 J Clin Aesthet Dermatol. 2024;17(3):12–17.
J Clin Aesthet Dermatol. 2024;17(3):12–17.
by Stefano Veraldi, MD; Valentina Rosca, MD; Remus Ioan Orasan, MD, PhD; Magda Constantin, MD;
and Roni P. Dodiuk-Gad, MD
Dr. Veraldi is with the Dermatological Center in Milan, Italy. Dr. Rosca is with Emergency County Hospital in Targoviste, Romania. Dr. Orasan is with Individual Medical Office of Dermatology in Cluj, Romania. Dr. Constantin is with Colentina Clinical Hospital in Bucharest, Romania. Dr. Dodiuk-Gad is with the Department of Dermatology at Emek Medical Center in Afula, Israel, the Division of Dermatology and Department of Medicine at the University of Toronto in Toronto, Canada, and the Department of Dermatology and Bruce Rappaport Faculty of Medicine at the Technion Institute of Technology in Haifa, Israel.
FUNDING: No funding was provided for this study.
DISCLOSURES: Dr. Dodiuk-Gad is a consultant/investigator for Sanofi, AbbVie, Pfizer, Janssen, Novartis, La Roche-Posay, Dexcel, and Eli Lilly
ABSTRACT: Objective. Psoriasis is a chronic, inflammatory skin disease, requiring local and systemic drugs according to disease severity. This study aims to investigate the efficacy and safety of a topical treatment containing xyloglucan, pea proteins and Opuntia ficus-indica extracts (XPO) compared to calcipotriol 50mcg/betamethasone 0.5mg ointment (CB).
Methods. Forty-two patients diagnosed with mild-to-moderate plaque psoriasis were assigned 1:1 to XPO treatment or CB for 28 days. Disease status was assessed at baseline (V1), monitored every two weeks (V2, V3), and at follow-up (V4). Disease severity was assessed by PASI (Psoriasis Area and Severity Index), PGA (Physician’s Global Assessment), and VAS (Visual Analog Scale for itching). Photos were taken before and after XPO treatment. Treatment efficacy was determined by comparing psoriasis severity at baseline to V3. Tolerability was assessed by monitoring the occurrence of adverse events.
Results. Both groups showed a statistically significant difference in PASI score from V1 to V2 (p=0.001, XPO; p=0.008, CB) and to V3 (p=0.001, XPO; p=0.004, CB). XPO achieved a PASI 50 score of 24 percent at V2 and 52 percent at V3 compared to CB (0% at V2 and 19% at V3). At V3, PGA was significantly reduced in both groups (p=0.003, XPO; p=0.001 CB). Both treatments significantly reduced itching at V2 (p=0.001, XPO; p=0.003, CB) and V3 (p=0.001, XPO; p=0.0005, CB). Conclusion. XPO showed similar efficacy to CB, significantly reducing disease severity, erythema, itching, induration, and scaling with an excellent tolerability profile.
Keywords: Psoriasis, xyloglucan, pea protein, Opuntia ficus-indica extract, calcipotriol/ betamethasone, PASI
Introduction
Psoriasis is a chronic, immune-mediated, inflammatory skin condition that affects approximately 2 to 3 percent of the global population.1 Approximately 85 to 90 percent of patients suffer from plaque psoriasis.2 Currently, there is no definitive cure for psoriasis, and existing treatments only reduce the severity of the pathology and manage the symptoms.2 Psoriasis severity (mild, moderate, severe) depends on many factors, such as the status of the disease, which is assessed by the patient’s medical history, the location of the lesions, the percentage of body surface area affected, and the presence of comorbidities like psoriatic arthritis.
The therapy of choice for mild to moderate psoriasis, which affects up to 90 percent of all patients,3 consists of topical treatments (e.g., topical corticosteroids, vitamin D analogs, or fixed combinations of these two components, anthralin [dithranol], topical calcineurin inhibitors, salicylic acid, and moisturizers).4,5 In most of these cases, topical treatments alone are enough to manage the disease;5,6 they require minimal monitoring since they act directly on plaques, and hardly have systemic side effects. Emollients, for example, have no known contraindications, are safe, and are especially recommended for face and intertriginous areas.4,6 In addition, they help reduce pruritus and desquamation by promoting the integrity of the epidermal barrier in psoriatic patients.4,7
Despite the large number of available topical treatments, patient satisfaction in terms of effectiveness, seems to be low,3,5 which suggests a need for alternative topical treatments to improve psoriasis management, patient satisfaction, and adherence. The interest in acquiring more clinical evidence on the efficacy of products acting with a non-pharmacological mechanism of action, with the aim of finding alternative approaches with a positive risk-benefit ratio, is on the rise. In the last several years, naturally derived non-pharmacological ingredients have gained interest. Xyloglucan (XG), a hemicellulose derived from the seeds of Tamarindus indica, and pea protein (PP), derived from the plant Pisum sativum, have been shown to preserve the physiological function of the skin barrier. Thanks to their mucoadhesive and mucomimetic properties, XG and PP form a protective layer that prevents pathological aggression after skin damage, as demonstrated by in vitro and in vivo studies.8,9 Moreover, a double-blind, parallel, randomized, placebo-controlled clinical trial in adult patients with atopic dermatitis (AD) showed that a XG/PP-based formulation significantly improved AD severity within eight days of treatment.10 Opuntia ficus-indica is a well-known plant used in the pharmaceutical, cosmetic, and food industries, characterized by its antioxidant properties and water-retaining capacity. In Latin America, it is used in traditional medicine as a remedy for bruises, burns, wounds, and infections.11 Interestingly, the healing properties of Opuntia ficus-indica polysaccharides were investigated in a murine model of dermal wounds, where they displayed beneficial properties when applied topically for six days, accelerating reepithelization of the skin.12 Moreover, in vitro and in vivo studies have demonstrated that a topical treatment based on naturally derived components (xyloglucan, pea protein, and Opuntia ficus-indica) significantly improves inflammatory hallmarks associated with psoriasis, managing the skin barrier dysfunction, with a similar efficacy to a topical corticosteroid.13 Therefore, the purpose of this prospective, multicenter, open-label study was to evaluate the clinical efficacy and tolerability of XPO, a topical treatment based on naturally derived components (xyloglucan, pea protein and Opuntia ficus-indica), in relieving the severity of mild to moderate plaque psoriasis, compared to calcipotriol 50mcg/betamethasone 0.5mg ointment (CB).
Methods
Study protocol. The study was carried out in adherence to the revised Declaration of Helsinki for biomedical research involving human subjects, with the principles of ICH-Good Clinical Practice of the European Community and/or US FDA, CPMP (CPMP/ICH/135/95; ICH Topic E6), ISO 14155, and in accordance with the Guidelines MED DEV 2.7/1 rev. 3, MED DEV 2.7/3, MED DEV 2.7/4. The National Committee of Medical Bioethics for clinical trials from Romania gave its favorable opinion on May 6th, 2020. The study was registered with the following ISCRTN Number: ISRCTN28479470.
This was a prospective, multicenter, open-label clinical trial. It was planned to recruit adult patients from three accredited hospitals in Romania. The study was carried out between May and November 2020. Patients with a previous diagnosis of mild to moderate plaque psoriasis, over 18 years of age, able to comply with all clinic visits and study-related procedures, and willing to cooperate and to give their written informed consent, were included in the study. Patients were excluded if they used systemic or topical anti-psoriatic therapy in the two weeks preceding the start of the study, used systemic anti-psoriatic therapy (e.g. methotrexate, biologics), phototherapy or topical corticosteroids during the study, presented serious dermatological disease other than psoriasis or any dermatological disease that might interfere with the clinical evaluation of psoriasis or bring the subject in danger, had known hypersensitivity or allergy to any of the investigation medical product ingredients, were pregnant, lactating or planning to become pregnant or lactating during the study. All patients who signed the informed consent form were screened for eligibility and enrolled. Patients were assigned to XPO, containing xyloglucan, pea proteins and Opuntia ficus-indica extract, or calcipotriol 50mcg/betamethasone 0.5mg (CB) in a 1:1 ratio. XPO was provided by Devintec Sagl (Lugano, Switzerland). CB is considered the standard of care in current clinical practice in Romania according to the Summary of Product Characteristics approved by the National Drug Agency.14 The investigators decided to prescribe XPO or CB based on patient medical history and their best medical judgement at the time the patient presented himself/herself at the medical center as a new patient or as an existing one. All selected patients were invited to attend the study, and received the subject information sheet describing the procedure (objectives, methodology, potential risks, expected benefits) and a sufficient amount for the entire treatment period. XPO and CB treatments were applied topically twice daily for four weeks. Patients were examined at baseline (Visit 1), after 15 and 29 days of treatment (Visit 2 and Visit 3, respectively), and at follow-up after 43 days (Visit 4). During the first baseline visit, the patient underwent a physical examination (weight, height, BMI), and clinical data, including medical history, demographics, and medications taken were recorded; while the investigator assessed psoriasis status using the following questionnaires: PASI, PGA, and VAS.
Study endpoints. Evaluation methods and scoring. The primary objective of the study was to evaluate the clinical efficacy of XPO in relieving the severity of mild to moderate psoriasis, compared to calcipotriol 50mcg/betamethasone 0.5mg ointment. Treatment efficacy was assessed using PASI, PGA, and VAS. The secondary objective of the study was to assess the tolerability of XPO by monitoring the occurrence of adverse events and the number of drop-outs due to adverse events.
PASI. The Psoriasis Area and Severity Index evaluates the percentage of Body Surface Area (BSA) affected by plaque psoriasis, as well as the degree of erythema, scaling, and thickness of lesions located on different parts of the body. The PASI score ranges from 0 to 72, with higher numbers representing the most severe disease. To calculate a patient’s PASI score, the body is divided into 4 areas: head, trunk, arms, and legs. Each area receives a score of 0 to 6 based on the amount of skin affected (0=no involvement; 1=<10%; 2=≥10%, <30%; 3=≥30%, <50%; 4=≥ 50%,<70%; 5=≥70%,<90%; 6=90%–100%). Each area also receives separate scores for the assessment of erythema, induration (localized hardening), and scaling: 0=no involvement; 1=slight; 2=moderate; 3=marked; 4=very marked. The final PASI score is determined by combining the scores for each area according to a formula. Mild psoriasis: 0≤ PASI≤5; Moderate: 5<PASI≤12; Severe: 12<PASI≤20; Very Severe: PASI>20. PASI-50, PASI-75, and PASI-90 refer to improvements in the PASI score of 50 percent, 75 percent, and 90 percent, respectively.15,16
PGA. The Physician’s Global Assessment method provides a subjective overall assessment of the severity of psoriasis. It uses a 6-point scale ranging from clear to severe: 0=none; 1=minimal; 2=mild; 3=moderate; 4=severe; 5=very severe.17
VAS. The Visual Analog Scale pruritus consists of a 10cm-long line measuring itch intensity in the last 24 hours. The left endpoint of the scale represents “no itch” and the right endpoint the “worst imaginable itch”. VAS=0: no pruritus; 0 < VAS < 4: mild pruritus; 4 ≤ VAS < 7: moderate pruritus; 7 ≤ VAS < 9: severe pruritus.18
SAF. The Safety Analysis Set was used for all safety analyses to evaluate tolerability. For each study treatment, the numbers and incidence rates of adverse events (AEs) were recorded, including serious adverse events leading to withdrawal from the study or death.
Statistical analysis. Assuming a true difference in means between the test and the reference group of -5 (i.e., 20–25) units, a pooled standard deviation of 13 units, the study would require a sample size of 21 for each group (i.e., a total sample size of 42, assuming equal group sizes), to achieve a power of 80 percent and a level of significance of 5 percent, for declaring that the test drug is superior to the active control drug at 5 units margin of superiority (assuming that a larger mean is desirable).
Continuous variables were reported as mean, standard deviation (SD), median, minimum, maximum, and number of observations unless otherwise stated. Categorical variables were reported in terms of number of patients providing data at the relevant time point (n), frequencies, and percentages. Demographics and baseline disease characteristics were summarized by descriptive statistics. Changes in clinical symptoms at Visit 2 and Visit 3 compared to baseline (intra-group comparisons) were analyzed using the Wilcoxon Signed Ranks test. Mann-Whitney Test for inter-group comparisons was used to compare the treatment arms on mean absolute variation (i.e. mean difference of a parameter between two time points).
The number and percentage of patients with recurrences and/or AE at Visits 2, 3, and 4 were tabulated. Statistical analysis was performed using IBM SPSS Statistics 20 for Windows; p values < 0.05 were considered statistically significant.
Results
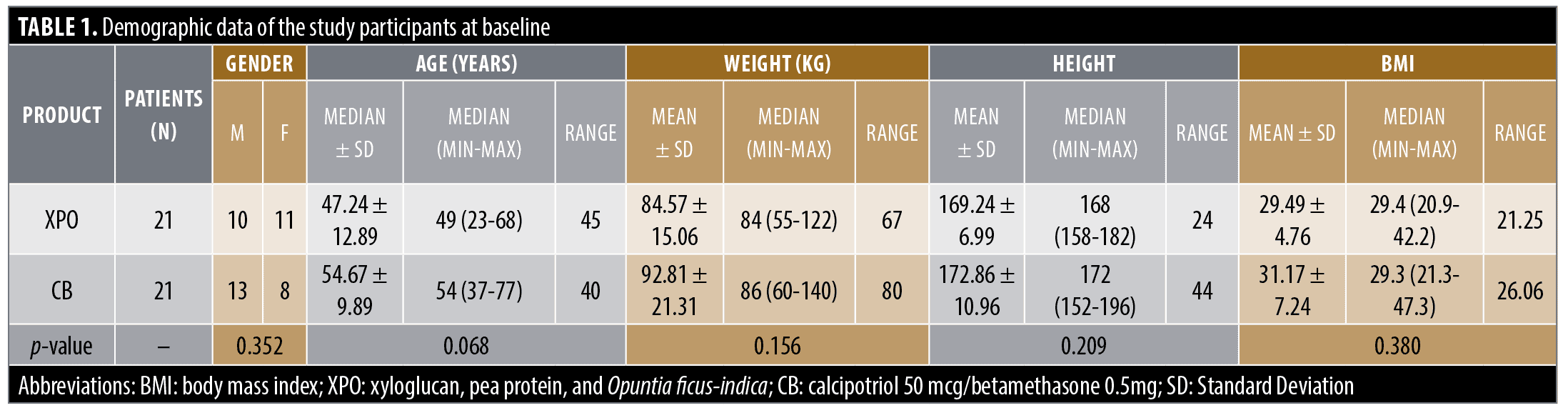
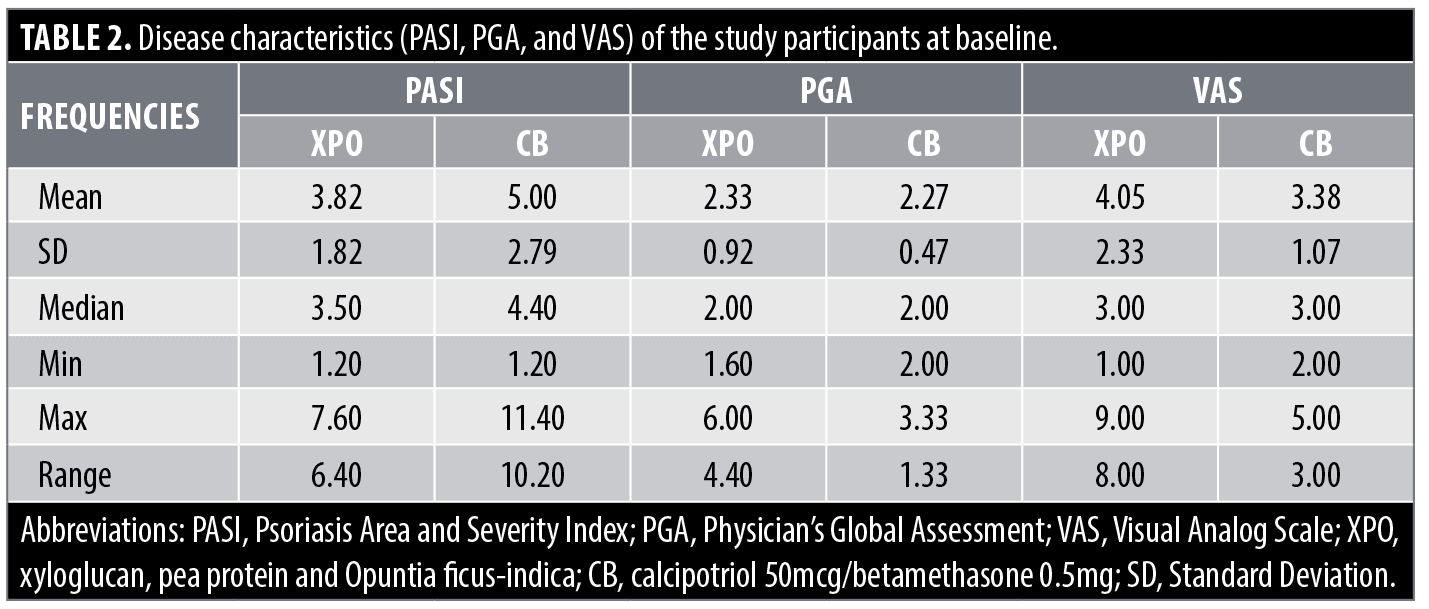
Demographics and baseline disease characteristics. 42 patients were enrolled: 21 were assigned to the test substance (XPO) and 21 to the standard of care (CB). All participants completed the study. The demographic data and disease characteristics (PASI, PGA, and VAS) at Visit 1 (Baseline) are reported in Table 1 and Table 2, respectively.
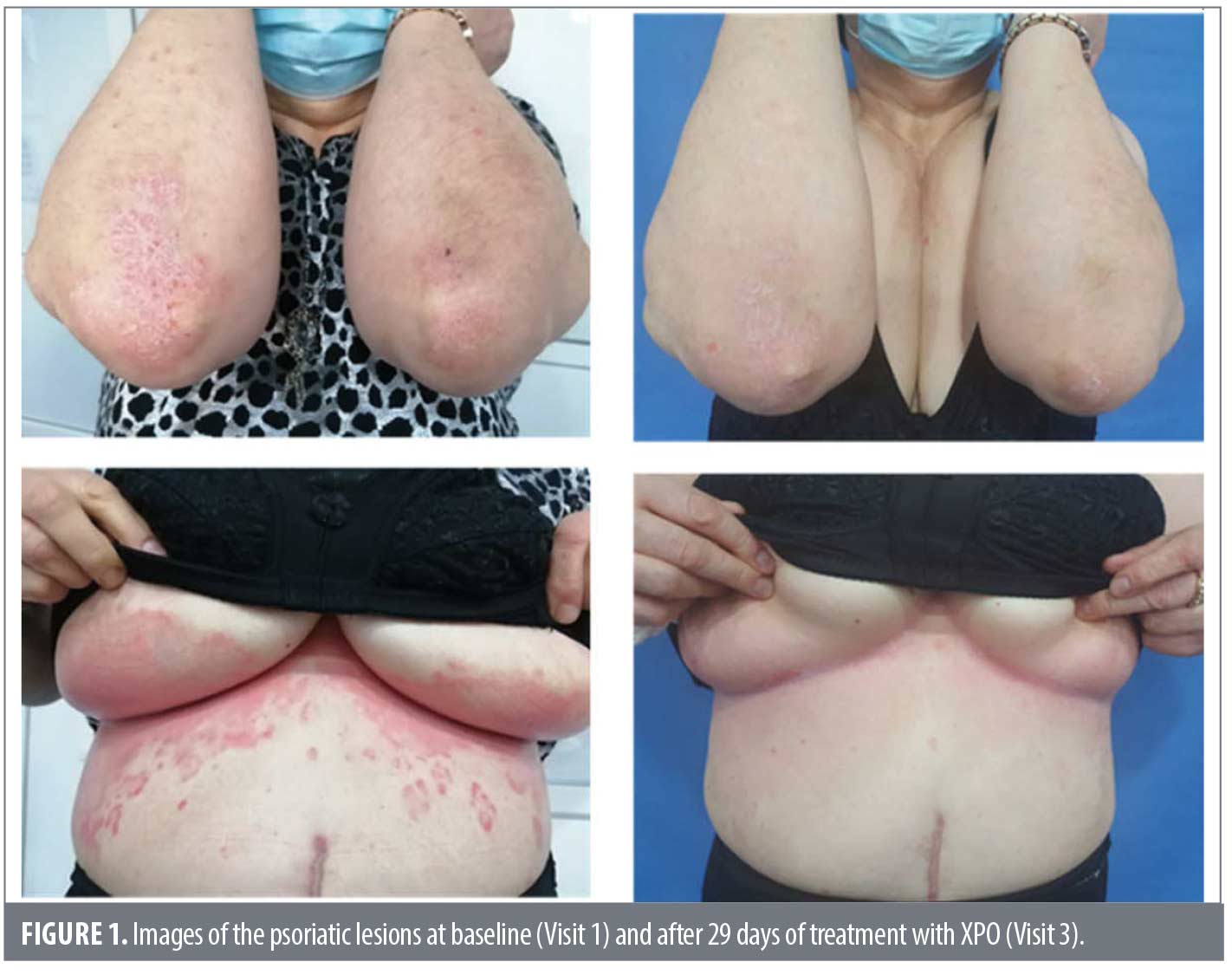
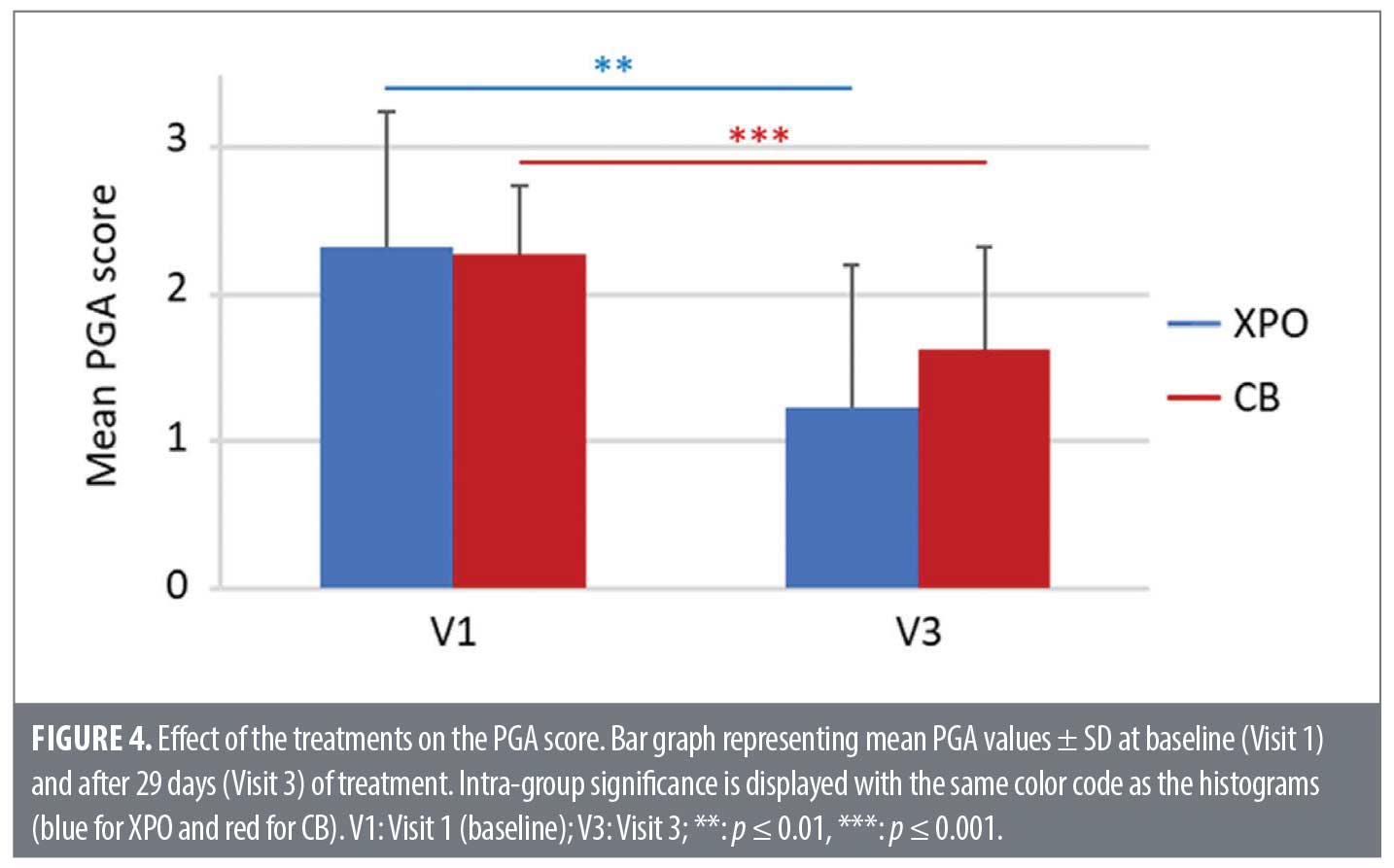
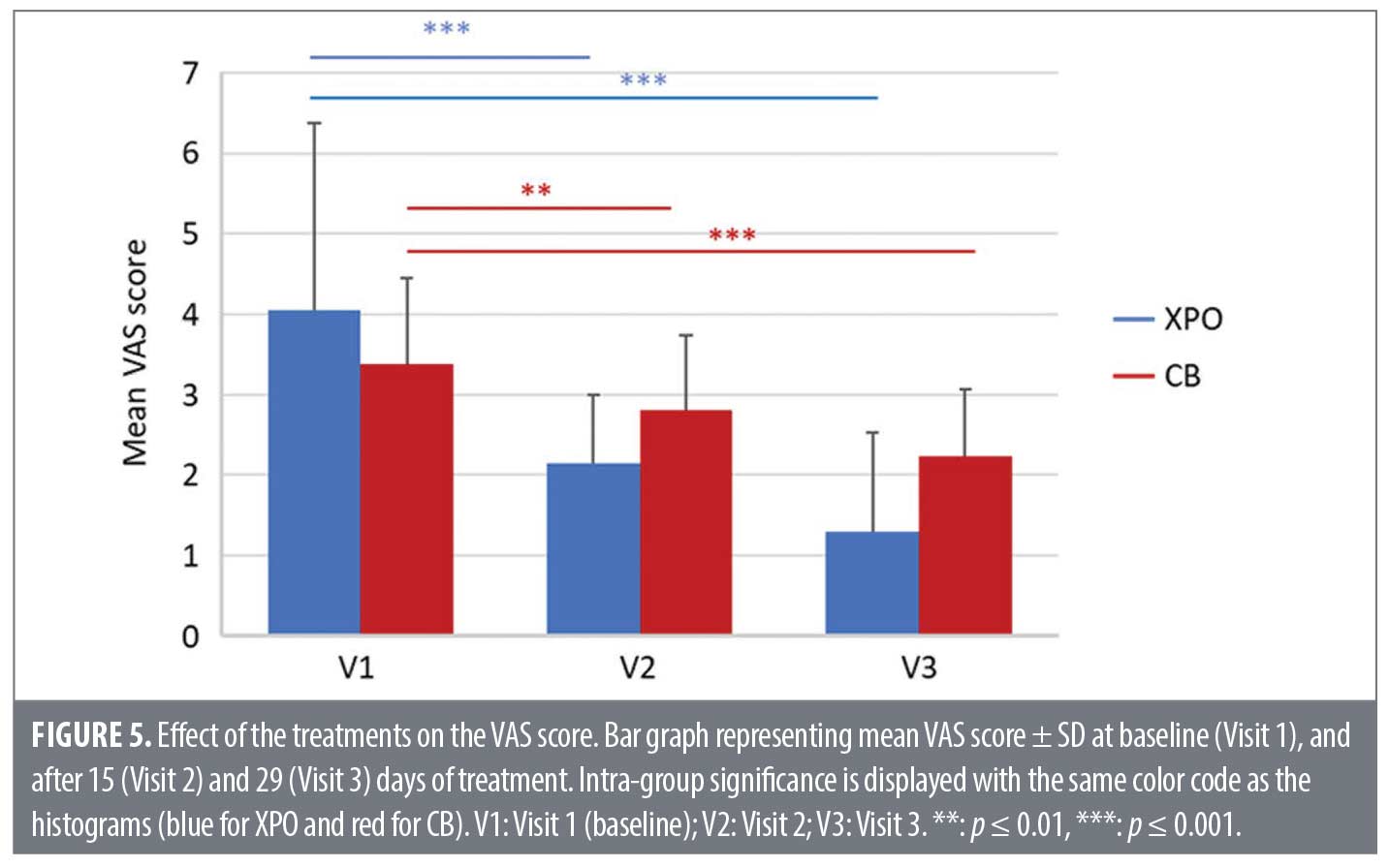
Efficacy results. The effect of the treatments on the severity of psoriasis was assessed at each visit by measuring the degree of erythema, thickness, and scales based on area coverage and plaque appearance (PASI score) or averaged over the whole body (PGA score); the effect on itching was evaluated by measuring intensity in the last 24 hours (VAS score). A qualitative assessment of the clinical efficacy was performed by visual inspection of the lesions. Figure 1 shows representative images of a patient at baseline (Visit 1) and after 29 days of treatment with XPO (Visit 3).
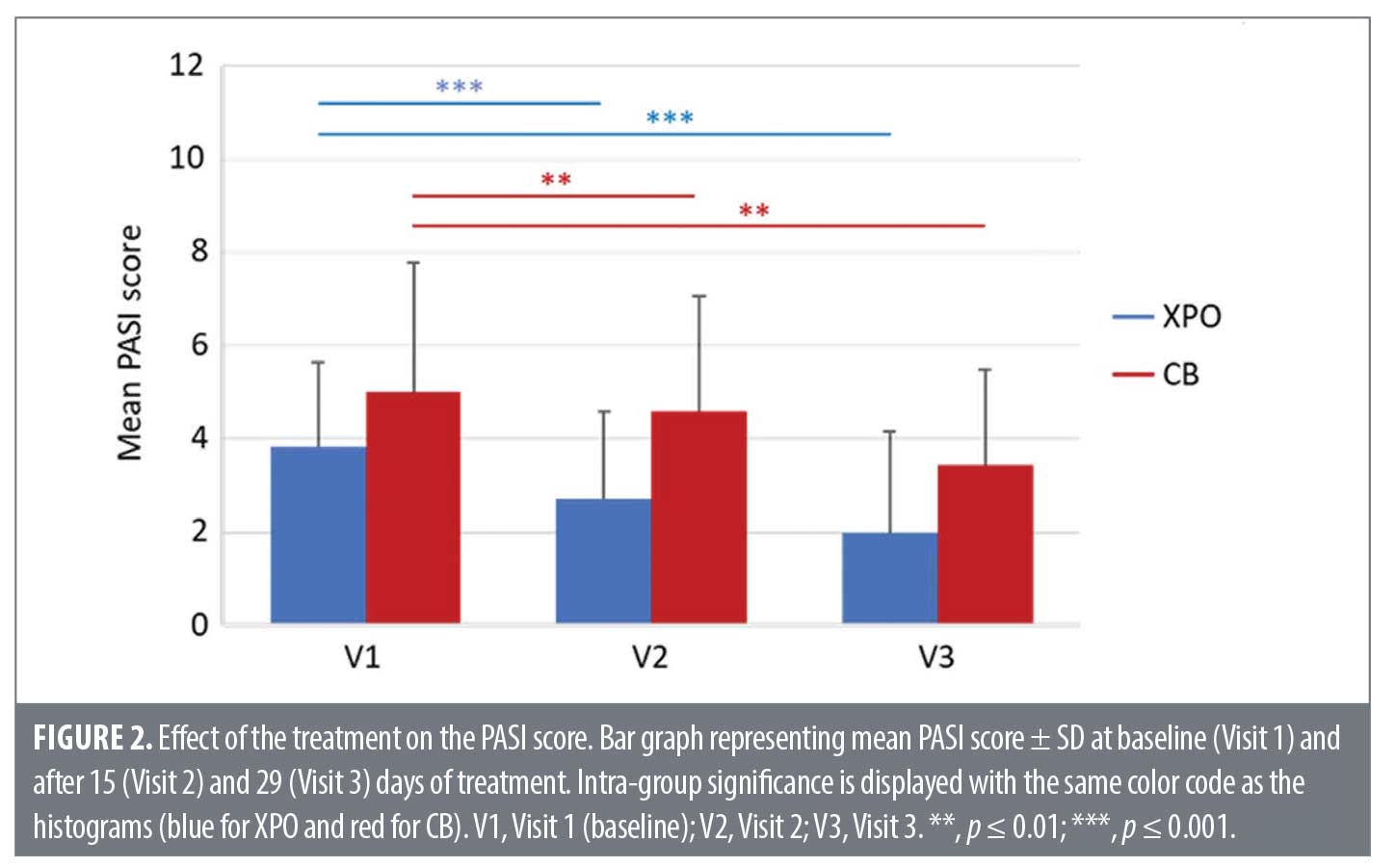
PASI score. As shown in Figure 2, XPO (blue) and CB (red) significantly decreased the mean PASI score compared to baseline values at Visit 2 and Visit 3. No statistically significant difference in PASI mean absolute variation score was observed between the study arms at V1–V3 (p=0.659).
At Visit 2, 23.8 percent of patients treated with XPO achieved a PASI score improvement of 50 percent, while no patients treated with CB reported such an improvement. At Visit 3, PASI 50 was achieved by 52.4 percent of patients treated with XPO compared with 19 percent of patients treated with CB (Figure 3). 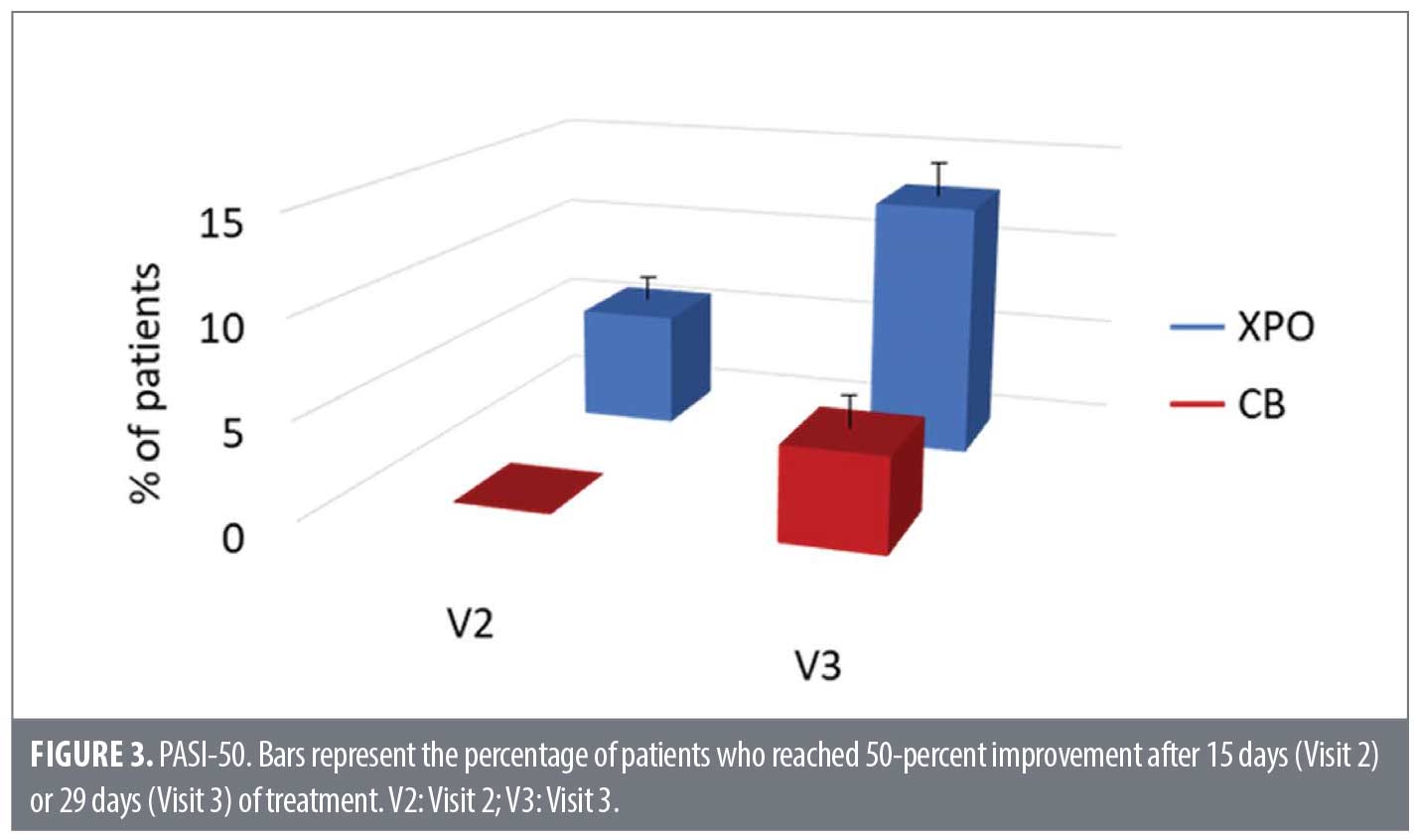
PGA score. At the end of the study, a significant decrease in the PGA mean score was observed in both treatment groups (XPO, p=0.003; CB, p=0.001; shown in Figure 4). There was no statistically significant difference in PGA mean absolute variation score between the study arms at V1-V3 (p=0.099).
VAS score. As shown in Figure 5, both treatments significantly decreased the VAS score both at Visit 2 and Visit 3. There was a statistically significant difference in VAS mean absolute variation score between the study arms at V1-V2 (p= 0.026) and V1-V3 (p=0.020). Notably, at Visit 2, XPO reduced the mean VAS score by 47 percent compared to 17 percent in the CB group. By the end of the study, a mean VAS score reduction of 68 percent was observed in the XPO treatment group compared to a 38 percent reduction in the CB group.
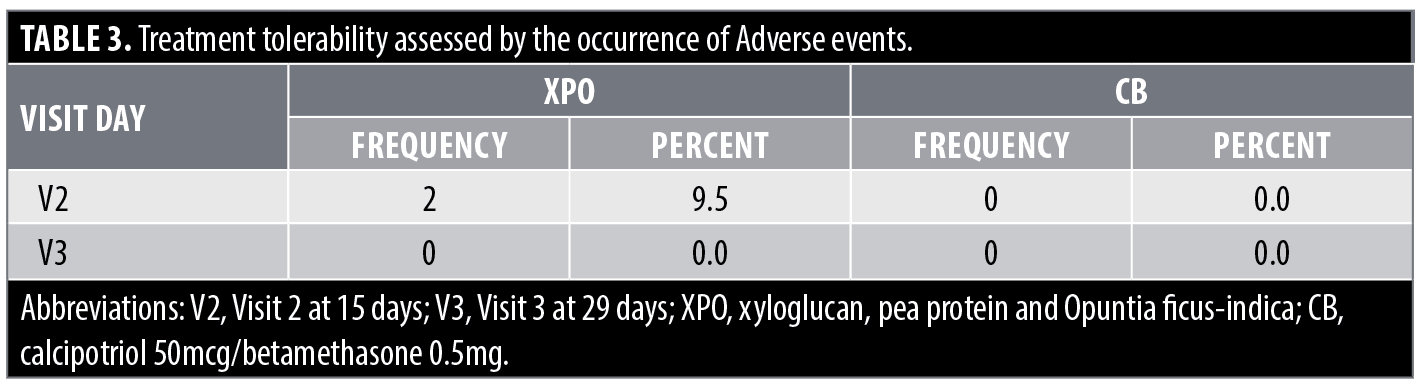
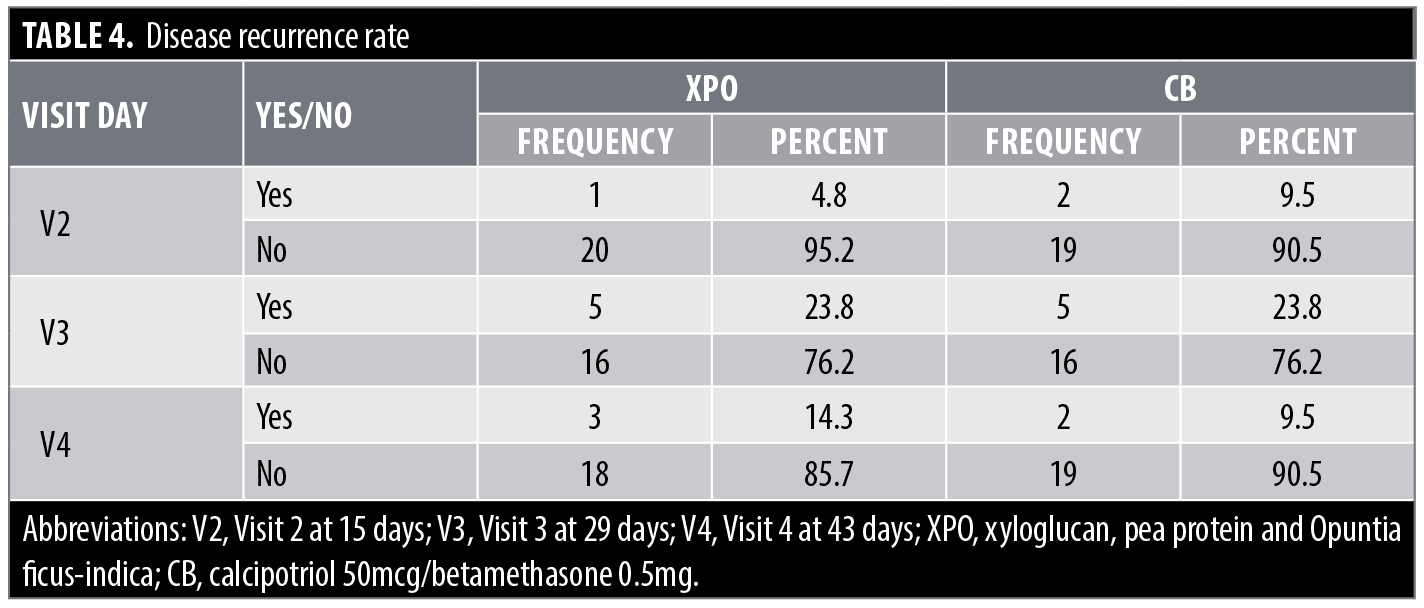
Tolerability results. The tolerability of XPO was assessed by monitoring the occurrence of Adverse Events (AE). Two patients in the XPO group (9.5%) reported non-serious AEs (moderate burning sensation) at Visit 2, which disappeared by the end of the study (Table 3). No subject dropped out of the study due to adverse events in either treatment arm. The disease recurrence rates for the XPO and CB treatment groups are shown in Table 4.
Discussion
A growing body of evidence supports the notion that the pathogenesis of psoriasis involves a complicated interplay between unbalanced immune activation and impaired skin barrier function, associated with a strong genetic predisposition and exposure to certain environmental stimuli. A vicious cycle involving both immune and epithelial cells is generated within the psoriatic lesion, in which a defective barrier allows pathogens and environmental antigens to penetrate the skin, resulting in additional activation of immune cells, further triggering inflammation.19,20 Therefore, a therapeutic approach aimed at normalizing the skin barrier function represents a valuable target to manage this disease.20,21 Xyloglucan (XG) possesses a ‘mucin-like’ molecular structure able to form a protective film that preserves the integrity of epithelial tissues,22 while pea proteins (PP) play an important role in tissue maintenance.9 Finally, the complex polysaccharides contained in Opuntia cladode extracts form molecular networks capable of retaining large amounts of water. The aforementioned ingredients are known to display protective effects when applied topically.12 It was previously shown that an XPO pre-treatment of human keratinocytes, infected with S. aureus in vitro, can preserve cell membrane integrity and barrier function, as assessed by lactate dehydrogenase (LDH) assay, Lucifer Yellow (LY) permeation assay, and measurement of Trans-Epithelial Electrical Resistance (TEER).13
The results of this multicenter clinical study show that XPO significantly reduces the degree of erythema, induration, scaling, and the severity of pruritus in adult patients with mild-to-moderate psoriasis, with a comparable efficacy to CB when used short-term. In addition, the treatment was very well tolerated, which was reflected by the good adherence to the study treatment. The only adverse event, reported by two patients was a moderate burning sensation, which disappeared by the end of the study. Given the results, the use of natural substances for skin conditions seems promising.
While XPO has been shown to be an effective and safe therapy for patients suffering from plaque psoriasis, the trial may have some limitations. These include a small sample size and an open-label, non-randomized study design. Due to the characteristic of CB, it was decided to conduct an open-label study rather than a randomized one evaluating the patient medical history and the investigators best medical judgement at the time the patient presented himself/herself at the medical center to prescribe one of the two treatments.
In the future, a randomized clinical trial with an extended sample size could be performed to confirm and corroborate these results. A further limitation was the short duration of the study, although the four weeks treatment period was sufficient to show the efficacy of the treatment, and in line with topical solutions available to patients. However, long-term studies are required to gain information about the duration of treatment benefits and tolerability. Since psoriasis is a chronic relapsing disease, it would be of particular interest to design ‘preventive’ trials, to assess the ability of XPO to delay the onset of symptoms, while decreasing their severity. For example, XPO could be applied independently by patients at the first signs of disease recurrence, to minimize the severity of flare-ups, thus delaying or avoiding the use of strong corticosteroids or other treatments that may cause side effects. It may be also noteworthy to investigate the efficacy and safety of XPO used in combination with systemic drugs for the treatment of severe forms of psoriasis.
Conclusion
This prospective, multicenter, open-label study demonstrated that XPO has similar efficacy to CB in the treatment of mild-to-moderate psoriasis in adult patients, by effectively reducing the severity of psoriatic lesions, the extent of erythema, induration, and scaling, and the intensity of itching. It also showed an excellent safety profile, as highlighted by the absence of adverse events. These results support the use of XPO for the treatment of patients with mild-to-moderate psoriasis.
References
- Blume-Peytavi U, Kanti V. Androgenetic alopecia. Egeberg A, See K, Garrelts A, Burge R. Epidemiology of psoriasis in hard-to-treat body locations: data from the Danish skin cohort. BMC Dermatol. 2020;20:3.
- Di Meglio P, Villanova F, Nestle FO. Psoriasis. Cold Spring Harb Perspect Med. 2014;4:a015354.
- PSORIASIS JOURNEY TO STABILITY National Report – Canadians’ Journey Living with Psoriasis – Winter 2018. Available from: www.canadianpsoriasisnetwork.com/wp-content/uploads/2018/09/JTSFinal-r.pdf
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432–470.
- Ninosu N, Hoelker S, Kappenstein M, et al. Treatment satisfaction of patients with psoriasis with topical therapy in a real-world setting: unmet need for higher effectiveness. J Dermatolog Treat. 2023;34:2200570.
- Chat VS, Kearns DG, Uppal SK, et al. Management of Psoriasis With Topicals: Applying the 2020 AAD-NPF Guidelines of Care to Clinical Practice. Cutis. 2022;110:8–14.
- Maroto-Morales D, Montero-Vilchez T, Arias-Santiago S. Study of Skin Barrier Function in Psoriasis: The Impact of Emollients. Life (Basel). 2021;11:651.
- Campolo M, Lanza M, Filippone A, et al. Evaluation of a Product Containing Xyloglucan and Pea Protein on Skin Barrier Permeability. Skin Pharmacol Physiol. 2020;33:231–236.
- Campolo M, Casili G, Paterniti I, et al. Effect of a Product Containing Xyloglucan and Pea Protein on a Murine Model of Atopic Dermatitis. Int J Mol Sci. 2020;21:3596.
- Sowlati M, Orzan O-A, Morariu SH, et al. Topical application of a formulation containing pea proteins and xyloglucan in adult patients with atopic dermatitis: a double-blind, parallel, randomized, placebo-controlled, multicenter study. J Clin Aesthet Dermatol. 2023;16:35–41.
- Martins M, Ribeiro MH, Almeida CMM. Physicochemical, Nutritional, and Medicinal Properties of Opuntia ficus-indica (L.) Mill. and Its Main Agro-Industrial Use: A Review. Plants (Basel). 2023;12:1512.
- Trombetta D, Puglia C, Perri D, et al. Effect of polysaccharides from Opuntia ficus-indica (L.) cladodes on the healing of dermal wounds in the rat. Phytomedicine. 2006;13:352–358.
- Filippone A, Casili G, Lanza M, et al. Evaluation of the Efficacy of Xyloglucan, Pea Protein and Opuntia ficus-indica Extract in a Preclinical Model of Psoriasis. Int J Mol Sci. 2023;24:3122.
- European Medicines Agency, Science Medicines Health. Daivobet. 30 September 2010 EMA/CHMP/466386/2010 rev. EMEA/H/A-30/001266. Available from: www.ema.europa.eu (accessed 05 Jul 2023)
- Feldman SR, Krueger GG. Psoriasis assessment tools in clinical trials. Ann Rheum Dis. 2005;64 Suppl 2:ii65-ii68; discussion ii69-ii73.
- Fredriksson T, Pettersson U. Severe psoriasis–oral therapy with a new retinoid. Dermatologica.1978;157:238–244.
- Pascoe VL, Enamandram M, Corey KC, et al. Using the Physician Global Assessment in a clinical setting to measure and track patient outcomes. JAMA Dermatol. 2015;151:375–381.
- Pedersen CB, McHorney CA, Larsen LS, et al. Reliability and validity of the Psoriasis Itch Visual Analog Scale in psoriasis vulgaris. J Dermatolog Treat. 2017;28:213–220.
- Bergboer JGM, Zeeuwen PLJM, Schalkwijk J. Genetics of psoriasis: evidence for epistatic interaction between skin barrier abnormalities and immune deviation. J Invest Dermatol. 2012;132:2320–2331.
- Ye L, Lv C, Man G, et al. Abnormal Epidermal Barrier Recovery in Uninvolved Skin supports the Notion of an Epidermal Pathogenesis of Psoriasis. J Invest Dermatol. 2014;134: 2843–2846.
- Sano S. Psoriasis as a barrier disease. Dermatol Sinica. 2015;33:64–69.
- Piqué N, Gómez-Guillén MDC, Montero MP. Xyloglucan, a Plant Polymer with Barrier Protective Properties over the Mucous Membranes: An Overview. Int J Mol Sci. 2018;19:673.