 J Clin Aesthet Dermatol. 2022;15(5):12–18.
J Clin Aesthet Dermatol. 2022;15(5):12–18.
by Elizabeth Grieshaber, MD and Alison Glorioso, CMA
Both authors are with Terezakis and Grieshaber Dermatology in Metarie, Louisiana
FUNDING: This study was sponsored by Colorescience, Inc.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. A post-treatment skincare protocol (Finishing Touch™ Protocol; Colorescience®, Inc.) comprising several commercial products was developed for use after minimally invasive facial procedures.
Objective. Our objective was to assess the effect of this post-treatment protocol on subject experiences following radiofrequency microneedling including confidence to resume normal activities, treatment satisfaction and likelihood for retreatment.
Methods. Healthy female subjects, aged 21 to 65 years with Fitzpatrick Skin Types I to IV seeking facial rejuvenation with radiofrequency microneedling were enrolled (N=29). The microneedling procedure was performed during the baseline visit. Digital images were obtained before and immediately following microneedling, and after post-procedure application of the skincare protocol. A nonmedicated barrier ointment was also applied prior to the skincare protocol immediately following treatment in a third group of subjects. Subsequently, the skincare protocol was applied each morning after regular skincare routines. Subjects returned after four weeks for a final assessment, a self-assessment questionnaire, and treatment satisfaction ratings.
Results. Following skincare protocol application, most subjects (97%) observed improvements in skin redness and/or treatment site marks, felt more confident (90%) and were very or extremely comfortable resuming normal activities (86%). Mild-to-moderate adverse events (n=4) resolved and were consistent with adverse events reported in the literature with no bleeding immediately following the microneedling with radiofrequency treatment.
Limitations. Primary limitations were the small number of subjects and self-reported outcomes.
Conclusion. Based on our results, the studied post-treatment skincare protocol appeared to improve subject confidence, comfort, satisfaction, and the likelihood of a repeat radiofrequency microneedling procedure among the included patients. No reported adverse events incremental to standard microneedling were observed.
Keywords: microneedling with radiofrequency, minimally invasive procedures, post-treatment skincare, skincare protocol
The number of patients seeking treatment for facial skin wrinkles, hyperpigmentation, sun damage, and acne scars continues to rise. Since 2000, minimally-invasive procedures have increased by 237 percent and now exceed 16 million annually.1 To meet this demand, numerous minimally-invasive procedures have been developed, such as laser and radiofrequency resurfacing,2, 3 dermal fillers,4 chemical peels,5 microfocused ultrasound,6 helium plasma,7 intense pulsed light,8 and microneedling.9
Microneedling, also known as collagen induction therapy, is a common dermal therapy that utilizes devices that penetrate the dermis to a uniform depth, creating a controlled skin injury. This injury induces rapidly healing micropunctures with subsequent stimulation of collagen and elastin fiber production, resulting in skin remodeling and rejuvenation.9 Microneedling was originally performed with handheld rollers for treating acne scars, stretch marks, wrinkles and facial rejuvenation;10–12 however, microneedling devices have more recently been combined with the delivery of radiofrequency energy to heat underlying layers of skin and enhance dermal remodeling and improved clinical outcomes.13
Microneedling is generally regarded as a safe and relatively inexpensive alternative to other forms of skin rejuvenation.9 A topical anesthetic is usually applied prior to the procedure to minimize discomfort.9 In a recent review, the most common side effects associated with this treatment include transient pain or discomfort, erythema, and edema.14 Although these effects are mild and spontaneously resolve, they can lead to patient concerns about their appearance immediately following the procedure, which might adversely affect self-confidence and impact their social lives.
In an unpublished study (Data on file. Colorescience®, Inc., Carlsbad, CA.), 34 subjects underwent 42 minimally invasive facial cosmetic procedures in six clinics, including chemical peels (n=13), intense pulsed light (n=7), laser resurfacing (n=7), microneedling (n=6), dermal fillers (n=5), and other minimally invasive procedures (n=4). Digital images were obtained prior to the cosmetic procedure, immediately post-treatment, after the post-treatment application of a series of skin protection and skin care products (Colorescience®, Inc. Carlsbad, CA) and after four weeks of use. After the post-procedure application of skincare products, subjects were shown their pre- and post-treatment images and asked to respond to several questions about their treatment. Although 50 percent of subjects were very or extremely uncomfortable immediately following their procedure, 94 percent would otherwise have been very or extremely self-conscious in public. Also, 88 percent of subjects reported their skin felt more comfortable following application of the skincare products, 95 percent would be less self-conscious in public, 94 percent reported the products immediately made their skin look better, 97 percent would be more likely to consider another having treatment again and 87 percent believed it improved their overall impression of their treatment procedure. After four weeks, most subjects reported their skin felt younger and healthier (94%), were more confident about repeating their procedure (94%) and would continue using their skincare as part of their daily routine (94%).
Based on these promising results, a post-treatment skincare protocol was developed specifically for use after minimally-invasive facial procedures (Finishing Touch™ Protocol; Colorescience®, Inc., Carlsbad, CA). The objective of the following study was to assess product safety and the effect of this post-treatment protocol on several subject experiences including confidence to resume normal activities, comfort, treatment satisfaction and likelihood to undergo retreatment following facial radiofrequency microneedling.
Methods
Study subjects. Female subjects, aged 21 to 65 years with Fitzpatrick Skin Types I to IV seeking facial rejuvenation with radiofrequency microneedling were enrolled. Subjects were required to be in generally good health and free of any disorder or condition that might place the subject at risk or impair facial evaluations. Each subject expressed their willingness to follow all study requirements and commit to all follow-up visits. Subjects were permitted to continue using their usual cosmetic products or medications but not add any new products to avoid any confounding effects. Usual skin products included over-the-counter and physician-dispensed cleansers, serums, lotions, creams, or sunscreens. Subjects of child-bearing potential provided a negative urine pregnancy test result at the Screening Visit and agreed to use an effective method of birth control during the study.
Reasons for exclusion from the study included the use of a systemic retinoid within 180 days or topical retinoid within 60 days; presence of open facial wounds, neurotic excoriations, or dermatitis; skin cancer or suspicious lesions on the planned treatment area; allergy or sensitivity to study product ingredients; active facial psoriasis, eczema, sunburn, excessive scarring, tattoos, or other skin condition that could interfere with study assessments; uncontrolled systemic illness; ablative laser resurfacing, facial peel, microdermabrasion, or a microneedling treatment within 30 days; facial non-ablative laser or intense pulsed light treatment within 60 days; participation in another study within 30 days; concurrent participation in another research study; nursing, pregnant, or planning to become pregnant.
The study was designed to compare subject responses regarding their pre- and post-treatment experience with microneedling with radiofrequency immediately before and after application of the skincare protocol and after four weeks post-procedure application. As topical products are often applied following facial microneedling,16 the study also included a third group of subjects who applied a non-medicated barrier ointment prior to the skincare protocol application following the microneedling procedure.
Ethics. This study protocol and related materials were approved by a commercial institutional review board (WCG – Aspire IRB, Santee, CA). Each subject provided signed informed consent and photography release prior to participating in any study-related activities. This study was conducted in compliance with International Conference of Harmonization Good Clinical Practice Guidelines and Federal Policy for the Protection of Human Subjects, or the Common Rule (45 CFR 46).
Study procedures. The screening visit occurred two weeks prior to the radiofrequency microneedling procedure. During the screening visit, subjects provided written informed consent and HIPAA and photographic release. Inclusion and exclusion criteria, medical history, and current medications were reviewed. Baseline standardized digital images were obtained approximately 20 minutes after cleansing the facial skin. Subjects were randomized to one of three treatment groups (Table 1) and instructions were provided for applying the full-face post-microneedling skincare protocol (Finishing Touch™ Protocol; Colorescience, Inc., Carlsbad, CA).
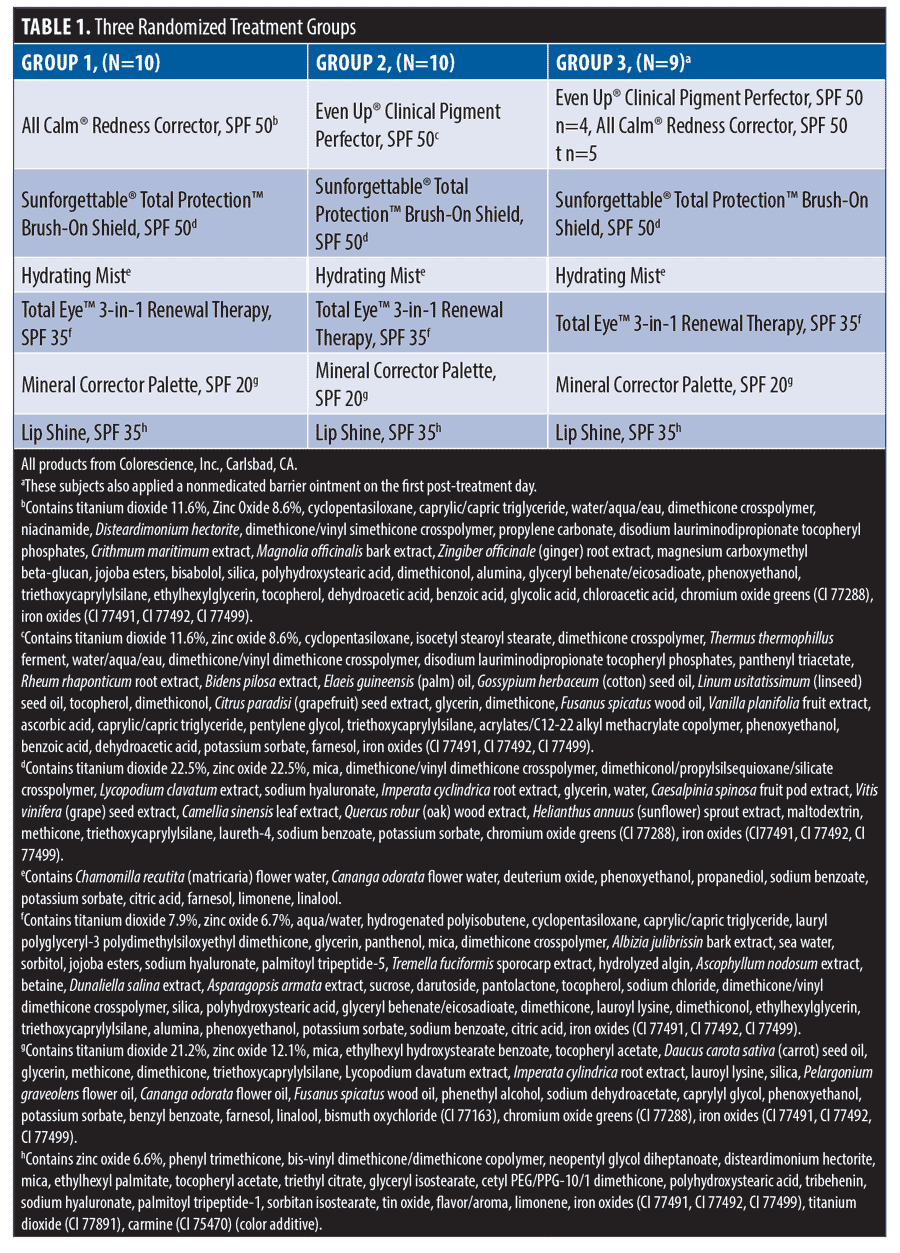
The microneedling with radiofrequency procedure was performed during the baseline visit (Legend Pro™; Lumenis® Inc., San Jose, CA) using a 6×6 needle array, 150 µm width, 0.6 mm length, at a frequency of 1 MHz and power up to 17.5 W. The skincare protocol was applied following the procedure and every morning. Immediately following the procedure, a subgroup of subjects applied a nonmedicated barrier ointment prior to the skincare protocol. Subsequently, products were applied each morning after regular skin care routines and before applying makeup. The subjects followed their regular evening skincare regimen. Subjects received a supply of the skincare protocol for use during the study.
Subjects returned after four weeks for a final assessment visit when digital images were obtained, the investigator assessed skin health and treatment recovery, current medications and adverse events were reviewed, and subjects completed a self-assessment questionnaire and rated treatment satisfaction from 1 (Excellent, very satisfied) to 4 (Poor, not satisfied at all).
Statistical Analysis. Changes from baseline through Week 4 were analyzed using Chi-square or Fisher’s exact tests for small sample sizes to compare responses between product groups (SPSS Statistics for Windows, v 27.0; IBM Corporation, Armonk, NY). Statistical testing was two-sided and interpreted with an α=0.05.
Results
The mean age of enrolled subjects (N=29) was 42.7 years (range, 23-63 years). Subjects self-identified as Caucasian (n=26) and Hispanic (n=3) with Fitzpatrick Skin Types I (n=4), II (n=21), III (n=3) and IV (n=1). The third group of subjects (n=9) applied a non-medicated barrier ointment prior to the skincare protocol application on post-treatment Day 1.
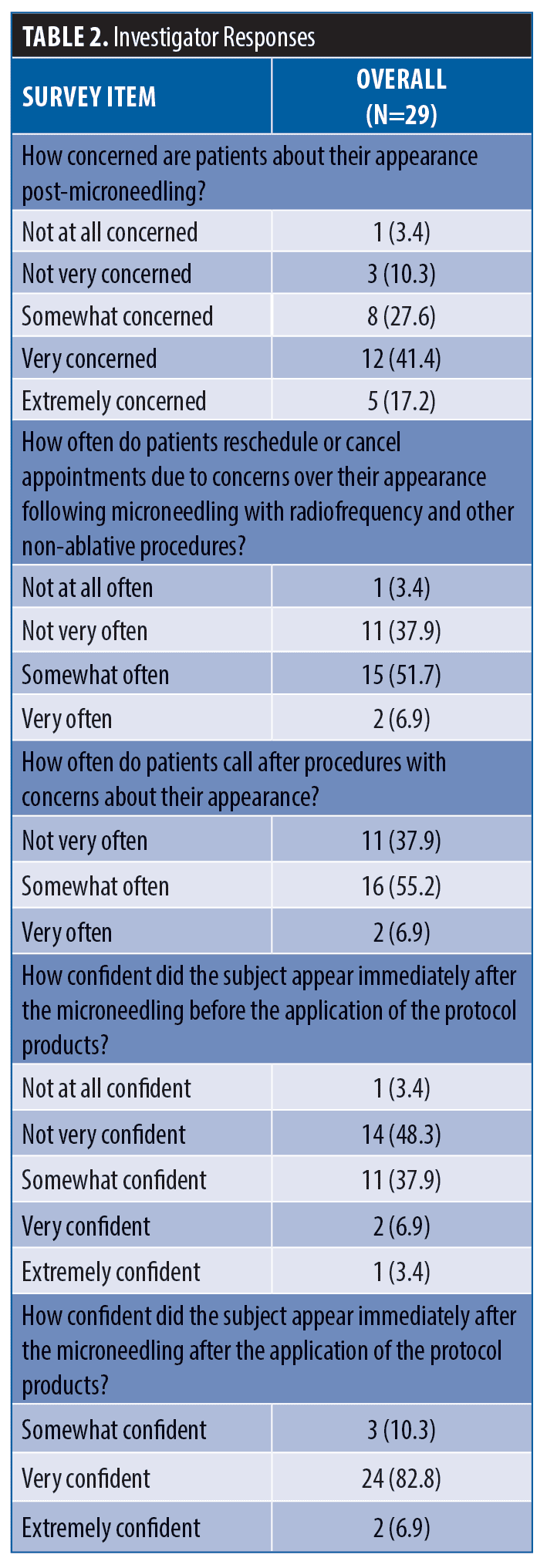
Responses to the Investigator Questionnaire are summarized in Table 2. Prior to treatment, more than half of subjects (59%) expressed being very or extremely concerned about their post-procedure appearance. Immediately following the procedure, 52 percent of subjects did not appear confident about their appearance after the radiofrequency microneedling. In general, a similar number of patients (59%) have cancelled or reschedule procedure appointments due to concerns over their post-procedure appearance and 62 percent of patients have contacted their office over treatment-related concerns.
Among treated subjects, 14 (43%) were hesitant to undergo microneedling due to concerns over needing to take time off from normal activities. Pre- and post-treated subject questionnaire responses are summarized in Table 3. Immediately after microneedling, 59 percent of subjects said their skin felt uncomfortable. Skin redness was observed by 86 percent of subjects and microneedling marks were seen by 41 percent. Nearly half of treated subjects (49%) were somewhat or very bothered by their appearance.
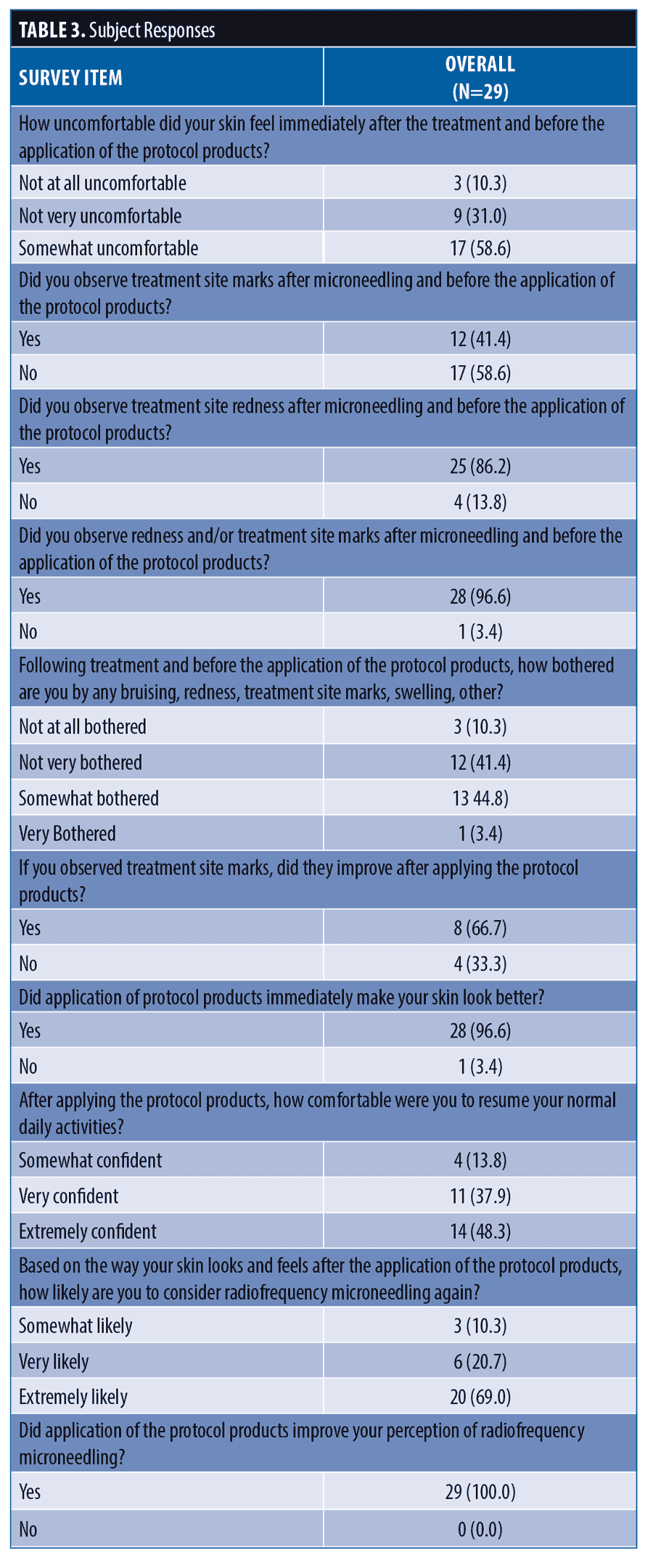
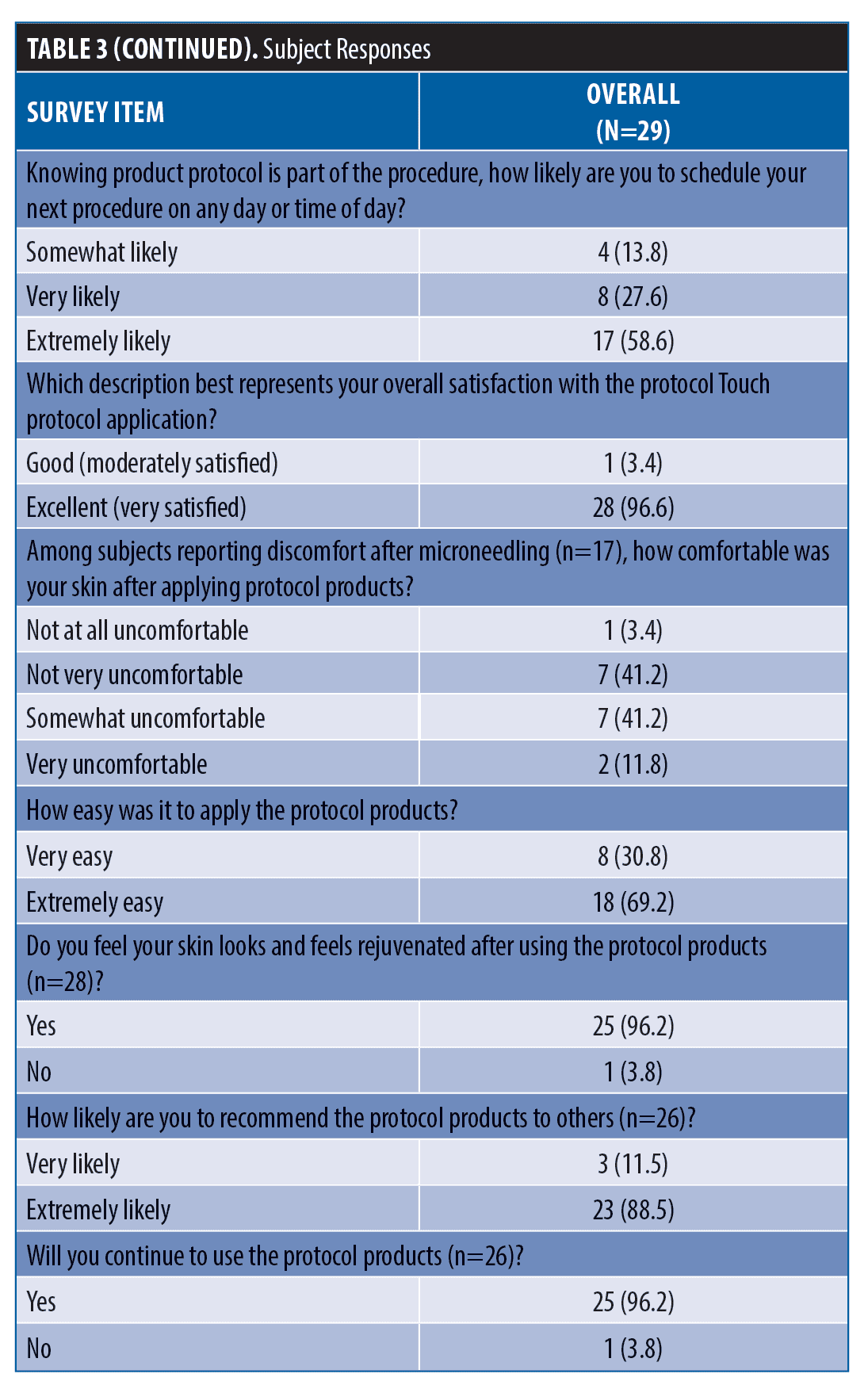
After application of the skincare protocol, 68 percent of subjects observed an improvement in skin redness, 68 percent saw improvement in treatment site marks and 97 percent saw an overall improvement in redness and/or treatment site marks. Most subjects (97%) thought the skincare protocol made their skin look better after the procedure, 90 percent felt more confident, and 86 percent were very or extremely comfortable resuming normal activities.
Most subjects (90%) were very or extremely likely to have a microneedling procedure again after receiving the skincare protocol and all subjects (100%) said the skincare protocol improved their perception of the radiofrequency microneedling procedure. After this experience,
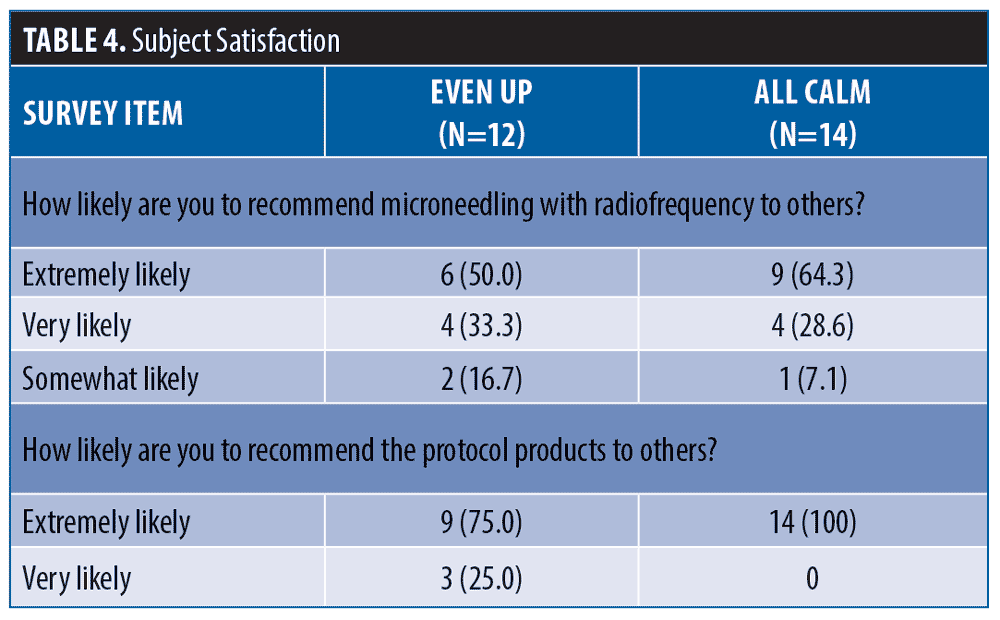
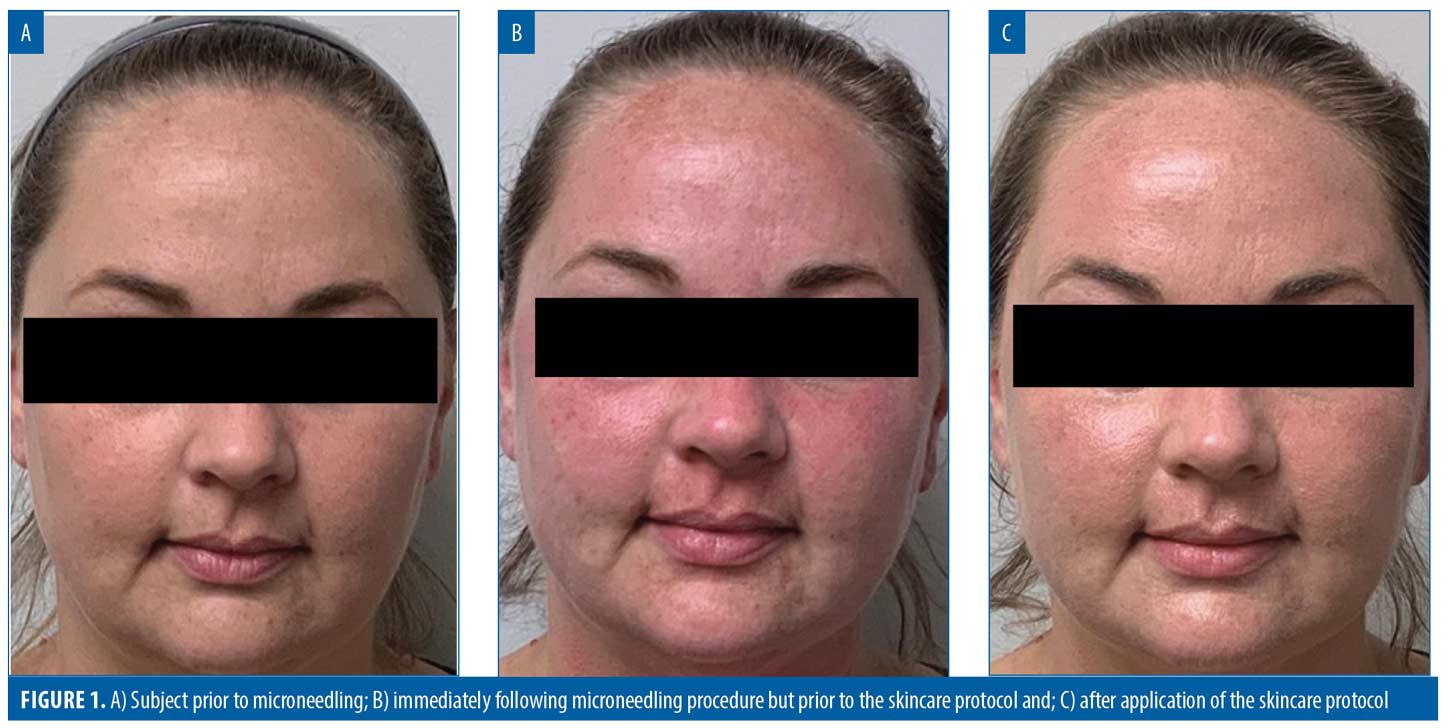
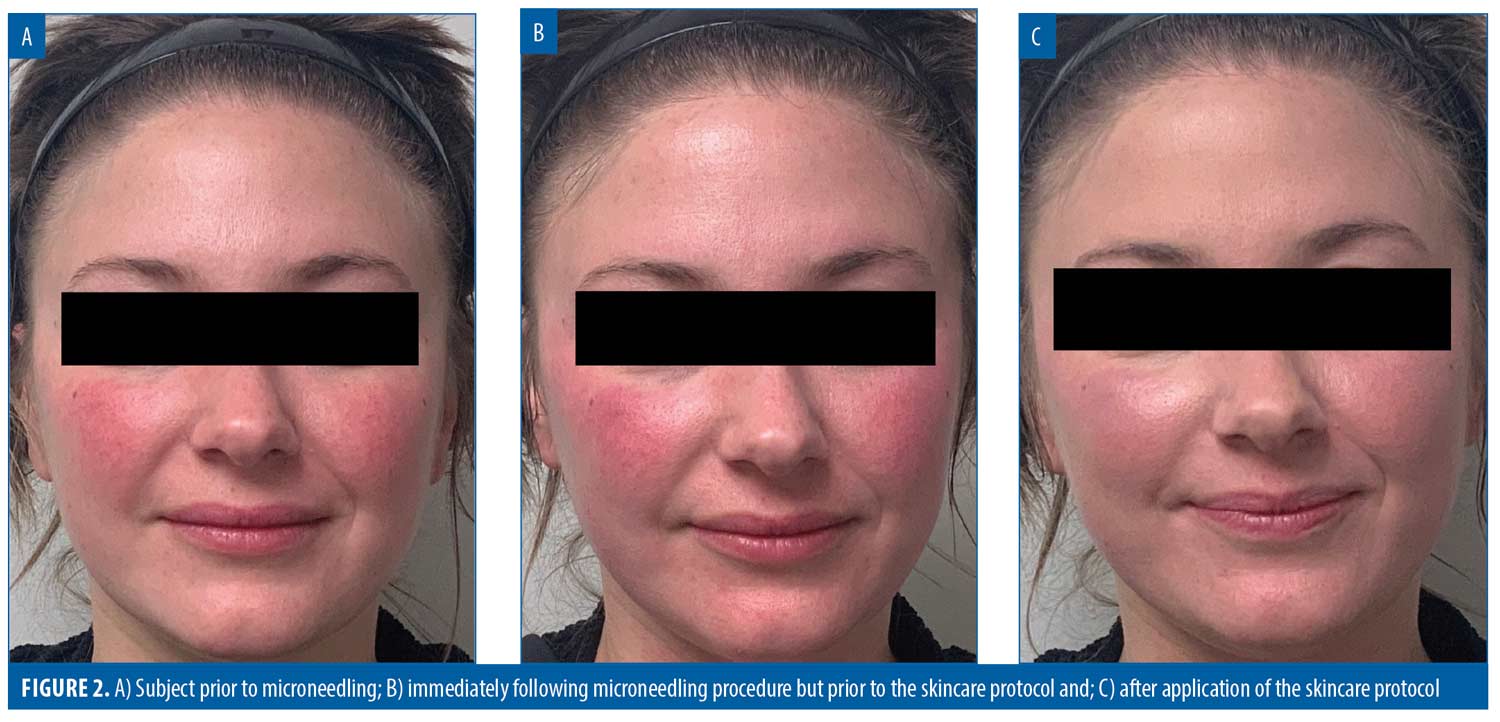
86% of subjects would be very or extremely comfortable booking their next microneedling procedure on any day of the week or time of day. Overall, 96 percent of subjects were extremely satisfied with their experience. The results for three representative subjects are shown in Figures 1 to 3.
Among subjects experiencing initial treatment discomfort (n=17), 47 percent said the skincare protocol made their skin feel more comfortable. All subjects (100%) reported applying the skincare products at home was easy and most (96%) believed continued use of the products at home made their skin look and feel rejuvenated. All subjects (100%) said they would recommend the skincare protocol products to others and 96 percent plan to continue to use these products at home. Responses to subject satisfaction questions are summarized in Table 4.
Safety. Four subjects reported mild-to-moderate adverse events of redness, itching, burning and swelling which are consistent with the previously reported adverse event profile of microneedling with radiofrequency therapy.17 Three of these subjects were treated with a steroid cream or injection. There were no reports of nodules or granulomas. There were no adverse events among subjects that applied a barrier product prior to the study protocol products (n=9). All adverse events resolved, and all subjects continued application of the study protocol products.
Discussion
Patients undergoing radiofrequency microneedling commonly experience mild-to-moderate erythema and mild edema which may persist for several days.17, 18 For this reason, many of the subjects in the current study expressed concern about their appearance after the procedure and many patients cancel or reschedule their procedures due to concerns about their post-procedure appearance. While more than half of treated subjects did not appear confident about their appearance after their microneedling procedure based on investigator impressions, the results of this study demonstrated that the application of a skincare protocol following treatment enhanced subject experiences including confidence to resume normal activities, comfort, treatment satisfaction, and likelihood to undergo repeat treatments.
The concerns of patients over their post-treatment facial appearance are similar to individuals with facial conditions associated with skin disorders such as rosacea, melasma and port-wine stains. Numerous studies have demonstrated the psychological impact of facial blemishes and their effect on quality of life in these individuals.19-25 Camouflaging facial skin imperfections with cosmetics is a viable treatment.26-28 Cosmetic camouflage provides a significant emotional benefit for patients with facial skin conditions such as rosacea and melasma20, 29 and has been shown to improve the appearance30 and quality of life among patients with vitiligo.31
Radiofrequency microneedling is just one of several minimally invasive procedures used for facial rejuvenation. Other methods including chemical peels, lasers, light and ultrasound devices, neuromodulators and injectable fillers, all of which may be associated with treatment-related complications.32, 33 Laser resurfacing may causes edema, erythema, and discoloration lasting 2 to 3 weeks.34, 35 Injection of dermal fillers can cause erythema, bruising/ecchymosis and trauma-related edema36-38 that can persist for 1 to 2 weeks.39 It is not uncommon to experience erythema, irritation and burning following chemical peels.40
The skincare protocol may also be useful for camouflaging the unwanted effects of these minimally invasive aesthetic procedures. The application of a tinted broad-spectrum sunscreen following intense pulsed light procedures immediately improved facial appearance, decreased self-consciousness and decreased treatment-related discomfort41 In a previous unpublished study (Data on file. Colorescience®, Inc., Carlsbad, CA.), a skincare protocol similar to that used in the current study improved facial appearance and decreased self-consciousness following chemical peels, intense pulsed light, laser resurfacing, microneedling, and dermal fillers.
Importantly, the skincare protocol used in this study includes products which provide mineral-based SPF-50 protection against damaging ultraviolet and high energy visible light42 and environment insults.43
Limitations. The primary limitations to this study were the modest number of enrolled subjects and the use of subjective, self-reported outcomes.
Conclusion
A skincare protocol has been developed for use following facial radiofrequency microneedling procedures. When applied immediately following the procedure, it increases subject confidence to resume normal activities, comfort, camouflage, overall treatment satisfaction and likelihood to undergo retreatment while providing essential mineral SPF, HEV and pollution protection. This skincare protocol may also be suitable for use following other minimally-invasive facial procedures.
Acknowledgments
The authors express their gratitude to Colorescience®, Inc. Carlsbad, CA for providing the skincare products used in this study and to Lumenis® Inc., San Jose, CA for providing the radiofrequency microneedling device consumables. The authors acknowledge Julie C. McCauley, MPHc, who performed the statistical analysis and the editorial assistance of Dr. Carl S. Hornfeldt, Apothekon, Inc., during the preparation of this manuscript.
References
- American Society of Plastic Surgeons®. Plastic Surgery Statistics, 2019. Available: https://www.plasticsurgery.org/news/plastic-surgery-statistics. Accessed 14 January 2021.
- Borges J, Araújo L, Cuzzi T, et al. Fractional laser resurfacing treats photoaging by promoting neocollegenesis and cutaneous edema. J Clin Aesthet Dermatol. 2020;13:22–27.
- Narurkar VA. Nonablative fractional laser resurfacing. Dermatol Clin. 2009;27:473–478.
- Braz A, Eduardo CCP. Reshaping the lower face using injectable fillers. Indian J Plast Surg. 2020;53:207–218.
- Lee KC, Wambier CG, Soon SL, et al. Basic chemical peeling: superficial and medium-depth peels. J Am Acad Dermatol. 2019;81:313–324.
- Chang YC, Croix J, Javvaji S, et al. Patient satisfaction and our clinical experience with 459 microfocused ultrasound treatments. Lasers Surg Med. 2019;51:495–499.
- Gentile RD. Renuvion RF-helium plasma for subdermal skin tightening, facial contouring and skin rejuvenation of the face and neck. Facial Plast Surg Aesthet Med. 2020;22:304–306.
- El-Domyati M, El-Ammawi TS, Moawad O, et al. Intense pulsed light photorejuvenation: a histological and immunohistochemical evaluation. J Drugs Dermatol. 2011;10:1246–1252.
- Litchman G, Nair PA, Badri T, et al. Microneedling. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing. 2020
- Iriarte C, Awosika O, Rengifo-Pardo M, et al. Review of applications of microneedling in dermatology. Clin Cosmet Investig Dermatol. 2017;10:289–298.
- Doddaballapur S. Microneedling with dermaroller. J Cutan Aesthet Surg. 2009;2:110–111.
- Wu SZ, Muddasani S, Alam M. A Systematic review of the efficacy and safety of microneedling in the treatment of melasma. Dermatol Surg. 2020;46:1636–1641.
- Kim JK, Roh MR, Park GH, et al. Fractionated microneedle radiofrequency for the treatment of periorbital wrinkles. J Dermatol 2013;40:172–176.
- Juhasz MLW, Cohen JL. Microneedling for the treatment of scars: an update for clinicians. Clin Cosmet Investig Dermatol. 2020;13:997–1003.
- Zahr AS, Kononov T, Sensing W, et al. An open-label, single-site study to evaluate the tolerability, safety, and efficacy of using a novel facial moisturizer for preparation and accelerated healing pre and post a single full-face radiofrequency microneedling treatment. J Cosmet Dermatol. 2019;18:94–106.
- Gold MH, Adelglass J. Evaluation of safety and efficacy of the TriFractional RF technology for treatment of facial wrinkles. J Cosmet Laser Ther. 2014;16:2–7.
- Bloom BS, Emer J, Goldberg DJ. Assessment of safety and efficacy of a bipolar fractionated radiofrequency device in the treatment of photodamaged skin. J Cosmet Laser Ther. 2012;14:208–211.
- Raveendra L, Sidappa H, Shree S. A study of quality of life in patients with facial melanoses. Indian Dermatol Online J. 2020;11:154–157.
- Balkrishnan R, McMichael AJ, Hu JY, et al. Corrective cosmetics are effective for women with facial pigmentary disorders. Cutis. 2005;75:181–187.
- Oussedik E, Bourcier M, Tan J. Psychosocial burden and other impacts of rosacea on patients’ quality of life. Dermatol Clin. 2018;36:103–113.
- Bewley A, Fowler J, Schöfer H, et al. Erythema of rosacea impairs health-related quality of life: results of a meta-analysis. Dermatol Ther (Heidelb). 2016;6:237–247.
- Pawaskar MD, Parikh P, Markowski T, et al. Melasma and its impact on health-related quality of life in Hispanic women. J Dermatolog Treat .2007;18:5–9.
- Ongenae K, Van Geel N, De Schepper S, et al. Effect of vitiligo on self-reported health-related quality of life. Br J Dermatol. 2005;152:1165–1172.
- Porter JR, Beuf AH, Lerner A, et al. Psychosocial effect of vitiligo: a comparison of vitiligo patients with “normal” control subjects, with psoriasis patients, and with patients with other pigmentary disorders. J Am Acad Dermatol. 1986;15:220–224.
- Andra C, Suwalska A, Dumitrescu AM, et al. A corrective cosmetic improves the quality of life and skin quality of subjects with facial blemishes caused by skin disorders. Clin Cosmet Investig Dermatol. 2020;13:253–257.
- Boehncke WH, Ochsendorf F, Paeslack I, et al. Decorative cosmetics improve the quality of life in patients with disfiguring skin diseases. Eur J Dermatol. 2002;12:577–580.
- Shear NH, Graff L. Camouflage cosmetics in dermatologic therapy. Can Fam Physician 1987;33:2343–2346.
- Levy LL, Emer JJ. Emotional benefit of cosmetic camouflage in the treatment of facial skin conditions: personal experience and review. Clin Cosmet Investig Dermatol. 2012;5:173–182.
- Hsu S. Camouflaging vitiligo with dihydroxyacetone. Dermatol Online J. 2008;14:8):23.
- Kaliyadan F, Kumar A. Camouflage for patients with vitiligo. Indian J Dermatol Venereol Leprol. 2012;78:8–15.
- Vanaman M, Fabi SG, Carruthers J. Complications in the cosmetic dermatology patient: a review and our experience (Part 1). Dermatol Surg. 2016;42:1–11.
- Vanaman M, Fabi SG, Carruthers J. Complications in the cosmetic dermatology patient: a review and our experience (Part 2). Dermatol Surg. 2016;42:12–20.
- Guida S, Nisticò SP, Farnetani F, et al. Resurfacing with ablation of periorbital skin technique: indications, efficacy, safety, and 3D assessment from a pilot study. Photomed Laser Surg. 2018;36:541–547.
- Chathra N, Mysore V. Resurfacing of facial acne scars with a new variable-pulsed Er:YAG laser in Fitzpatrick skin types IV and V. J Cutan Aesthet Surg. 2018;11:20–25.
- Cassuto D, Pignatti M, Pacchioni L, et al. Management of complications caused by permanent fillers in the face: a treatment algorithm. Plast Reconstr Surg. 2016;138:215–27e.
- Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Plast Surg Nurs. 2015;35:13–32.
- Lafaille P, Benedetto A. Fillers: contraindications, side effects and precautions. J Cutan Aesthet Surg. 2010;3:16–19.
- Moradi A, Shirazi A, Moradi-Poehler J, et al. A blinded, randomized, split-face pilot study of bruising and pain with hyaluronic acid for correction of perioral lines using no lidocaine, lidocaine alone, and lidocaine and epinephrine. Aesthet Surg J. 2015;35:443–455.
- Levy LL, Emer JJ. Complications of minimally invasive cosmetic procedures: prevention and management. J Cutan Aesthet Surg. 2012;5:121–132.
- Jones IT, Guiha I, Fabi SG. Open-label study assessing the efficacy and tolerability of topical skincare and sun protection products following intense pulsed light treatment. J Cosmet Dermatol. 2017;17:441–447.
- Bernstein EF, Sarkas HW, Boland P. Iron oxides in novel skin care formulations attenuate blue light for enhanced protection against skin damage. J Cosmet Dermatol. 2021;20:532–537.
- Bernstein EF, Sarkas HW, Boland P, et al. Beyond sun protection factor: An approach to environmental protection with novel mineral coatings in a vehicle containing a blend of skincare ingredients. J Cosmet Dermatol. 2020;19:407–415.

