 by Ryan R. Riahi, MD, and Philip R. Cohen, MD
by Ryan R. Riahi, MD, and Philip R. Cohen, MD
Dr. Riahi is with DermSurgery Associates, Houston, Texas; and Dr. Cohen is with the Department of Dermatology, University of California San Diego, La Jolla, California.
J Clin Aesthet Dermatol. 2017;10(7):23–27
Funding: No funding was provided.
Disclosures: The authors have no conflicts of interest relevant to the contents of this article.
Keywords: Antineoplastic, BRAF, chemotherapy, cutaneous, c-kit, dasatinib, melanoma, mucocutaneous, mucosal, seborrheic dermatitis, side effect, tyrosine kinase inhibitors
Abstract: Dasatinib is an oral tyrosine kinase inhibitor approved for imatinib-resistant chronic myelogenous leukemia. It has been investigated in treating other neoplasms, including non-small-cell lung cancer and a subset of melanomas. Seborrheic dermatitis is characterized by erythematous patches or plaques with scaling typically affecting the external ear, glabella, hair-bearing areas of the face, nasolabial fold, and scalp. Antitumor agents are often associated with mucocutaneous side effects, including seborrheic dermatitis.
We describe the case of a 79-year-old woman with a history of sinonasal melanoma who developed a seborrheic dermatitis-like eruption while taking dasatinib. We also review the molecular abnormalities associated with melanoma, summarize the mucocutaneous side effects of dasatinib, and list the other antineoplastic agents associated with a seborrheic dermatitis-like eruption.
Introduction
Adverse cutaneous reactions frequently occur with chemotherapy and antineoplastic biologic therapies.[1] As novel agents are introduced, new mucocutaneous side effects are discovered. Common forms of skin toxicity include acneiform eruptions, alopecia, generalized rashes, hand-foot syndrome, hyperpigmentation, mucositis, and nail changes.[1] Adverse cutaneous reactions frequently occur with chemotherapy and antineoplastic biologic therapies.[1] As novel agents are introduced, new mucocutaneous side effects are discovered. Common forms of skin toxicity include acneiform eruptions, alopecia, generalized rashes, hand-foot syndrome, hyperpigmentation, mucositis, and nail changes.[1]
Seborrheic dermatitis is a chronic skin disorder characterized by erythematous, occasionally pruritic patches with greasy scales in areas with increased sebaceous glands.[2] Seborrheic dermatitis is common, affecting 1 to 5 percent of immunocompetent individuals, and is more frequent in men. The condition most commonly affects the face, including the beard area, external ear, eyebrows, nasolabial folds, and scalp.[3]
Dasatinib is a second-generation, multitargeted tyrosine kinase inhibitor with implications for use in several neoplasms.[4,5] Dasatinib may be an effective agent in tumors containing a mutation in the c-kit receptor tyrosine kinase gene.[6] Anti-tumor agents may be associated with mucocutaneous side effects, including seborrheic dermatitis. We describe the case of a 79-year-old woman with a history of sinonasal melanoma who developed a seborrheic dermatitis-like eruption two days after initiation of dasatinib. Written consent was obtained from the patient to use the photos. We also review the molecular abnormalities associated with melanoma. In addition, we summarize the mucocutaneous side effects of dasatinib and list the antineoplastic agents associated with a seborrheic dermatitis-like eruption.
Case Report
A 79-year-old woman had a two-year history of intermittent epistaxis that was controlled conservatively. She subsequently developed severe nasal bleeding in February 2012. She required a surgical procedure to control the bleeding. Biopsies were taken of the nasal sinus during surgery; the pathology revealed sinonasal melanoma. A mutation analysis panel report demonstrated a c-kit mutation.
The patient underwent a medial maxillectomy and had postoperative radiation to the left nose and maxillary sinus. A subsequent positron emission tomography (PET) scan revealed metastases to the liver and lung with increased uptake noted along the left nasal maxillary region, which was consistent with melanoma recurrence. The patient was treated with dasatinib since her mucosal melanoma expressed a c-kit mutation.[6,7]
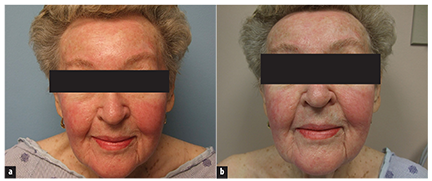
The patient presented to the dermatology clinic for evaluation of facial redness that had occurred since beginning treatment with dasatinib (Figure 1). The patient noted swelling of her face and erythema of the cheeks, forehead, and chin 1 to 2 days after initiation of the drug. She reported that that the areas did not itch and were not painful.
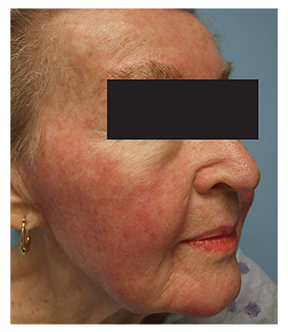
Cutaneous examination demonstrated erythema with slight scaling on the forehead, malar cheeks, upper lip, and chin area of the face (Figure 2). The facial erythema and scaling favored the diagnosis of a seborrheic dermatitis-like eruption. The sequence of events and time course suggested that this facial eruption occurred secondary to dasatinib. Topical desonide 0.05% ointment was initiated twice daily.Within a few weeks, the patient developed dehydration and failure to thrive requiring hospitalization for rehydration and correction of her electrolyte imbalance. Dasatinib was discontinued and her facial rash resolved.
Discussion
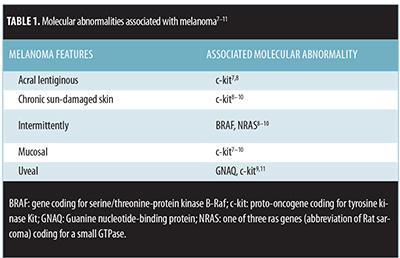
Oncogenic mutations associated with melanoma. Melanoma is a malignant tumor that can be associated with several oncogenic mutations (Table 1).[7–11] Activating BRAF, gene coding for serine/threonine-protein kinase B-Raf, mutations can lead to a mutated protein kinase with downstream effects of activating mitogen-activated protein kinase (MAPK).[12] BRAF mutations are present in approximately 40 to 60 percent of cutaneous melanomas and are more common in melanomas arising in skin that did not receive chronic sun damage.[7,12] Tumor regression has been demonstrated in some patients with a BRAF V600E mutation who were treated with vemurafenib, a BRAF inhibitor.[7–9,12]
C-kit mutations in melanoma. In contrast, some mucosal melanomas have been associated with other mutations and respond to different therapies. C-kit mutations are generally not found in cutaneous melanomas occurring in intermittently sun-exposed skin.[9] Indeed, a subset of acral lentiginous, chronic sun-damaged, and mucosal melanomas have been associated with mutations in the c-kit gene.[7–9] Activating c-kit mutations signal through the phosphatidylinositol 3-kinase pathway; inhibition of this pathway can result in decreased migration and proliferation.[7] Dasatinib, a small molecule inhibitor of tyrosine kinases, including bcr-abl, c-kit, and src-family kinases (SFK), has been demonstrated to be effective in a subset of patients with c-kit mutation positive melanomas.[7,9,10]
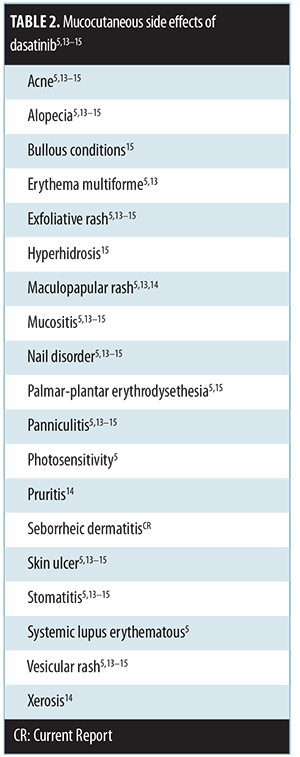
Mucocutaneous side-effects of dasatinib. Mucocutaneous side effects of dasatinib have been described (Table 2).[5,13–15] The most common mucocutaneous side effects include erythematous macular, papular, and/or exfoliative rash, mucositis/stomatitis, and pruritus.[14] To the best of our knowledge, a seborrheic dermatitis-like eruption associated with dasatinib treatment has not previously been described in the literature.
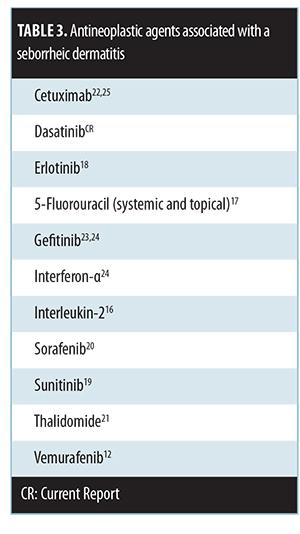
Anti-tumor agents with seborrheic dermatitis-like eruption as a mucocutaneous side-effect. Seborrheic dermatitis-like eruptions have been reported with other antineoplastic agents (Table 3)[12,16–25] Our patient’s seborrheic dermatitis-like eruption was temporally related to the administration of dasatinib. The skin lesions appeared shortly after the drug was started and resolved promptly after it was discontinued.
Conclusion
Medications have been associated with mucocutaneous side effects, including seborrheic dermatitis. Dasatinib can be added to the list of antineoplastic agents with a seborrheic dermatitis-like eruption as a side effect. Although the presentation of medication-associated seborrheic dermatitis can be dramatic, its presence does not require discontinuation of the offending agent in oncology patients whose malignancy is responding to the antineoplastic drug.
References
- Wyatt AJ, Leonard GD, Sachs DL. Cutaneous reactions to chemotherapy and their management. Am J Clin Dermatol. 2006;7:45–63.
- Naldi L, Rebora A. Clinical practice. Seborrheic dermatitis. N Engl J Med. 2009;360:387–396.
- Bikowski J. Facial seborrheic dermatitis: a report on current status and therapeutic horizons. J Drugs Dermatol. 2009;8:125–133.
- Montero JC, Seoane S, Ocaña A, Pandiella A. Inhibition of SRC family kinases and receptor tyrosine kinases by dasatinib: possible combinations in solid tumors. Clin Cancer Res. 201;17:5546–5552.
- Gnoni A, Marech I, Silvestris N, et al. Dasatinib: an anti-tumour agent via Src inhibition. Curr Drug Targets. 2011;12:563–578.
- Woodman SE, Trent JC, Stemke-Hale K, et al. Activity of dasatinib against L576P KIT mutant melanoma: molecular, cellular, and clinical correlates. Mol Cancer Ther. 2009;8:2079–2085.
- Kluger HM, Dudek AZ, McCann C, et al. A phase 2 trial of dasatinib in advanced melanoma. Cancer. 2011;117:2202–2208.
- Antonescu CR, Busam KJ, Francone TD, et al. L576P KIT mutation in anal melanomas correlates with KIT protein expression and is sensitive to specific kinase inhibition. Int J Cancer. 2007;121:257–264.
- Liang R, Wallace AR, Schadendorf D, Rubin BP. The phosphatidyl inositol 3-kinase pathway is central to the pathogenesis of Kit-activated melanoma. Pigment Cell Melanoma Res. 2011;24:714–723.
- deBlacam C, Byrne C, Hughes E, et al. HOXC11-SRC-1 regulation of S100beta in cutaneous melanoma: new targets for the kinase inhibitor dasatinib. Br J Cancer. 201;105:118–123.
- All-Ericsson C, Girnita L, Müller-Brunotte A, et al. c-Kit-dependent growth of uveal melanoma cells: a potential therapeutic target? Invest Ophthalmol Vis Sci. 2004;45:2075–2082.
- Huang V, Hepper D, Anadkat M, Cornelius L. Cutaneous toxic effects associated with vemurafenib and inhibition of the BRAF pathway. Arch Dermatol. 2012;148:628–633.
- Huang X, Patel S, Ahmed N, Seiter K, Liu D. Severe toxicity of skin rash, fever and diarrhea associated with imatinib: case report and review of skin toxicities associated with tyrosine kinase inhibitors. Drug Des Devel Ther. 2009;2:215–219.
- Haura EB, Tanvetyanon T, Chiappori A, et al. Phase I/II study of the Src inhibitor dasatinib in combination with erlotinib in advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:1387–1394.
- Amitay-Laish I, Stemmer SM, Lacouture ME. Adverse cutaneous reactions secondary to tyrosine kinase inhibitors including imatinib mesylate, nilotinib, and dasatinib. Dermatol Ther. 2011;24:386–395.
- Kawakami Y, Nakamura-Wakatsuki T, Yamamoto T. Seborrheic dermatitis-like eruption following interleukin-2 administration. Dermatol Online J. 2010;16(9):12.
- Brodell EE, Smith E, Brodell RT. Exacerbation of seborrheic dermatitis by topical fluorouracil. Arch Dermatol. 2011;147:245–246.
- Benomar S, Boutayeb S, Afifi Y, et al. Hand-foot syndrome and seborrheic dermatitis-like eruption induced by erlotinib. Dermatol Online J. 2009;15(11):2.
- Tsai KY, Yang CH, Kuo TT, et al. Hand-foot syndrome and seborrheic dermatitis-like rash induced by sunitinib in a patient with advanced renal cell carcinoma. J Clin Oncol. 2006;24:5786–5788.
- Yang CH, Lin WC, Chuang CK, et al. Hand-foot skin reaction in patients treated with sorafenib: a clinicopathological study of cutaneous manifestations due to multitargeted kinase inhibitor therapy. Br J Dermatol. 2008;158:592–596.
- Hall VC, El-Azhary RA, Bouwhuis S, Rajkumar SV. Dermatologic side effects of thalidomide in patients with multiple myeloma. J Am Acad Dermatol. 2003;48:548–552.
- Pinto C, Barone CA, Girolomoni G, et al. Management of skin toxicity associated with cetuximab treatment in combination with chemotherapy or radiotherapy. Oncologist. 2011;16:228–238.
- Graves JE, Jones BF, Lind AC, Heffernan MP. Nonscarring inflammatory alopecia associated with the epidermal growth factor receptor inhibitor gefitinib. J Am Acad Dermatol. 2006;55:349–353.
- Scheinfeld NS. Seborrheic dermatitis. Skinmed. 2005;4:49–50.
- Cohen PR, Escudier SM, Kurzrock R. Cetuximab-associated elongation of the eyelashes: case report and review of eyelash trichomegaly secondary to epidermal growth factor receptor inhibitors. Am J Clin Dermatol. 2011;12:63–67.

