J Clin Aesthet Dermatol. 2025;18(8):16–17.
by Jiana Wyche, MHS, and Crystal Aguh, MD
Ms. Wyche and Dr. Aguh are with the Johns Hopkins School of Medicine in Baltimore, Maryland.
FUNDING: No funding was provided for this article.
DISCLOSURES: Dr. Aguh is an advisor for Lilly, Pfizer, Olaplex, and Myovant Sciences. She is the author of the UpToDate section on Central Centrifugal Cicatricial Alopecia and directs the Ethnic Skin Program at Johns Hopkins which is funded through an educational grant by Janssen. Ms. Wyche has no conflicts of interest to disclose.
Abstract: Introduction: This study highlights the experiences and clinical outcomes of Black women treated with compounded topical minoxidil (CTM), containing a steroid and retinoid, compared to over the counter (OTC) minoxidil. Methods: A retrospective chart review included 66 Black women treated at Johns Hopkins Hospital between 2020 and 2024. Patients previously used OTC minoxidil and were currently using CTM. Data collected included patient demographics, formulation, side effects, follow-up visits, and patient-reported outcomes. Adherence was assessed at follow-up appointments based on self-reported CTM use. Results: Of the 66 patients, 33.3 percent had traction alopecia (TA), 37.9 percent had androgenetic alopecia (AGA), and 28.8 percent had combined AGA and TA. Side effects from OTC minoxidil were reported by 21.2 percent of patients, with scalp irritation being the most common. Only 10.6 percent of patients reported side effects while using CTM, with 4.5 percent reporting scalp irritation and four reporting hypertrichosis. After receiving treatment with CTM for at least six months, patients had an overall adherence rate of 84.7 percent, compared to only 44.7 percent of patients who adhered to OTC minoxidil (p<0.001). Clinical improvement was reported by 69.6 percent of patients using CTM, compared to 45.0 percent of patients using OTC minoxidil (p<0.001). Discussion: CTM was associated with higher adherence, fewer side effects, and clinical improvement in majority of patients treated with CTM compared to their previous OTC minoxidil use. Limitations: This is a single institution study. Conclusion: When treating AGA or TA in Black women, special consideration should be taken when selecting minoxidil formulation, as it may have an impact on adherence and efficacy. Keywords: Androgenetic alopecia, traction alopecia, nonscarring alopecia, minoxidil, skin of color
Topical minoxidil is the only Food and Drug Administration (FDA) approved treatment for androgenetic alopecia (AGA) in women, with off-label use extended to other forms of hair loss, including traction alopecia (TA).1 Although topical minoxidil is a widely used therapy when treating hair loss, adherence remains a challenge, particularly among patients with textured hair, due in part to factors such as product formulation.2,3 Over the counter (OTC) minoxidil is available in 2% and 5% foams and solutions, which often contain alcohols that can cause dryness and irritation.4 This can be especially problematic for individuals with textured hair, which already has the tendency to be drier and more prone to breakage.5 Compounded topical minoxidil (CTM) can be formulated in a variety of vehicles, including as an oil or ointment, and may contain adjunct ingredients such as retinoids and corticosteroids to enhance product absorption, while minimizing scalp irritation.6,7 Limited research has examined adherence patterns and treatment experiences with CTM and OTC minoxidil, particularly in Black women with textured hair.
To better understand adherence patterns, side effect profiles, and clinical outcomes between OTC minoxidil and CTM, we conducted a retrospective chart review including all Black women with TA, AGA, or both treated at a tertiary care center between August 2020 and August 2024. Patients included in the study had a documented history of OTC minoxidil use and were currently being treated with CTM. Data collected included patient demographics, CTM formulation, patient-reported side effects, duration of use, and both patient- and physician-reported outcomes. Adherence was assessed at follow-up appointments based on self-reported CTM use. Chi-square analysis was used to assess associations between adherence patterns and clinical outcomes, with a p-value of less than 0.05 considered significant.
A total of 66 Black women were included: 33.3 percent (n=22) had TA, 37.9 percent (n=25) had AGA, and 28.8 percent (n=19) had a combination of both (Table 1). The most commonly used preparations were 7% minoxidil/0.025% retinoic acid/fluocinolone 0.01% oil, 8% minoxidil/0.025% retinoic acid/triamcinolone 0.1% ointment, and 10% minoxidil/0.025% retinoic acid/triamcinolone 0.1% ointment. Side effects from OTC minoxidil were reported by 21.2 percent (n=14) of patients, with scalp irritation being the most common. In contrast, only 10.6 percent (n=7) of patients reported side effects while using CTM, with 4.5 percent reporting scalp irritation and 6.1 percent reporting hypertrichosis. After receiving treatment with CTM for at least six months, patients had an overall adherence rate of 84.7 percent, compared to only 44.7 percent of patients who adhered to OTC minoxidil (p<0.001) (Table 2). Notably, clinical improvement was reported by 69.6 percent of patients using CTM, compared to 45.0 percent of patients using OTC minoxidil treatment (p<0.001).
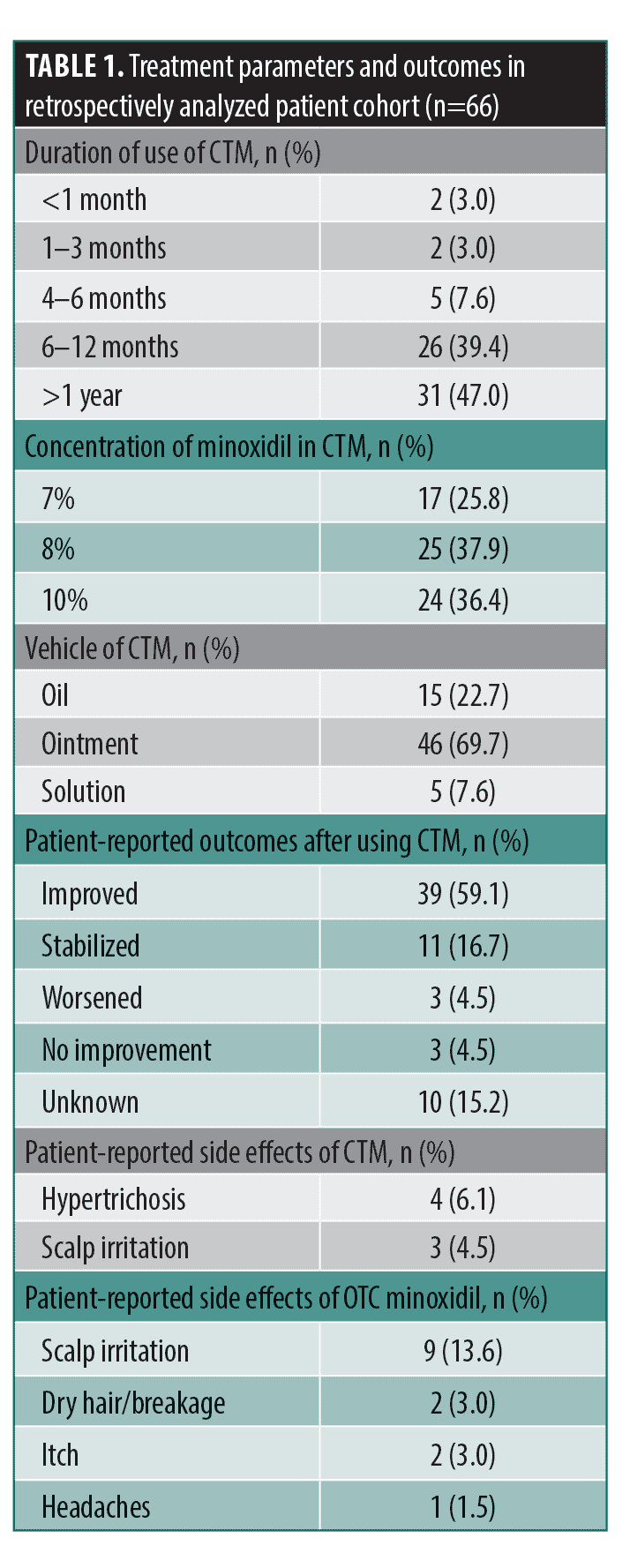
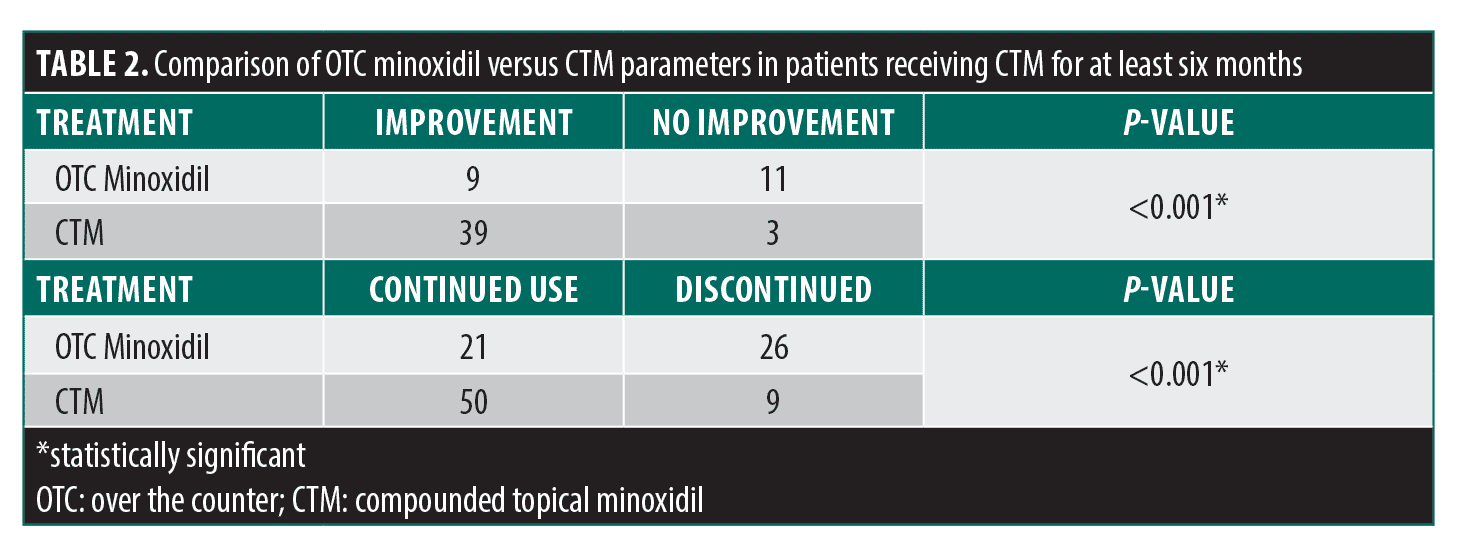
Our study found that CTM was associated with higher adherence, fewer side effects, and improved clinical outcomes in the majority of patients compared to their previous OTC minoxidil use. These differences may be attributed to a multitude of factors. One of the most commonly reported reasons for discontinuing OTC minoxidil is scalp irritation.2 This is often attributed to propylene glycol, a vehicle commonly used in OTC minoxidil solution to increase the hydrophilicity of minoxidil that is known to induce contact dermatitis.2,4 In contrast, the CTM formulation used in this study was propylene glycol-free and contained a corticosteroid, which likely helped to reduce irritation and enhance overall tolerability.
In addition to improved tolerability, CTM formulations may be more cosmetically compatible with textured hair. Textured hair is characterized by a tightly coiled, spring-like appearance in part due to the elliptical nature of the hair shaft.5 These structural features make textured hair more prone to dryness and breakage, as sebum has difficulty traveling from the scalp down the hair strand.5,8 Oil or ointment-based formulations may improve manageability and softness of hair strands, provide scalp lubrication, and coat the surface of the cuticle as the oils work their way down the hair strand, preventing moisture loss.5 In addition to this cosmetic compatibility, CTM may also align with cultural hair care practices. Applying oil or “grease” to the scalp is a longstanding tradition in the Black community. Together, these factors may contribute to increased long-term CTM treatment adherence in Black women.
In addition to tolerability, cosmetic compatibility, and cultural relevance, perceived lack of efficacy also plays a major role in treatment adherence.2 A recent study found that over two-thirds of participants discontinued OTC minoxidil due to lack of efficacy.2 Similarly, in our cohort, nearly one third of patients who reported discontinuing OTC minoxidil cited the same reason. However, over half of the patients in our cohort using CTM reported clinical improvement. One possible explanation for the perceived increase in efficacy is the inclusion of retinoic acid in the compounded formulation. Tretinoin has been shown to upregulate follicular sulfotransferase, an enzyme responsible for converting minoxidil to its bioactive metabolite, minoxidil sulfate. This may enhance efficacy, particularly in patients who are poor responders to topical minoxidil.4,9
Overall, our findings highlight several factors, both clinical and cultural, that may contribute to increased adherence of Black women using CTM compared to their previous OTC minoxidil use. When treating AGA or TA in Black women, special consideration should be taken when selecting minoxidil formulation, as it may significantly impact adherence and perceived efficacy. CTM’s improved tolerability, alignment with traditional haircare practices, consideration of hair texture, and enhancement of minoxidil activation may make it a more practical and effective treatment option for this population. Although this is a single institution study, our results reinforce the importance of tailoring treatment to patients’ hair type, preferences, and cultural haircare practices to support long-term, consistent use.
References
- Stoehr JR, Choi JN, Colavincenzo M, Vanderweil S. Off-Label Use of Topical Minoxidil in Alopecia: A Review. Am J Clin Dermatol. 2019;20(2):237–250.
- Shadi Z. Compliance to Topical Minoxidil and Reasons for Discontinuation among Patients with Androgenetic Alopecia. Dermatol Ther (Heidelb). 2023;13(5):1157–1169.
- Singh S, Patil A, Kianfar N, et al. Does topical minoxidil at concentrations higher than 5% provide additional clinical benefit? Clin Exp Dermatol. 2022;47(11):1951–1955.
- Sattur S, Talathi A, Shetty G, et al. Comparative Clinical Study Evaluating the Efficacy and Safety of Topical 5% Cetosomal Minoxidil and Topical 5% Alcohol-Based Minoxidil Solutions for the Treatment of Androgenetic Alopecia in Indian Men. Cureus. 2023;15(10):e46568.
- Aguh C, Okoye GA, editors. Fundamentals of ethnic hair: the dermatologist’s perspective. 1st ed. Cham: Springer International Publishing: Imprint: Springer; 2017.
- Sharma A, Goren A, Dhurat R, et al. Tretinoin enhances minoxidil response in androgenetic alopecia patients by upregulating follicular sulfotransferase enzymes. Dermatol Ther. 2019;32(3):e12915.
- Coman GC, Holliday AC, Mazloom SE, et al. A randomized, split-face, controlled, double-blind, single-centre clinical study: transient addition of a topical corticosteroid to a topical retinoid in patients with acne to reduce initial irritation. Br J Dermatol. 2017;177(2):567–569.
- Quaresma MV, Martinez Velasco MA, Tosti A. Hair Breakage in Patients of African Descent: Role of Dermoscopy. Skin Appendage Disord. 2015;1(2):99–104.
- Mehta N, Huang S, Dhura R, et al. Minoxidil sulfotransferase enzymatical activity in plants: A novel paradigm in increasing minoxidil response in androgenetic alopecia. J Cosmet Dermatol. 2024;23(1):339–343.
