J Clin Aesthet Dermatol. 2025;18(10):18–24.
by Hawasatu Dumbuya, PhD; Andrew Alexis, MD; Stephen Lynch, PhD; Valerie Callender, MD; Seemal R. Desai, MD; and Zoe Diana Draelos, MD
Dr. Dumbuya is with La Roche-Posay Laboratoire Dermatologique, L’Oreal USA in New York, New York. Dr. Alexis is with the Department of Dermatology at Weill Cornell Medicine in New York, New York. Dr. Lynch is with L’Oréal USA Research and Innovation in Clark, New Jersey. Dr. Callender is with Callender Dermatology and Cosmetic Center in Glenn Dale, Maryland. Dr. Desai is with Innovative Dermatology in Plano, Texas and the Department of Dermatology, The University of Texas Southwestern Medical Center in Dallas, Texas. Dr. Draelos is with Dermatology Consulting Services, PLLC, High Point, North Carolina.
FUNDING: Research was funded by La Roche-Posay, L’Oreal, USA.
DISCLOSURES: Dr. Dumbuya and Dr. Lynch are employees of L’Oreal USA. Drs. Alexis, Callender, Desai, and Draelos are researchers and consultants for L’Oreal.
Abstract: Objective: Populations with skin of color remain under-represented in dermatology clinical trials. Here, we first evaluated the efficacy of a serum and sunscreen regimen, containing 2-mercaptonicotinoyl glycine (2-MNG, MelasylTM), a new ingredient that quenches melanin precursors, on improving facial dyschromia in women with skin of color. Methods: This open-label study completed with 60 female participants, aged 25-70 years old, from diverse racial/ethnic backgrounds with skin phototypes IV-VI and presenting with mild to moderate hyperpigmentation and skin roughness. After completing a 1-week washout period, all participants used a 2-MNG-containing serum and sunscreen SPF30 regimen for 12 weeks. Evaluations included clinical assessments, quality of life questionnaires, plus imaging at several time points. Results: After 2 weeks of treatment, dermatological assessments showed significant improvement in skin brightness and radiance in all participants. At Week 4, we observed significant reduction in dyschromia and hyperpigmentation. Expert grading and clinical imaging also demonstrated significant improvement in skin smoothness and pores appearances. By Week 12, all participants perceived a significant improvement in quality of life: from feeling less unattractive to decreasing the use of camouflage to cover up skin concerns. Lastly, skincare regimen was overall well tolerated by all participants. Limitations: This study evaluated the 2-MNG containing skincare regimen efficacy and did not specifically address the effectiveness of individual ingredients, nor include a comparison to a gold standard treatment. Conclusion: Overall, our results demonstrate that a 2-MNG-containing serum and sunscreen SPF30 regimen effectively reduces the appearance of facial dyschromia, while improving skin texture and quality of life among women with skin of color. Keywords: Skin of color, dyschromia, hyperpigmentation, skincare, photoprotection
Introduction
Populations with skin of color (SOC), typically defined as belonging to a self-identified racial/ethnic group other than White and having Fitzpatrick phototype IV-VI, constitute a large and growing segment of the United States (US) and global population.1 As the prevalence, clinical appearance, and quality of life impact of dermatologic disorders can vary across the range of racial/ethnic groups, skin tones and phototypes, strategies to treatment involve a tailored and individualized approach.2 Women with SOC show fewer visible signs of photoaging (wrinkles and fine lines) compared to light skinned women.3 However, they are more susceptible to facial dyschromia or facial discoloration, such as uneven skin tone or hyperpigmentation.2-4 Facial dyschromia can be caused by increased melanin synthesis induced by various factors, including sun exposure, inflammation, hormonal changes, and certain medical conditions.5-7 Many studies have demonstrated that one of the major skin concerns of women with SOC is related to dyschromia, which has significant impact on their quality of life.2,6
Melanin, the main pigment that determines eyes, hair and skin color, is synthesized by the initial conversion of L-tyrosine amino acid into different melanin precursors through a process catalyzed by the rate-limiting tyrosinase enzyme.8 These precursors ultimately polymerize into different melanin pigments.8 Currently, topical formulations, containing hydroquinone, kojic acid, arbutin, azelaic acid, or other skin-lightening agents, remain a common option for the management of hyperpigmentary disorders, such as melasma and postinflammatory hyperpigmentation (PIH).9 Most of these ingredients work by inhibiting tyrosinase activity, preventing the conversion of L-3,4-dihydroxyphenylalanine (L-DOPA) to melanin.9,10 Despite their clinical efficacy, many of these tyrosinase inhibitors are associated with cutaneous adverse reactions, such as skin irritation, skin allergies, and less frequently, ochronosis, especially after long-term use of hydroquinone.9,11-14 In addition to cosmeceuticals, many studies have reported the benefits of adequate sun protection with broad-spectrum sunscreens in improving the appearance of facial dyschromia by preventing further skin darkening and neomelanogenesis.15-17
A novel molecule, 2-mercaptonicotinoyl glycine (2-MNG, MelasylTM) was recently identified and characterized in vitro and ex vivo, where it exhibited a high performance in reducing excess melanin.18 Unlike other conventional agents, 2-MNG shows an innovative mode of action: it intercepts certain melanin precursors, such as Dopaquinone, and inhibits their conversions into eumelanin and pheomelanin pigments, without inhibiting tyrosinase activity.19 This leads to a balanced inhibition of excess eumelanin and pheomelanin synthesis, without compromising the integrity of melanocytes.18,19 In addition, 2-MNG was also shown to exhibit superior efficacy in inhibiting both immediate and long-lasting UV-induced skin pigmentation compared to 13 other antipigmentation ingredients in a mini-zone clinical protocol.20 Another study recently demonstrated that 2-MNG can synergistically interact with other agents to further enhance the UV-induced skin pigmentation reduction.21 Despite skin discoloration being considered as one of the top dermatological concerns for populations with SOC, they remain underrepresented in clinical trials focused on skin-aging prevention and photoprotection.22 In this view, the objective of this study was to first evaluate the efficacy of a new skincare regimen, consisting of a 2-MNG-containing-serum and sunscreen, in improving overall facial dyschromia, as well as quality of life in women with SOC over 12 weeks.
Methods
Clinical protocol and demographics. This monocenter, open-label study was performed in accordance with the Good Clinical Practices and the principles of the Declaration of Helsinki (Dermatology Consulting Services, PLLC). The procedures used in this study were approved by Allendale Institutional Review Board. Before any study procedure, the participants received the necessary written and verbal information, including an informed consent form. Eligibility was determined by physical examination and confirmation of all inclusion/exclusion criteria. Female participants, aged 25-70 years old, from diverse racial/ethnic backgrounds, as self-identified, were enrolled into study. Participants were allowed to report more than one race, including mixed with White. Skin phototype was determined by Fitzpatrick questionnaires. The study completed with 60 female participants from Black and/or African American decent with skin phototypes IV-VI (20% FITZ IV, 53.3% FITZ V, 26.7% FITZ VI), and presenting with mild to moderate uneven skin tone, hyperpigmentation, and skin roughness. The study consisted of a 7-day washout period, followed by 12 weeks of treatment phase.
Investigational products and treatment. After completing a 1-week washout period, all participants used a 2-MNG-containing serum and sunscreen SPF30 regimen for 12 weeks. The tested serum contained 0.5% 2-MNG, combined with 10% niacinamide, cystoseira tamariscifolia extract, lipohydroxy acid, carnosine, retinyl palmitate and dipotassium glycyrrhizate (Mela B3 Dark Spot Serum, La Roche-Posay Laboratoire Dermatologique). The tested organic sunscreen SPF30, also contained 2-MNG and niacianamide (Mela B3 UV Daily Moisturizer SPF 30, La Roche-Posay Laboratoire Dermatologique). Participants were instructed to apply the serum twice a day (morning and evening), while the sunscreen was applied at least once a day 15 minutes before sun exposure. An auxiliary moisturizer was also provided to all participants for use as needed. Prior to starting treatment phase, all participants carried out a 7-day washout period, during which they only use their respected cleanser and auxiliary moisturizer. After completing the washout period, all participants self-applied investigational products at the test center on evaluation visits, and then at-home in the mornings and evenings as instructed for 12 weeks. In addition, all participants continue to use their cleanser and provided moisturizer at home as needed.
Dermatological clinical assessments. Clinical grading for facial dyschromia and skin texture related-attributes were performed on all participants on full face at baseline, Week 2, 4, 8 and 12 by a board-certified dermatologist, using a Modified Griffiths and internal grading scales respectively, both using a 10-point scale (0=none, 1-3=mild, 4-6=moderate, and 7-9=severe).23
Self-assessment questionnaires. Questions, using an internal 5-point scale, regarding quality of life improvements (1=not bothered at all to 5=constantly bothered), and perceived product efficacy on several skin attributes (1=completely disagree to 5=completely agree), were completed by all the participants at different time points compared to baseline.
Clinical imaging. VISIA-CR 4.3 (Canfield Scientific) imaging for photography of participants’ full face were taken at baseline and after 12 weeks of treatment.
Safety and tolerance assessments. A board-certified dermatologist evaluated local cutaneous tolerability by assessing the signs and symptoms of erythema, edema, peeling and dryness, and the participants reported the degree of burning, stinging, tingling, tightness, and itching on the treatment area. A self-assessment diary sheet was also provided for participants to record any local intolerance at home. The following scales and definitions were used for tolerability evaluations: 0-None, 1-Mild, 2-Moderate, 3-Severe.
Statistical methods. All clinical endpoints of efficacy, participant self-assessment questionnaires, and tolerance parameters were expressed as change from baseline data and time. The data were analyzed using a linear mixed model. For clinical grading improvements at Weeks 2, 4, 8, and 12 were calculated from baseline and plotted as “mean clinical grading score.” A decrease in the mean clinical score indicated improvement with the treatment for each evaluated parameter at indicated time point. For product efficacy self-assessment, participant self-perceived improvements at Weeks 4 and 12 were calculated as percentage from baseline. An increase indicated improvements in product self-perceived efficacy. Improvement in quality of life at Week 12 compared to baseline was calculated and plotted. A decrease in the grading score indicated self-perceived improvement in quality of life. All data was calculated as mean and statistical significance was expressed as statistically significant different vs. baseline.
Results
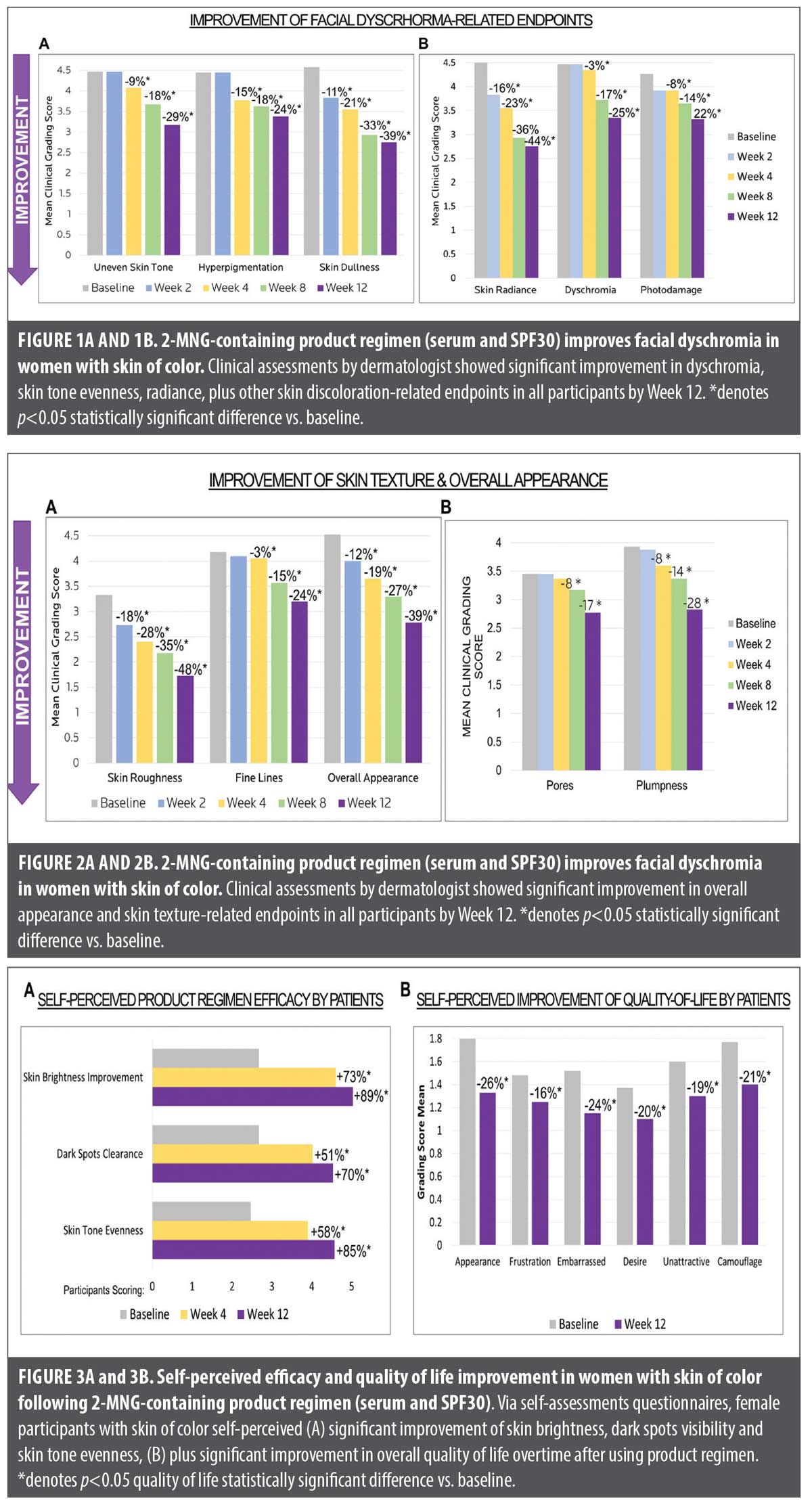
Improvement of facial dyschromia in women with skin of color. Figures 1A and 1B demonstrate the clinical assessments by a board-certified dermatologist of facial dyschromia-related skin attributes overtime, following 12 weeks of treatment with 2-MNG-containing skincare regimen in female participants with skin of color.
Statistically significant improvement in uneven skin tone was observed starting at Week 4, continued to Week 8 (18% improvement), reaching 29% improvement by Week 12. Significant decrease in skin hyperpigmentation was observed starting at Week 4 (15%), continuing into Week 8 (18%), and reaching 24% reduction by 12 weeks. Significant reduction in skin dullness was observed as early as Week 2 (11%), reaching 39% by Week 12. Skin radiance was also shown to significantly improve as early as Week 2 (16%) and continued until Week 12, reaching 44% improvement. Decrease in facial dyschromia and overall skin photodamage were comparable overtime, reaching 25% and 22% significant reduction respectively by Week 12.
In summary, following 12 weeks of using a 2-MNG-containing serum and sunscreen SPF 30 regimen significantly reduces the appearance of facial dyschromia and related discoloration endpoints in women with SOC.
Improvement of skin texture in women with skin of color. Figures 2A and 2B show the clinical assessments by board-certified dermatologist of skin texture and quality parameters overtime, following 12 weeks of treatment with 2-MNG-containing skincare regimen in female participants with skin of color.
After just 2 weeks of treatment, visual and tactile skin roughness significantly decreased by 18%, reaching 48% reduction by Week 12, suggesting that the serum and sunscreen regimen smoothed the skin surface overtime. Starting at Week 4, clinical assessments revealed significant reduction in fine lines appearance and increase in skin plumpness, reaching 24% and 28% improvement respectively by Week 12. Consistent with these results, overall skin appearance significantly improved as early as 2 weeks (12%), reaching 39% improvement by Week 12.
In summary, 12 weeks of treatment with a 2-MNG-containing serum and sunscreen SPF30 regimen significantly improves the overall skin quality and texture-related endpoints in women with SOC.
Self-perceived efficacy and quality of life improvement in women with skin of color. Questionnaires were filled out by participants at the study center at baseline, Week 4 and Week 12 evaluation visits. Compared to baseline, participants self-perceived a 73% increase in skin brightness by Week 4, reaching 89% by Week 12. Participants also self-perceived dark spots clearance to improve by 51% at Week 4 and by 70% at Week 12. Skin tone evenness was self-perceived by the participants to be improved by 58% at Week 4, reaching 85% by Week 12 (Figure 3A). Additionally, participants were asked to rate their quality of life at baseline and following 12 weeks of treatment. In alignment with their perceptions on the serum and sunscreen regimen efficacy, by Week 12 they also self-perceived a significant improvement in quality of life: from feeling less unattractive to decreasing the use of camouflage to cover up skin discoloration-related concerns (Figure 3B).
In summary, as early as 4 weeks of using the 2-MNG-containing serum and sunscreen SPF 30 regimen, women with SOC were able to self-perceive improvement in facial dyschromia endpoints, which resulted in a significant improvement in their quality of life overtime.
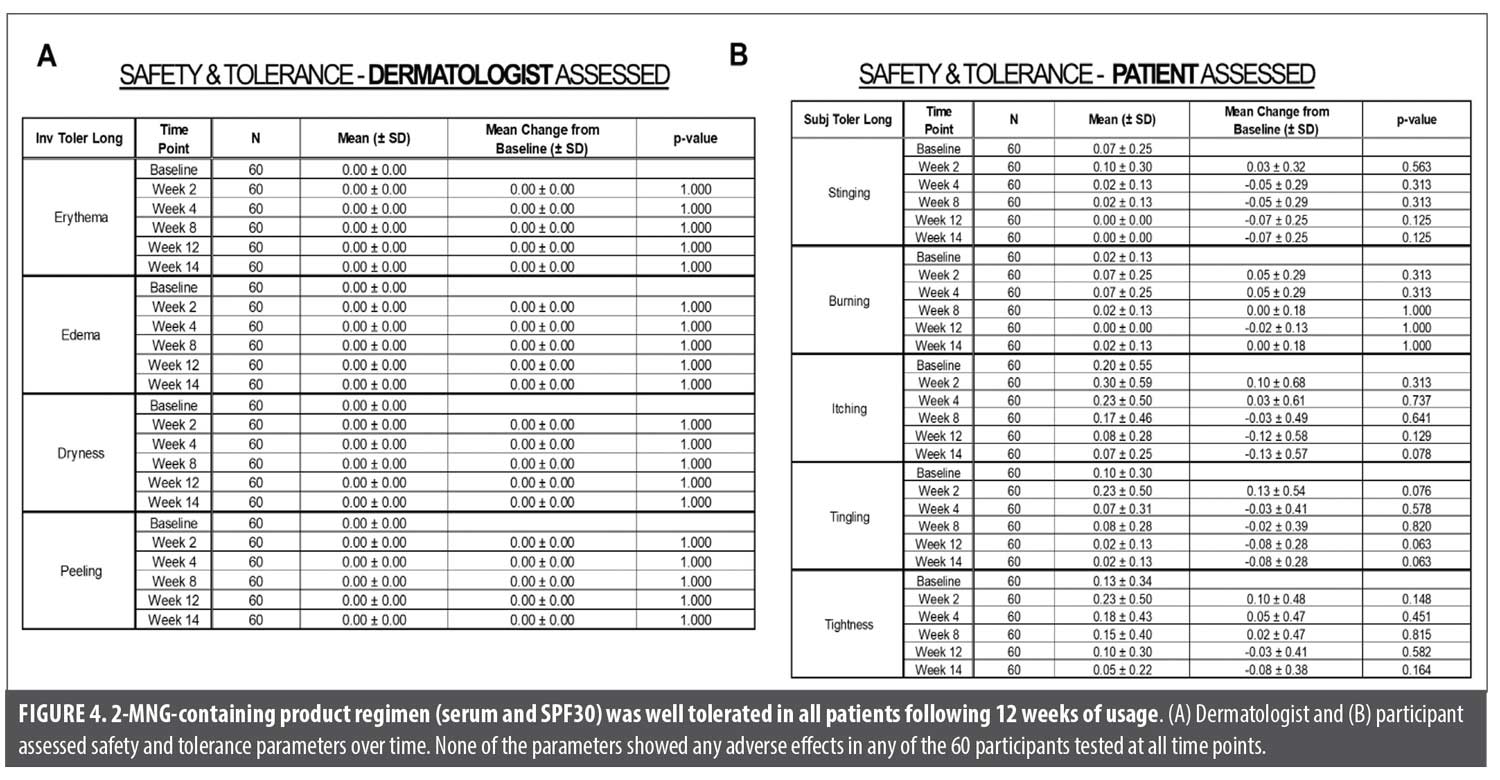
Safety and tolerance assessments in women with skin of color. Figures 4A and 4B show the safety and tolerance assessments, performed by board-certified dermatologist and clinical participants for the following: erythema, edema, dryness and peeling. No adverse effects in any of the 60 participants evaluated at all time points were observed throughout study. Overall, the 2-MNG-containing serum and sunscreen SPF30 regimen was well tolerated in women with SOC after 12 weeks of usage under study conditions.
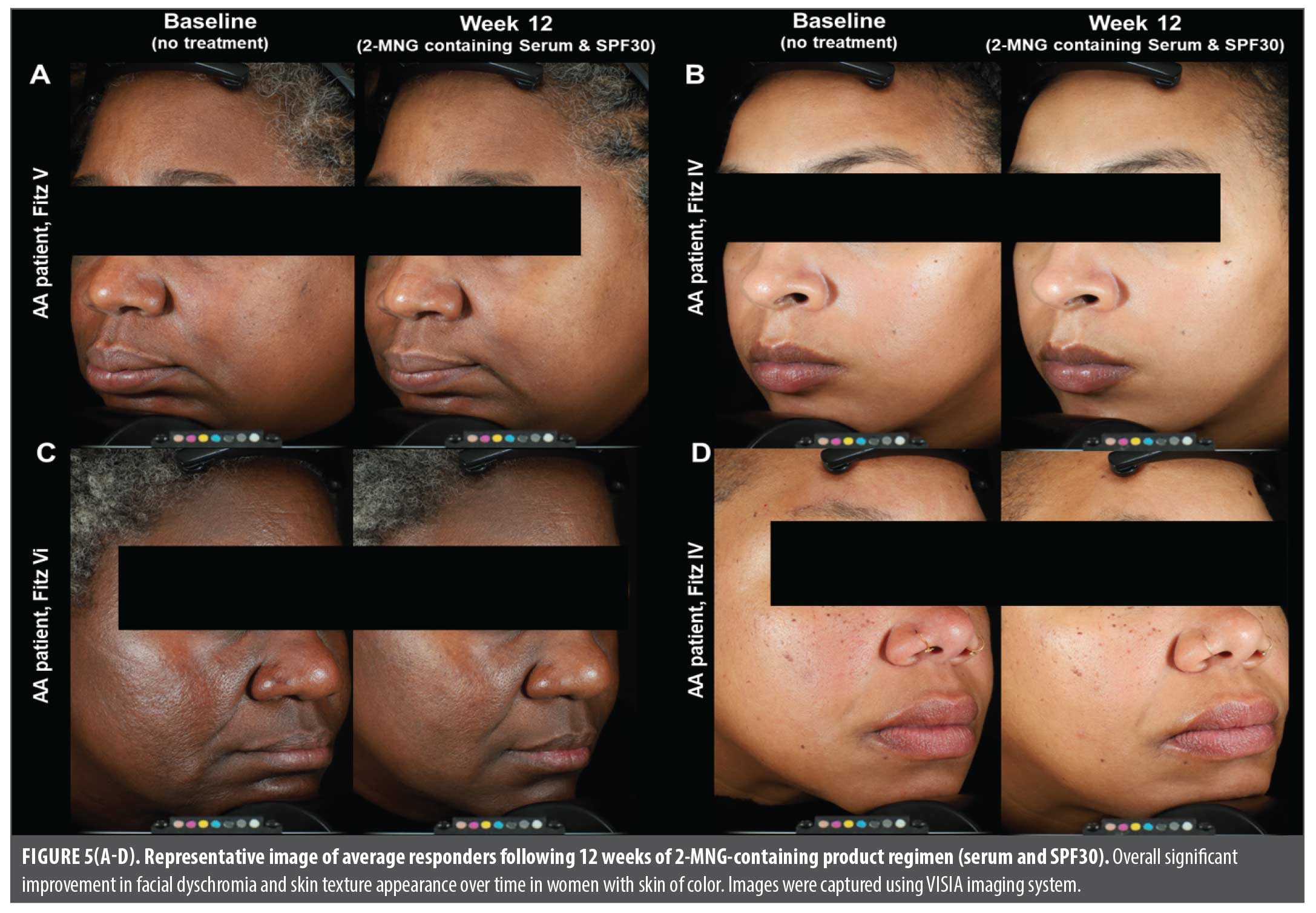
Clinical images of women with skin of color following 2-MNG-containing skincare regimen. Representative VISIA-CR standard clinical images of 4 average responders are shown in Figure 5. Overall significant improvement in facial dyschromia and skin texture, plus quality can be observed following 12 weeks of treatment with 2-MNG-containing serum and sunscreen SPF30 regimen.
Discussion
Dyschromias are a common subset of dermatologic disorders that cause significant distress and disfigurement in darker skinned ethnic groups, and especially pronounced in aging darker skin, presenting either as hypopigmentation or hyperpigmentation. The most common dyschromias in darkly pigmented patients include melasma and PIH.24,25 Both disorders are associated with psychosocial and emotional distress.26 Participants with pigmentary disorders have also been reported to have high prevalence of anxiety and depression that correlated with the disease severity.27 Many studies have reported that populations with SOC are more susceptible to experiencing dyschromia.2 Because of the chronic nature of the disease and established psychosocial and emotional impacts that affect the quality of life of patients, there is a need to ensure the best available treatment for individuals presenting with facial dyschromias.28 Despite skin discoloration being considered as one of the top dermatological concerns, plus highly prevalent in patients with skin of color, they remain underrepresented in clinical trials focused on skin-aging prevention and photoprotection.22 Here, we first demonstrate the clinical efficacy of a serum and sunscreen regimen containing 2-MNG, a new ingredient that intercepts melanin precursors without inhibiting tyrosinase, on improving facial dyschromia, skin texture and quality of life in women with SOC.
Several structural and functional differences exist among darkly pigmented skin types, many of which have important clinical implications for disease and aesthetics, plus may play a role in their response to cosmetic dermatologic treatments. Darker pigmented individuals’ epidermal skin contains an increased amount of melanin, derived from melanocytes located in the basal cell layer of the epidermis. Studies have shown that there is no major difference in the number of melanocytes between ethnic groups; however, melanosomes, are not only more numerous in darkly pigmented skin, but are also larger, more singly dispersed, plus higher in melanin content with a slower rate of degradation.8 Pigment lability, which manifests through exaggerated responses of melanocytes to cutaneous stimulation (eg, sunlight, irritating topical medications, or medical conditions), is also a prominent feature observed in populations with skin of color and contributes to the development of dyschromias.6,8
Melanin synthesis involves the initial conversion of L-tyrosine into different melanin precursors, which ultimately polymerize into melanin pigments; tyrosinase is the rate-limiting enzyme in this process.8 Currently, the most used topical depigmenting agents are hydroquinone, arbutin, niacinamide, and kojic acid.5,7,9,10 These agents work by preventing the conversion of L-3,4-dihydroxyphenylalanine (L-DOPA) to melanin by inhibiting tyrosinase. Other topical antioxidant treatments work by preventing the polymerization of melanin by scavenging free radicals.29 All these topical agents have the potential risk of developing adverse events in patients depending on the use.9,11-14 In addition, hydroquinone and derivatives, while reducing the formation of melanosomes, are also melanotoxic and carcinogenic at higher levels or excessive lengths of treatment.30 Therefore, there remains a need for effective nonhydroquinone actives to target the pigmentary mechanisms that are safer, show good tolerance overtime, and demonstrate fast clinical efficacy without harmful side effects.
Recent research from the L’Oreal group demonstrates the discovery of a novel molecule 2-MNG, using high throughput screening methods, that possesses superior melanogenic inhibitory activity.18-21 The effectiveness of 2-MNG was confirmed by topical application on pigmented reconstructed epidermis and human skin explants.18,19 Unlike most other melanogenesis inhibitors, such as kojic acid or hydroquinone, which inhibit tyrosinase, or antioxidants, such as vitamin C, which prevent polymerization of melanin, this new molecule has a different mode of action.18-21 2-MNG binds to certain melanin precursors to inhibit their conversion to eumelanin and pheomelanin pigments, while preserving the integrity of melanocytes.18,19 The superiority of this molecule over other traditional melanogenic inhibitors was also established using in vivo methods.20,21 The efficacy of 2-MNG in inhibiting both immediate darkening and delayed tanning, suggested two distinct but complementary mechanisms of action to prevent UV-induced skin pigmentation in vivo.19-21 Recently, the efficacy and safety of a new dermocosmetic serum, containing 0.5% 2-MNG, 10% niacinamide, and other cosmetic ingredients, was shown for the management of PIH in an open-label study, and melasma in comparison to gold-standard treatment, hydroquinone.31,32 In the latest study, the 2-MNG-containing dermocosmetic serum demonstrated parity in terms of efficacy and better tolerability versus hydroquinone at 4% after three months of treatment in patients presenting with mild-to-severe epidermal or mixed facial melasma.32 These studies provided us with an opportunity to first test this new compound 2-MNG in a skincare regimen system, consisting of a serum and an organic sunscreen on improving facial dyschromia in women with SOC.
Overall, our results indicated that after 2 weeks of using a 2-MNG skincare regimen (serum and organic sunscreen SPF30), dermatological assessments combined with clinical images, showed significant improvement in skin brightness and radiance, plus overall skin appearance in all participants (Figure 1 and Figure 5). Starting at Week 4, we observed significant reduction in hyperpigmentation, dyschromia, plus photodamage (Figure 1). Interestingly, expert grading and clinical imaging also demonstrated significant improvement in skin smoothness, fine lines, and pores appearances, which could be due to the comprehensive serum formula and key ingredients working to improve skin texture (Figure 2 and Figure 5). By Week 12, all participants self-perceived a significant improvement in quality of life: from feeling less unattractive to decreasing the use of camouflage to cover up skin discoloration-related concerns (Figure 3). Lastly, safety and tolerance assessments revealed no adverse effects throughout treatment phase, suggesting that 2-MNG-containing skincare regimen is well tolerated for both short and long-term used (Figure 4).
Based on our design, our study had limitations. Here, we only evaluated the 2-MNG-containing skincare regimen efficacy and did not specifically address the effectiveness of individual ingredients, particularly in the serum, nor individual tested products (serum vs. sunscreen). Therefore, we cannot conclude the clinical efficacy solely due to the 2-MNG ingredient nor serum, since broad-spectrum sunscreens are well documented to also help improve the appearance of facial dyschromia and skin texture.15-17 The study design also did not include a comparison to a gold standard treatment. Future studies are needed to further evaluate the suitability of combing this new skincare regimen with other topical agents or procedures in managing pigmentary concerns.
Conclusion
In summary, our findings demonstrate that a 2-MNG-containing serum and sunscreen SPF30 regimen can effectively and safely reduce the appearance of facial dyschromia-related concerns and improve skin texture and quality overtime in individuals with skin of color; these results indicate that this new skincare regimen could serve as an effective non-hydroquinone alternative to manage hyperpigmentary disorders in diverse racial/ethnic patients, with additional benefits in improving skin texture. The improvement in overall quality of life experienced by clinical participants after using skincare regimen overtime in our study, may help support clinicians on short and longer-term skin aging prevention and photoprotection strategies to consider for all patients, particularly for skin of color.
References
- Colby S, Ortman J. Projections of the Size and Composition of the US Population: 2014 to 2060. US Department of Commerce. US Census Bureau. https://www.census.gov/content/dam/Census /library/publications/2015/demo/p25-1143.pdf. Accessed July 1, 2025.
- Burgess CM. Global Perspectives of the Patient of Color. J Drugs Dermatol. 2019 18 (7):615.
- Campiche R, Trevisan S, Séroul P, et al. Appearance of aging signs in differently pigmented facial skin by a novel imaging system. J Cosmet Dermatol. 2019; 18(2) 614-627.
- Venkatesh S, Maymone MBC, Vashi NA. Aging in skin of color. Clin Dermatol. 2019 37(4):351-357.
- Nautiyal A, Wairkar S. Management of hyperpigmentation: current treatments and emerging therapies. Pigment Cell Melanoma Res. 2021;34(6):1000-14.
- Syder NC, Quarshie C, Elbuluk N. Disorders of facial hyperpigmentation. Dermatol Clin. 2023;41:393-405.
- Perez- Bernal A, Munoz- Perez MA, Camacho F. Management of facial hyperpigmentation. Am J Clin Dermatol. 2000;1:261–8.
- Bento-Lopes L, Cabaço LC, Charneca J, et al. Melanin’s Journey from Melanocytes to Keratinocytes: Uncovering the Molecular Mechanisms of Melanin Transfer and Processing. Int J Mol Sci. 2023;24(14):11289.
- Searle T, Al- Niaimi F, Ali FR. The top 10 cosmeceuticals for facial hyperpigmentation. Dermatol Ther. 2020;33(6):e14095
- Sofen B, Prado G, Emer J. Melasma and post inflammatory hyperpigmentation: management update and expert opinion. Skin Therapy Lett. 2016;21(1):1-7
- Ocampo‐Candiani J, Alas‐Carbajal R, Bonifaz‐Araujo JF, et al. Latin American consensus on the treatment of melasma. Int J Dermatol. 2025;64(3):499-512.
- Patel N, Shah H. Exogenous Ochronosis from Skin-Lightening Cream. N Engl J Med. 2024 18;390(3):254.
- Vernhet L, Dendooven E, Pasteur J, et al. First Two Cases of Allergic Contact Dermatitis From Isobutylamido Thiazolyl Resorcinol (‘Thiamidol’) in Depigmenting Skin Care Routine. Contact Dermatitis. 2025 Mar 28.
- Ishack S, Lipner SR. Exogenous ochronosis associated with hydroquinone: a systematic review. Int J Dermatol. 2022;61(6):675-684.
- Randhawa M, Wang S, Leyden JL, et al. Daily Use of a Facial Broad-Spectrum Sunscreen Over One-Year Significantly Improves Clinical Evaluation of Photoaging. Dermatol Surg. 2016, 42(12):1354-1361.
- Passeron T, Lim HW, Kang HY, et al. Photoprotection according to skin phototype and dermatoses: practical recommendations from an expert panel. J Eur Acad Dermatol Venereol. 2021;35(7):1460-1469.
- Grimes PE, Paturi J, Chen Y, et al. Photoprotection Efficacy of Sun Protection Factor and Iron Oxide Formulations in Diverse Skin With Melasma and Photodamage. J Drugs Dermatol. 2025;24(7):662-667.
- Sextius P, de Dormael R, Lereaux G, Warrick E, Kerob D, Marat S, Diridollou S. Discovery of 2-Mercapto Nicotinoyl glycine a new potent skin lightening with a proven clinical efficacy. Presented at: European Academy of Dermatology and Venereology Congress; October 11-14, 2023; Berlin.
- Sextius P, Warrick E, Lereaux G, et al. 2- Mercaptonicotinoyl glycine, a new potent melanogenesis inhibitor, exhibits a unique mode of action while preserving melanocyte integrity. Pigment Cell Melanoma Res. 2024;37:462-479.
- Muller B, Flament F, Jouni H, et al. A Bayesian network meta- analysis of 14 molecules inhibiting UV daylight-induced pigmentation. J Eur Acad Dermatol Venereol. 2024;38:1566-1574.
- de Dormael R, Sextius P, Bourokba N, et al. 2-Mercaptonicotinoyl glycine prevents UV- induced skin darkening and delayed tanning in healthy subjects: A randomized controlled clinical study. J Cosmet Dermatol. 2024;23:1745-1752.
- Callender VD, Harvey VM, Hartman CL, et al. Do Women with Skin of Color Think They Are Well Represented in Skin Aging Prevention Information? J Clin Aesthet Dermatol. 2024 Apr;17(4):18-22.
- Gold MH, Wilson A, Makino E, et al. Improvements in skin quality parameters in postmenopausal participants after use of topical growth factor serum, J Cosmet Dermatol, 22(1), 236-244.
- Bolognia JL, Pawelek JM, Biology of hypopigmentation. J Am Acad Dermatol. 1988 19(2 Pt 1):217-55.
- Marcelyn KC, Alexis AF. Cosmetic Concerns in Skin of Color, Part 1, Cosmet Dermatol; 2009 • Vol. 22 No. 7
- Desai SR, Alexis AF, Elbuluk N, et al. Best practices in the treatment of melasma with a focus on patients with skin of color. J Am Acad Dermatol. 2024, 90(2):269-279
- Dabas, G, Vinay, K, Parsad D, et al. Psychological disturbances in patients with pigmentary disorders: a cross-sectional study. J Eur Acad Dermatol Venereol. 2019, 34, 392-399.
- Nautiyal A, Wairkar S. Management of hyperpigmentation: current treatments and emerging therapies. Pigment Cell Melanoma Res. 2021;34(6):1000-14.
- Zilles JC, Dos Santos FL, Kulkamp-Guerreiro IC, et al. Biological activities and safety data of kojic acid and its derivatives: a review. Exper Dermatol. 2022;31(10):1500-21.
- Draelos ZD. Skin lightening preparations and the hydroquinone controversy. Dermatol Ther. 2007;20(5):308-313.
- Demessant-Flavigny AL, Petkar G, Jodun D, et al. Efficacy of a 2- MNG- Containing Depigmenting Serum in the Treatment of Post- Inflammatory Hyperpigmentation. J Cosmet Dermatol. 2025 Feb;24(2):e16735.
- Passeron T, Kerob D, Le Dantec G, et al. Efficacy and Tolerability of a New Facial 2-Mercaptonicotinoyl Glycine-Containing Depigmenting Serum Versus Hydroquinone 4% over 3-Month Treatment of Facial Melasma. Dermatol Ther (Heidelb). 2025. 2013;40:249-260
- Klassen AF, Cano SJ, Schwitzer JA, et al. FACE-Q scales for health-related quality of life, early life impact, satisfaction with outcomes, and decision to have treatment: development and validation. Plast Reconstr Surg. 2015;135:375-386
- Baumann L, Weiss RA, Grekin S, et al. Comparison of hyaluronic acid gel with (HARDL) and without lidocaine (HAJUP) in the treatment of moderate-to-severe nasolabial folds: A Randomized, Evaluator-Blinded Study. Dermatol Surg. 2018;44(6):833-840.