for all skin cancers and melanoma. Participating internal medicine and family medicine physicians reported increased confidence in their assessments with the device. The ESS device can improve PCP decision making when managing lesions suggestive of melanoma. Keywords: Primary care, elastic scattering spectroscopy, melanoma, skin cancer detection
Introduction
Skin cancer is the most common type of cancer in the United States (US), and, compared to basal cell and squamous cell cancers, melanoma has the highest mortality rate.1 Melanoma is projected to become the second most common cancer in the US by 2040 with 219,000 cases.2 Early detection reduces the likelihood of disease-specific morbidity and mortality.3 However, there are drastic shortages of dermatologists,4 and patients often seek care for dermatologic conditions in the primary care setting.
While the American Academy of Family Physicians includes some skin cancer detection training as an important part of all family medicine resident training, access to training can be limited and many primary care physicians (PCPs) feel ill-equipped to evaluate skin growths.5
Recent advances have been made in artificial intelligence (AI) technology to improve melanoma detection and have the potential to aid PCPs in the evaluation of skin growths.6-8 Efforts to implement AI algorithms for melanoma detection have also been applied in primary cancer screening.9 One such AI-based tool that has been investigated and cleared by the US Food and Drug Administration (FDA) is the elastic scattering spectroscopy (ESS) device (DermaSensor, Inc.), a handheld, noninvasive, and painless assistive tool for skin cancer detection. In a prospective multicenter study, the ESS device exhibited a sensitivity of 97.04% in detecting skin cancer lesions, including a sensitivity of 96.67% for melanoma, with sensitivity and accuracy (ie, area under the curve) found to be comparable to dermatologists’ in-person performance, which holds promise for improving PCPs’ ability to assess suspicious lesions and appropriately refer suspicious lesions to dermatologists.10 Results of the DermaSensor Use in the Assessment of Skin Lesions Suggestive of Melanoma III (DERM-ASSESS III) study, a prospective blinded melanoma validation study, demonstrated similar findings for device sensitivity and accuracy compared to dermatologists, reporting that the ESS device had a melanoma sensitivity of 95.5% and a negative predictive value (NPV) of 98.1%, highlighting its potential benefit as a nonspecialist adjunctive tool for melanoma detection at the point of care.11 Finally, results from studies involving PCPs found the device sensitivity to be 95.5% and NPV to be 96.6% across all skin cancers,12 with a companion utility study showing that PCPs’ use of the ESS device significantly improved diagnostic sensitivity (10.6%, p=0.0085), management sensitivity (9.4%, p=0.003), and physician confidence, indicating its potential to enhance PCP skin cancer diagnosis and confidence in management.13 Moreover, results from a study of the ESS device suggest that device use may improve PCP sensitivity for skin cancer from 83.0% to 95.5% for high-risk lesions, and could rule out 20.7% of suspicious lesions from further evaluation.14
Given the importance of PCP decision making regarding lesions suspicious of skin cancer, particularly melanoma, it is postulated that use of the ESS device may assist PCPs in referral decision making and enhance provider confidence in managing these cases. Here, we describe the findings of a multireader multicase (MRMC) study to assess the referral performance of PCPs when evaluating lesions suggestive of melanoma with and without the aid of the ESS device. Since previous studies were not adequately powered or designed to demonstrate the device impact on melanoma management, this study was accomplished with intentional inclusion of more melanoma cases.
Methods
ESS device. This handheld, noninvasive tool uses ESS and machine learning (ML) to aid in evaluation of skin lesions. The device’s algorithm has been trained on more than 10,000 recordings from more than 2,000 skin lesions to distinguish malignant from benign skin lesions, including histologically confirmed melanoma and keratinocyte carcinoma, as well as unbiopsied benign lesions, diagnosed by dermatologists. None of the ESS spectral recordings used in the training process were employed in the lesion testing set used in this study.
For each lesion, the device classifies the observed spectral pattern as either a positive result of having malignant characteristics (“investigate further”) or a negative result of having benign characteristics (“monitor”). For lesions classified as “investigate further,” a spectral similarity score from 1 to 10 is reported, with higher values corresponding to the amount of spectral similarity a lesion has to malignant lesions in prior studies used to develop the algorithm.10-12
Study design. This melanoma-focused reader study was a web-based, MRMC investigation using clinical information, digital images, and ESS device result data. PCPs were asked to perform 200 reads for 100 skin lesion cases, each presented without and then with ESS device output.
The study examined two aspects of PCP decisions concerning skin evaluation using the device: (1) the impact of the device result on the PCPs’ management decision on whether the lesion should be referred for further evaluation by a dermatologist, and (2) the device’s impact on the PCP’s diagnostic assessment of whether the lesion was malignant or benign. A study platform with traceability for each physician’s responses was used to conduct this reader study. The study sponsor was blinded to the physician lesion case responses during study enrollment.
For every lesion case evaluation (both unaided and aided), physicians completed a questionnaire about their diagnosis of the lesion, their recommended management decision, and their confidence level in that decision. Physicians were instructed to maintain consistent evaluation criteria, based on their own clinical judgement, throughout the study, including for the 1 to 10 confidence assessment. A series of 10 questions were included at the end of the study to assess physician perceptions of the device and its benefits. The questions were asked as a select multiple response or with a 5-point Likert scale assessment ranging from “strongly agree” to “strongly disagree.”
Study physicians. PCP reader eligibility was limited to currently practicing, board-certified PCPs (family medicine or internal medicine); they could not have participated in any of the clinical studies pertaining to this device from 2020 to 2022. Physicians with board certification in surgery or dermatology were excluded. A total of 118 PCP readers, none of whom participated in prior studies pertaining to the device, completed the study and were eligible for the effectiveness analysis. The study began after IRB approval, and proper informed consent was obtained from patients relating to the cases acquired as part of the previous clinical study.
Lesion image selection. This study used a randomly selected subset of lesion images and accompanying clinical information (ie, lesion cases) that were acquired during a previous clinical study (DERM-ASSESS III clinical study).11 Photos were acquired using a standardized approach via an iPad and clip-on handyscope.
The photography procedure and training provided to study sites were intended to produce high-quality lesion images that would be usable for reader studies. Study sites in the DERM-ASSESS III clinical study were representative of the demographics of the patient population in the US for the device’s intended use. The images and cases collected and used were therefore representative of those patients with evaluable lesions suggestive of melanoma.
The lesion images and the accompanying clinical information that met the quality standards of the DERM-ASSESS III study protocol (ie, in the study effectiveness population) were reviewed by a panel of three independent physicians (one dermatologist and two PCPs) to confirm quality eligibility for the reader study by confirming that the lesion photos allowed for an assessment clinically comparable to a real-world clinical care assessment. In total, 100 high-resolution digital clinical lesion cases for 50 malignant lesions including melanoma and non-melanoma skin cancers [NMSC; ie, basal cell carcinoma [BCC] and squamous cell carcinoma [SCC]]), and 50 benign lesions, all biopsy-proven, including nevi, seborrheic keratosis (SK), and other benign lesions that had passed evaluation by the physician panel were randomly selected. Given the limited number of BCC and SCC lesion cases enrolled for lesions suggestive of melanoma, an overrepresentation of BCCs and SCCs were included to limit physician bias toward melanoma diagnoses. To minimize bias, every physician reader had a unique, randomized order in which cases were reviewed. Case randomization was performed using an algorithm within the survey platform.
Study outcomes. The device was expected to significantly increase physician sensitivity and area under the receiver operating characteristic (AUROC) curve based on the results of prior reader studies.13,15 However, this study was conducted to provide supplemental evidence for regulatory purposes; thus, only noninferiority hypothesis tests were used as endpoints, but both noninferiority and superiority were tested for. The primary endpoint was to determine whether the AUROC of PCPs aided by knowledge of the ESS output was noninferior to the PCPs’ unaided AUROC, using biopsy-verified skin lesions as the reference standard.
The secondary endpoints were: (1) noninferiority of device-aided PCP sensitivity compared to unaided sensitivity for referral decisions for all malignancies included in the study; (2) noninferiority of device-aided PCP sensitivity for histopathology-confirmed melanoma only compared to unaided sensitivity for referral decisions; and (3) noninferiority of device-aided PCP specificity compared to unaided specificity for referral decisions.
All included lesions were biopsied and dermatopathology was used as the reference standard for sensitivity and specificity calculations. Further analyses included aided and unaided AUROC for melanoma, BCC, SCC, and NMSC (BCC and SCC collectively) and aided and unaided referral specificity for benign melanocytic nevi, seborrheic keratoses, and other benign lesions. In addition, physician confidence in their referral decisions was compared with and without the device output availability.
Results
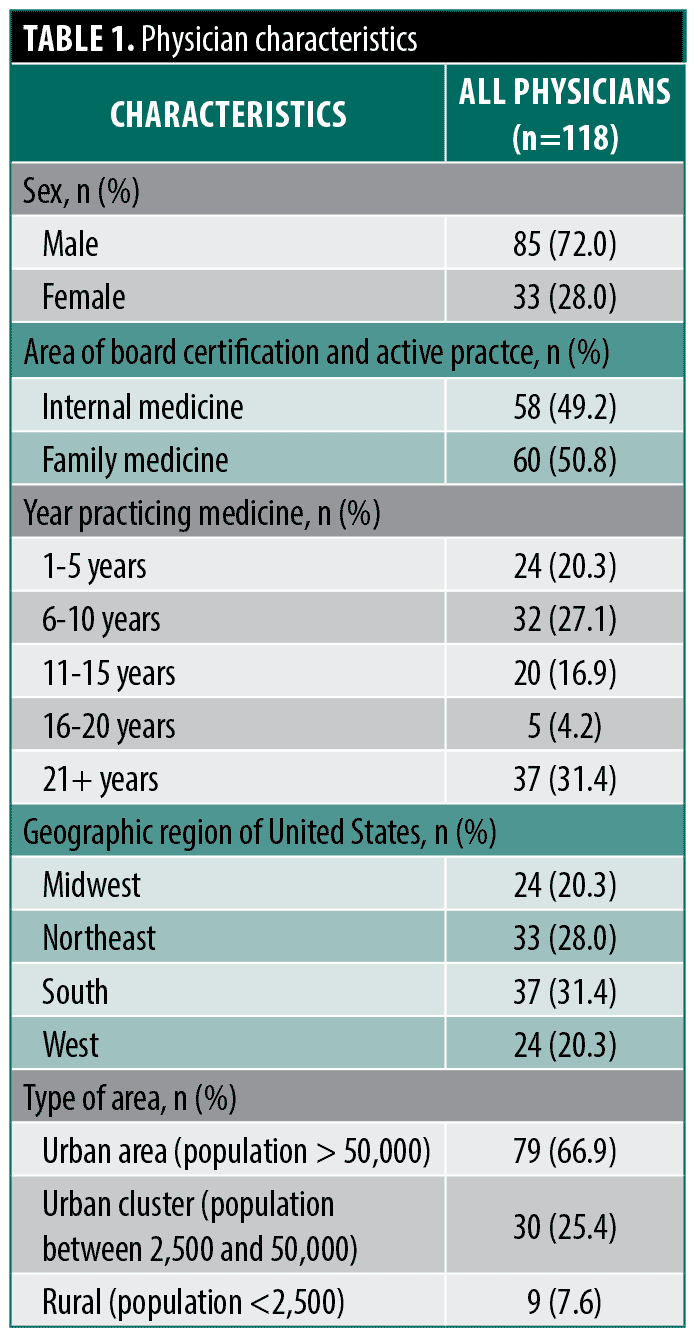
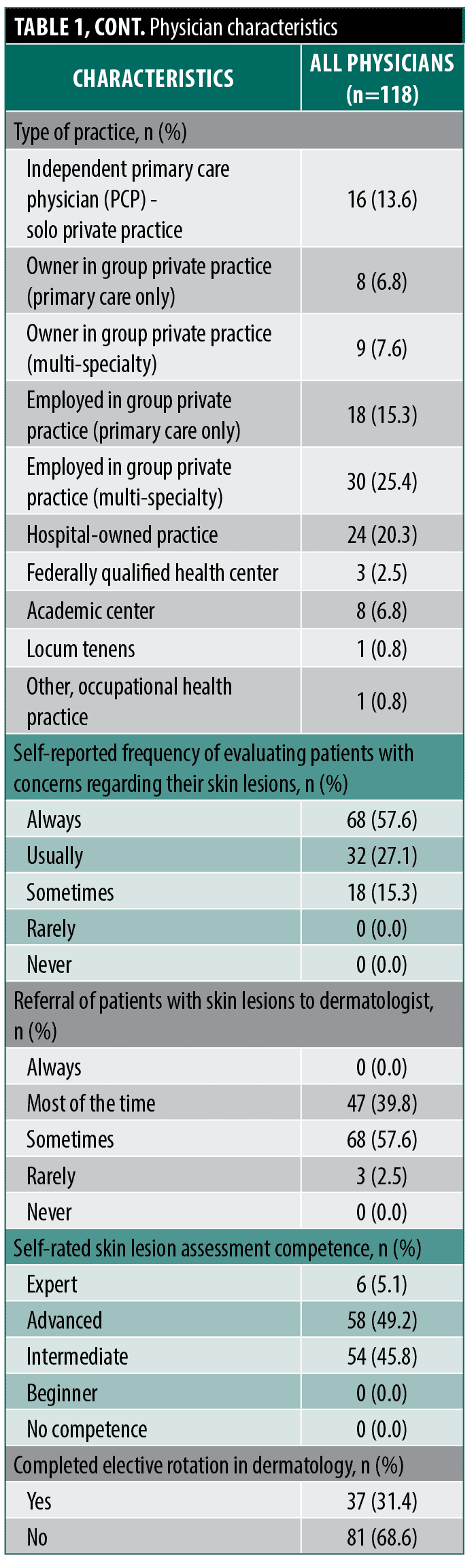
Study physicians. Overall, 118 participants included board-certified internal and family medicine physicians (49.2% and 50.8%, respectively) with a broad distribution of years in practice (range: 1-21+ years) (Table 1). There were 72.0% male and 28.0% female physicians. Years in practice varied with most physicians reporting 6 to 10 years and 21+ years in practice (27.1% and 31.4%, respectively), followed by 1 to 5 years (20.3%), 11 to 15 years (16.9%), and 16 to 20 years in practice (4.2%). Type of practice also varied, with most physicians reporting being employed in a multi-specialty group private practice (25.4%) or a hospital-owned practice (20.3%) and most commonly practicing in an urban area (66.9%). Physicians recruited from each state was reflective of US physician population numbers in each state, ranging from 0.8–12.7% of physicians recruited, with most physicians recruited from California (12.7%). The frequency they reported evaluating patients with concerns regarding their skin lesions was most often reported as “always” (57.6%) followed by “usually” (27.1%). With regards to referring patients to dermatology, 57.6% of physicians reported “sometimes,” followed by “most of the time” (39.8%) and “rarely” (2.5%). In accordance with the device indication for use and the study eligibility requirements, no physicians reported “never” or “always” referring patients for skin lesions. Self-rated competence in skin lesion assessment was most reported as “advanced” (49.2%) followed by “intermediate” (45.8%). Only 5.1% of physicians self-reported being an “expert” in skin lesion assessment. Nearly one-third of physicians reported completing an elective rotation in dermatology (31.4%).
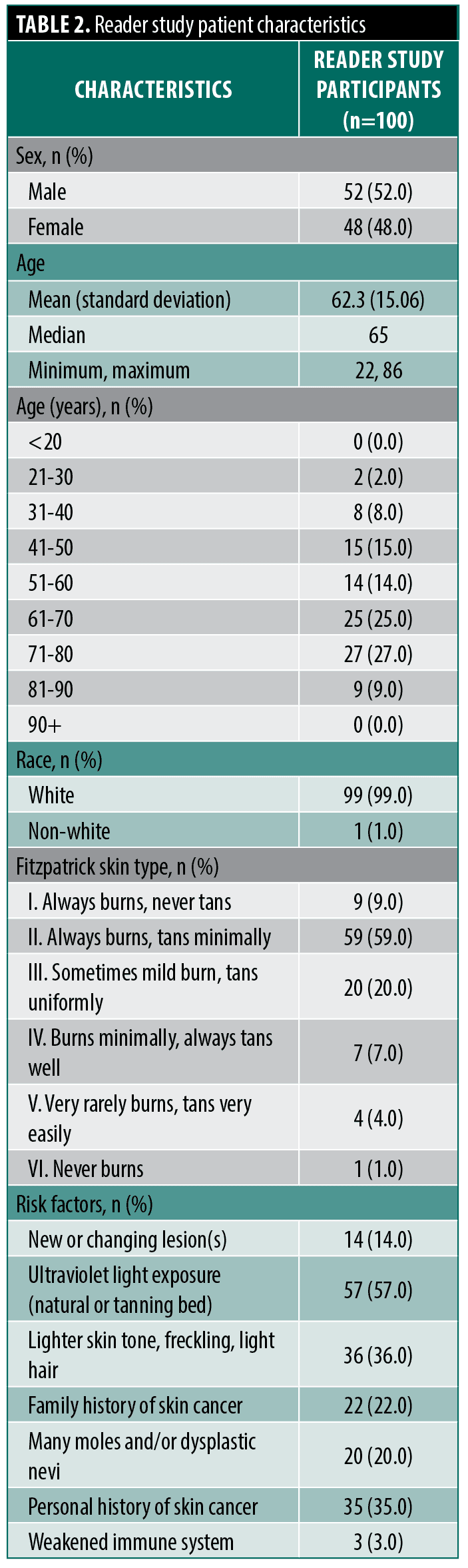
Subject and lesion demographics. The population of included images were from patients (n=100) that were 52.0% male and 48.0% female with a mean age of 62.3 (standard deviation [SD]: 15.06). Over half of patients were aged between 61-80 (52.0%). A total of 88 (88.0%) patients were of lighter skin types (ie, Fitzpatrick skin types I-III), with 59.0% designated as Fitzpatrick skin type II and 20.0% Fitzpatrick skin type III. For the subjects’ most often reported risk factors, 14.0% had a new or changing lesion, 57.0% had ultraviolet light exposure, 36.0% had lighter skin tone, freckling, and light hair, and 20.0% had many moles and/or dysplastic nevi.
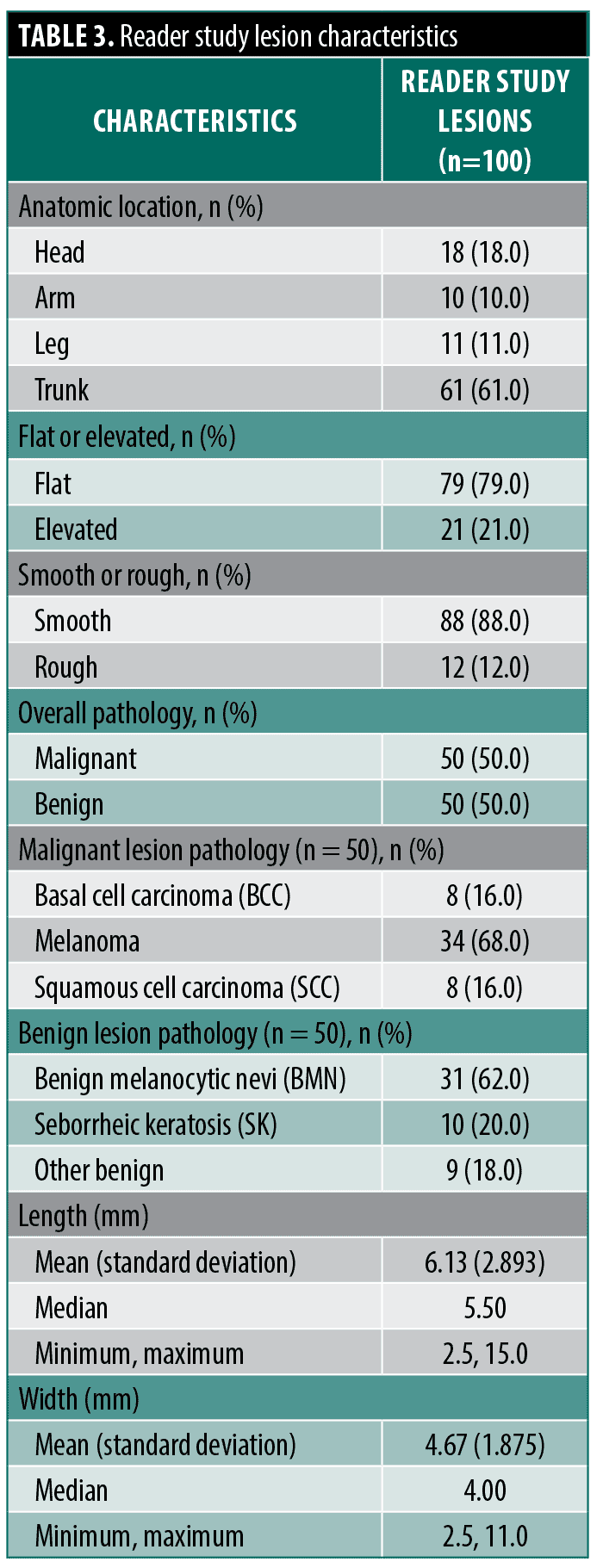
The reader study lesions (n=100) were mostly located on the trunk (61%), followed by head (18%), leg (11%), and arm (10%). Half of the 100 lesion cases were malignant (n=50). Among all lesions, 79% were flat (21% elevated), and 88% were smooth (12% rough). Among the malignant lesions, 68% were melanoma, with an equal proportion of BCC and SCC at 16% each. Among the histologically proven benign lesions, 62% were benign melanocytic nevi (BMN), and 20% were seborrheic keratosis (SK). The average lesion width was 4.67 mm (SD: 1.875) (Table 2 and Table 3).
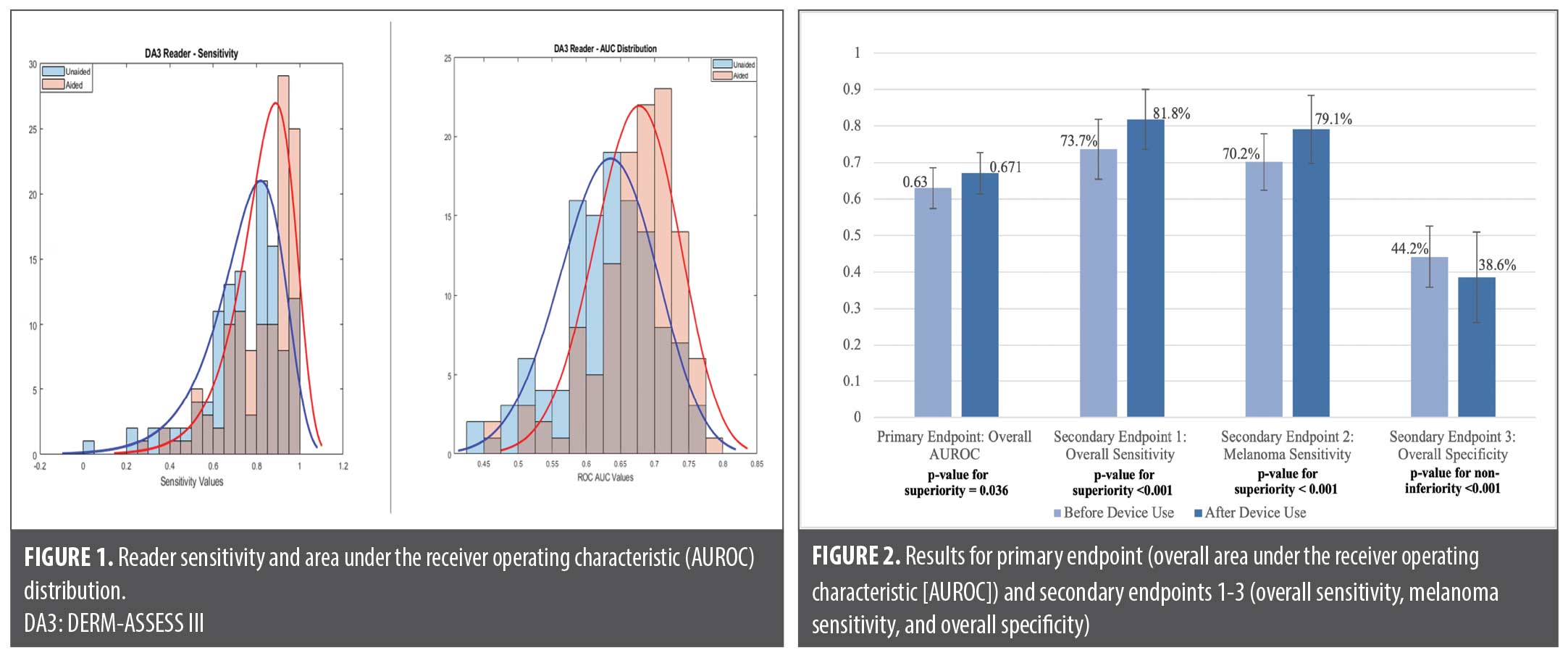
Primary outcome. A total of 50 malignant and 50 benign lesions were assessed by each of the 118 physicians, yielding 5,900 lesion assessments without the device and 5,900 with the ESS device result. The study met the primary endpoint; the AUROC for referral decisions regarding all lesions of the PCPs aided with the device was 0.671 (95% confidence interval [CI]: 0.611-0.732) compared to the AUROC unaided by the device of 0.630 (95% CI: 0.582-0.678). The aided AUROC for referral decisions regarding all lesions was noninferior to the unaided AUROC (p<0.001). Furthermore, a statistical test for superiority found that the aided AUROC was significantly higher than the unaided AUROC (absolute difference of 0.041; p=0.036). See Figure 1 for physician sensitivity and AUROC distributions and Figure 2 for primary and second outcomes.
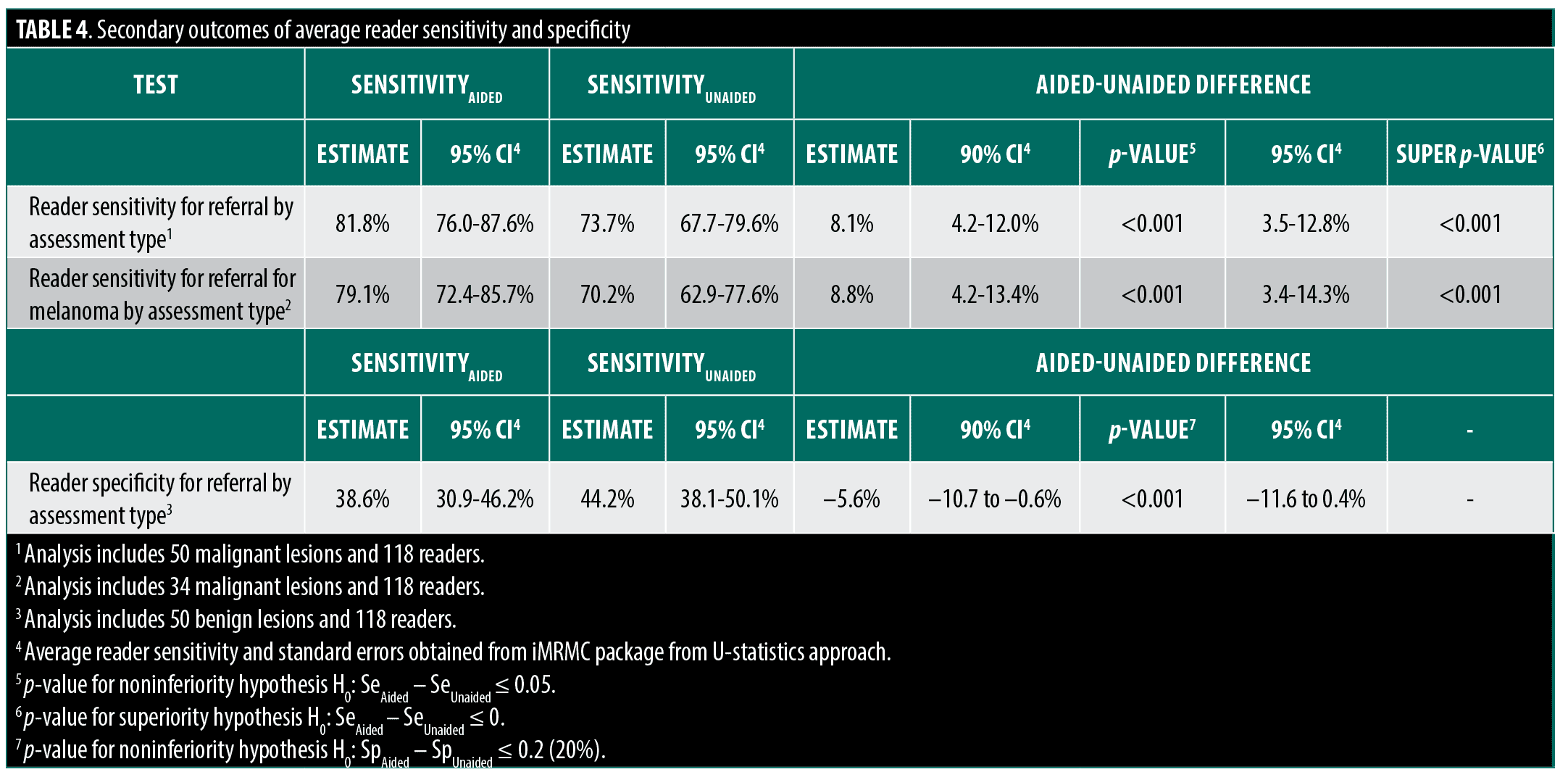
Secondary outcomes. Aided sensitivity of the PCPs was noninferior to unaided sensitivity across all skin cancer types, and superior to unaided assessments for melanoma detection. Specificity did not decrease significantly, as demonstrated through the noninferiority test (Table 4).
Physician confidence assessment. From unaided to aided assessments, the mean scores changed from 6.4±1.59 (range: 1-10) to 7.0±1.74 (range: 1-10). There was a significant difference in the mean score (0.67; 95% CI: 0.52-0.81; p<0.001) between aided and unaided equal. With the aid of the device result, confidence scores decreased in 16.0% (n=1890) of readings, while 33.1% (n=3903) of scores stayed the same and 50.9% (n=6007, p<0.001) of scores increased for all readings.
Sensitivity increased with increasing confidence levels. When evaluating confidence groupings into low (1-3), mid (4-7), and high (8-10) spectral scores, sensitivity increased with availability of a device result. For low device scores (1-3), PCPs’ unaided sensitivity of 77.3% increased to 84.2% for aided assessments, while specificity decreased from an unaided value of 33.0% to 25.2% when PCPs were aided with the device. For malignant lesions in which the PCPs’ had low confidence in their unaided assessments (242 lesions), the unaided sensitivity was 77.3% and aided sensitivity was 88.0%, a difference of 10.7%. For low confidence benign lesions (276), the unaided specificity was 33.0% and aided specificity was 31.9%, a difference of –1.1%.
Diagnostic sensitivity and specificity. The diagnostic sensitivity for PCPs in detecting malignancies with the device result was 62.1% (95% CI: 54.0-70.2%), which was higher than sensitivity without the device result of 59.8% (95% CI: 52.7-66.9%). Physician diagnostic specificity for benign lesions with the device output available at 64.2% (95% CI: 57.1-71.3%) was also higher than without the device output available at 62.4% (95% CI: 56.3-68.6%). While use of the device to inform diagnostic assessments is not the device’s indication for use, these results suggest that even if the device were used in this manner for lesions suggestive of melanoma, neither sensitivity nor specificity would decrease and that one or both may increase.
Subgroup analyses. While the present study was not powered to assess lesion subtypes and there was no formal hypothesis testing within subgroups, numerical increases in AUROC were seen and AUROC was noninferior for each cancer subtype. For example, when assessing sensitivity by cancer types (melanoma, BCC, SCC, NMSC), the study results demonstrated superior sensitivity for melanoma when aided by the device and a meaningful sensitivity increase for NMSC. Average physician AUROC for melanoma unaided by the device (0.605, 95% CI: 0.548-0.661) was numerically higher when aided by device use (0.637, 95% CI: 0.570-0.703). Moreover, PCPs’ sensitivity was observed to be higher for each malignant lesion subgroup when comparing their unaided sensitivity to aided sensitivity. Overall PCP sensitivity with device output for melanoma was 79.1% (95% CI: 72.4-85.7%), and for NMSC was 87.6% (95% CI: 77.2-97.9%). This was an increase from PCP sensitivity without device output of 70.2% (95% CI: 62.9- 77.6%) for melanoma and 80.9% (95% CI: 73.7-88.1%) for NMSC.
Specificity was observed to be lower across benign lesion subgroups when comparing PCP-aided specificity to unaided specificity. Specificity for benign melanocytic nevi was 42.5% (95% CI: 33.0-52.0%) with the device compared to 47.2% (95% CI: 40.5-53.8%) without the device. For SKs, specificity was 32.7% (95% CI: 17.5-47.9%) with device output and 35.6% (95% CI: 26.5-44.6%) without device output. For other benign lesions, specificity was 31.5% (95% CI: 18.0-44.9%) with device output and 43.4% (95% CI: 29.8-57.0%) without device output.
Physician perceptions of device. When asked about whether the device use would provide value to their decision making, 91.5% of respondents either agreed or strongly agreed, with 7.6% reporting neutral and 0.8% reporting they disagree or strongly disagree. When asked about the inclusion of the spectral score with the “investigate further” result, 4.2% disagreed there was a benefit, 14.4% were neutral, while 81.4% either agreed (47.5%) or strongly agreed (33.9%) that there was a benefit. In addition, 93.2% of respondents also agreed or strongly agreed that they would benefit from having NPV and positive predictive value (PPV) available in instructional materials associated with the device.
When asked about their own standard of care performance in assessing skin lesions, most respondents rated their unaided sensitivity between 61 to 80% (52.5%) and their specificity between 51 to 70% (39.8%). Respondents reported that the availability of the ESS device output would alter their behavior encouraging them to perform better quality skin lesion evaluations. Also, regarding expected benefits of the device in real-world care with their patients, 81% of respondents agreed that the device would provide an immediate, objective result to help with management of suspicious skin lesions, 75% agreed that they would detect more skin cancer, and 71% agreed they would have greater confidence in their clinical assessments and management decisions. Only 1% of respondents said they would not expect any benefit with the device.
Discussion
In this MRMC study, we found both a noninferior and superior increase in overall management sensitivity and in AUROC with physician use of the adjunctive device. Aided sensitivity of melanoma identification was also noninferior and superior to unaided sensitivity. While the specificity decreased by 5.6 to 38.6% aided, this was noninferior to the specificity endpoint. Diagnostic sensitivity also increased from 59.8 to 62.1% with the availability of device output, and specificity increased from 62.4 to 64.2%.
The only two AI tools to have been approved prior to this ESS device were MelaFind and Nevisense, and both were restricted for use to only dermatologists.16 Despite FDA approval in 2011, MelaFind was discontinued for sale and clinical use in 2017. The limitations of this device included unnecessary biopsies owing to low specificity of 10%, high device cost and workflow burden, and device use restricted to only pigmented lesions. Resembling prior studies of the ESS device, device use improves PCPs’ evaluation and management decisions regarding all skin cancers. However, this is the first study demonstrating the potential of the device to significantly improve melanoma detection.
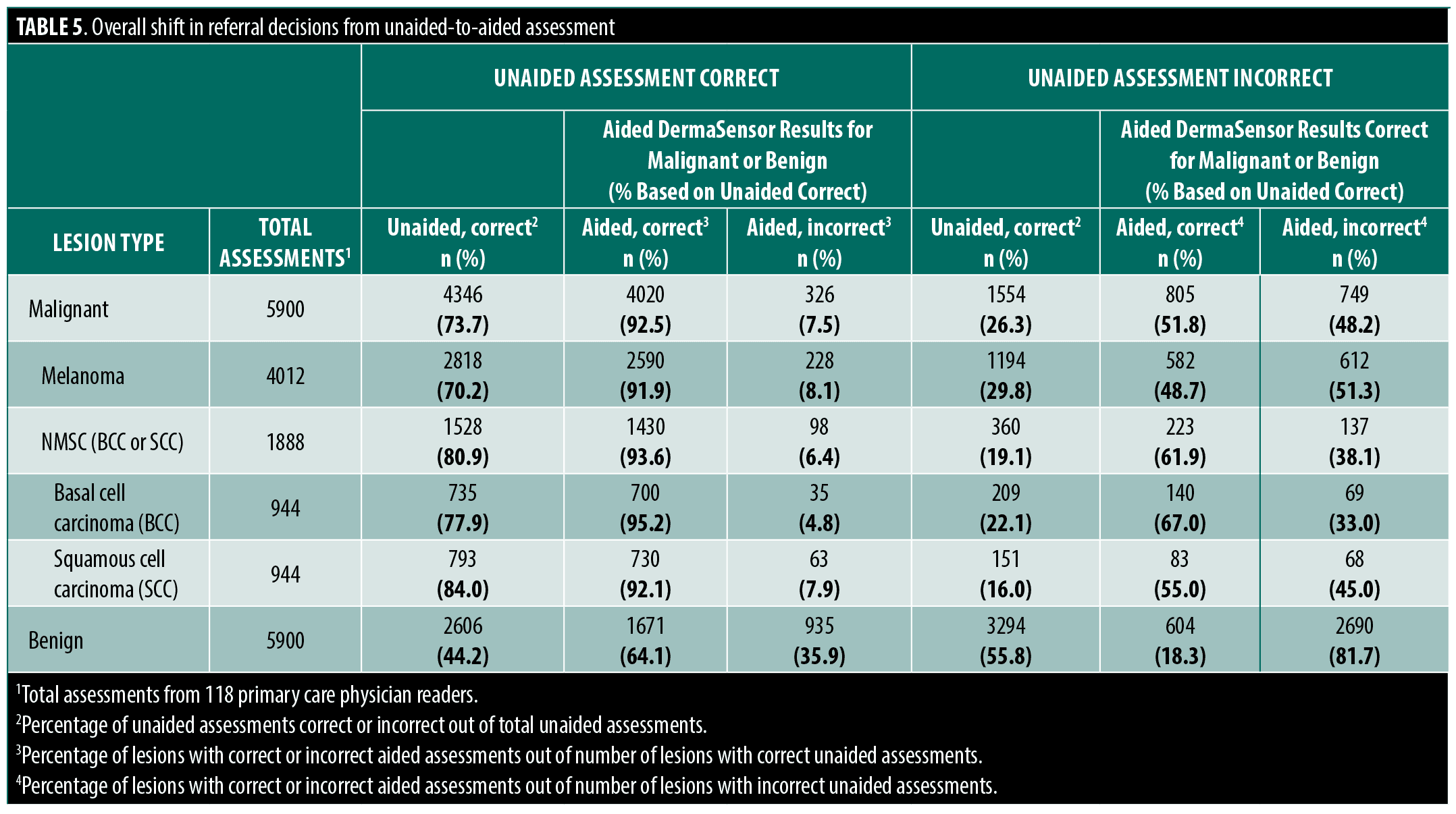
Clinical implications. PCP use of the ESS device may improve referral decisions. For example, correctly referred aided melanomas increased by 48.7% (n=582) for melanomas that would have been incorrectly managed if unaided. For each skin cancer subgroup, this trend was consistent and resulted in an overall increase in correctly aided referrals for melanoma as well as BCCs and SCCs (Table 5).
There has been a rising incidence of skin cancer in the US amid the changing climate. A 2ºC increase in temperature due to global warming has been postulated to increase skin cancer incidence by 10% annually; this temperature rise is expected by 2050. Therefore, the ESS device may help meet the need for PCPs to diagnose these skin cancers and refer patients appropriately.17,18
Noninvasive, point-of-care tools for PCPs have the potential to aid in rapid skin cancer detection and earlier access to dermatologists while also reducing unnecessary referrals of benign lesions. When the tool is used as an adjunct to careful consideration of the clinical appearance and available clinical information (patient skin phototype, age, location on the body, risk factors, changes of the lesion reported by the patient), there is greater potential for more accurate triage of potential skin cancers.
This study was strengthened by the multireader design, which allowed for multiple physicians to assess lesions, each with a different randomized order of lesion cases, potentially reducing individual bias. The study was also strengthened by its inclusion of a representative mix of common skin cancers, with two-thirds of cancerous lesions being melanoma. Limitations of our study impacted both sensitivity and specificity calculations. Limitations include the limited sample size in certain subgroups including those with Fitzpatrick skin type VI and I, limited sample size of patients ages 21-30, and limited sample of flat lesions. Additionally, this study was conducted in a theoretical setting, not direct patient care.
Conclusion
Skin cancer is the most common type of cancer in the US, with projected increases in diagnoses anticipated. While less common than BCCs and SCCs, melanoma spreads more aggressively and is associated with decreased survival rates. As the primary entry point into the healthcare system, PCPs play a pivotal role in connecting patients with specialized dermatological care when warranted.
We present the findings of a reader study assessing the referral performance of PCPs when evaluating lesions suggestive of melanoma with and without the ESS result. The device significantly improved physicians’ accuracy in managing lesions suggestive of melanoma, with significant increases in sensitivity for all skin cancers and melanoma, while not significantly raising the rate of unnecessary referrals. Moreover, the physicians reported increased confidence in their assessments with the availability of the device result. As such, the device can improve PCP decision making and confidence when managing lesions suggestive of melanoma.
By enhancing PCP management accuracy and confidence, this FDA-approved device has the potential to optimize resource utilization by minimizing unnecessary referrals and biopsies, saving healthcare dollars, and reducing patient morbidity and concern, while helping to prioritize access for patients with high-risk lesions. Given the rising rates of skin cancer in the US and limited access to dermatologic care, this device has the potential to make a significant impact in skin cancer detection in the primary care setting.
References
- Aggarwal P, Knabel P, Fleischer AB. United States burden of melanoma and non-melanoma skin cancer from 1990 to 2019. J Am Acad Dermatol. 2021;85(2):388-95.
- Rahib L, Wehner MR, Matrisian LM, Nead KT. Estimated projection of US cancer incidence and death to 2040. JAMA Netw Open. 2021;4(4):e214708.
- Schuchter L, Schultz DJ, Synnestvedt M, et al. A prognostic model for predicting 10-year survival in patients with primary melanoma. Ann Intern Med. 1996;125(5):369-75.
- Balboul S, Gronbeck C, Feng H. Dermatology workforce projections in the United States, 2021 to 2036. Arch Dermatol Res. 2024;316(5):192.
- Najmi M, Brown AE, Harrington SR. A systematic review and synthesis of qualitative and quantitative studies evaluating provider, patient, and health care system-related barriers to diagnostic skin cancer examinations. Arch Dermatol Res. 2022;314(4):329-40.
- Gareau DS, Correa da Rosa J, Yagerman S, et al. Digital imaging biomarkers feed machine learning for melanoma screening. Exp Dermatol. 2017;26(7):615-8.
- Gareau DS, Browning J, Correa Da Rosa J, et al. Deep learning-level melanoma detection by interpretable machine learning and imaging biomarker cues. J Biomed Opt. 2020;25(11):112906.
- Marchetti MA, Cowen EA, Kurtansky NR, et al. Prospective validation of dermoscopy-based open-source artificial intelligence for melanoma diagnosis (PROVE-AI study). NPJ Digit Med. 2023;6(1):1-11.
- Giavina-Bianchi M, Sousa RM de, Paciello VZ de A, et al. Implementation of artificial intelligence algorithms for melanoma screening in a primary care setting. PLoS One. 2021;16(9):e0257006.
- Manolakos D, Patrick G, Geisse JK, et al. Use of an elastic-scattering spectroscopy and artificial intelligence device in the assessment of lesions suggestive of skin cancer: a comparative effectiveness study. JAAD Int. 2024;14:52-8.
- Hartman RI, Trepanowski N, Chang MS, et al. Multicenter prospective blinded melanoma detection study with a handheld elastic scattering spectroscopy device. JAAD Int. 2024;15:24-31.
- Merry SP, Croghan IT, Dukes KA, et al. Primary Care Physician Use of Elastic Scattering Spectroscopy on Skin Lesions Suggestive of Skin Cancer. Journal of Primary Care & Community Health. 2025;16.
- Jaklitsch E, Thames T, de Campos Silva T, Coll P, Oliviero M, Ferris LK. Clinical utility of an AI-powered, handheld elastic scattering spectroscopy device on the diagnosis and management of skin cancer by primary care physicians. J Prim Care Community Health. 2023;14:21501319231205979.
- Merry SP, Chatha K, Croghan I, Nguyen V, McCormick B, Leffell D. Clinical performance of novel elastic scattering spectroscopy (ESS) in detection of skin cancer: a blinded, prospective, multi-center clinical trial. J Clin Aesthet Dermatol. 2023;16(4 Suppl 1):S5-31.
- Ferris LK, Jaklitsch E, Seiverling EV, et al. DERM-SUCCESS FDA pivotal study: a multireader multicase evaluation of primary care physicians’ skin cancer detection using AI-enabled elastic scattering spectroscopy. J Prim Care Community Health. 2025;16:21501319251342106.
- Beltrami EJ, Brown AC, Salmon PJM, Leffell DJ, Ko JM, Grant-Kels JM. Artificial intelligence in the detection of skin cancer. J Am Acad Dermatol. 2022;87(6):1336-42.
- Kaffenberger BH, Shetlar D, Norton SA, Rosenbach M. The effect of climate change on skin disease in North America. J Am Acad Dermatol. 2017;76(1):140-7.
- Lin MJ, Torbeck RL, Dubin DP, et al. Climate change and skin cancer. J Eur Acad of Dermatol Venereol. 2019;33(9):e324-e325.