J Clin Aesthet Dermatol. 2025;18(6):29–40.
by Promise Ufomadu, BSA; Bartley Joseph Gill, MD, PhD; Theodore Rosen, MD; Ikue Shmizu, MD; and Ida Orengo, MD
Mr. Ufomadu, Drs. Gill, Orengo, Rosen, and Shmizu are with the School of Medicine at Baylor College of Medicine in Houston, Texas. Drs. Orengo, Rosen, and Shmizu are also with the Department of Dermatology at Baylor College of Medicine in Houston, Texas. Dr. Gill is also with Memorial Hermann Health Systems in Houston, Texas.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors declare no conflicts of interest relevant to the content of this article.
Abstract: Background: Lately, there has been a growing demand for the utilization of complementary and alternative medicines (CAMs) with dermatological applications. This is true despite limited RCT-level studies on such agents. This presents a barrier for dermatologists and fellow clinicians in counseling patients who may be using or are tempted to use these CAM modalities. This review highlights CAM agents used by patients for applications in cosmetic and surgical dermatology, exploring their efficacy and toxicity profiles. Methods: A comprehensive review was conducted on the effectiveness of several CAMs utilized in cosmetic and surgical dermatology by patients. A literature search was performed using PubMed, Embase, Google Scholar, Web of Science, and Cochrane. Results: Most CAM agents studied had statistically insignificant results, and for CAM agents that had significant results in efficacy, the studies were questionable due to flawed randomization, lack of proper blinding, faulty data analysis, poor study design, suggestion of bias, small sample size, and limited clinical application. Conclusion: CAM agents have promising potential in dermatologic use; however, more RCT-level studies are needed. A study design that either emphasizes a comparison between the CAM agent and conventional therapy, or the CAM agent with or without conventional therapy should be incorporated in future studies. As of now, dermatologists should be cognizant of bias in published studies demonstrating the effectiveness of certain CAM agents, as well as the possible adverse effects. Keywords: Complementary and alternative medicine, mohs, photoaging, skin cancer, bcc, bowen, scars, Steven Johnson syndrome, melanoma, botanicals
Introduction
Complementary and alternative medicine (CAM) usage has grown substantially, peaking in 2019 at 80 percent in the United States (US) and 91 percent in Europe.1 In surgical settings, 50 to 67 percent of outpatients report perioperative CAM use.2,3 Specific to dermatologic surgery, 20 percent of CAM usage was reported among Mohs micrographic surgery patients.4 Given such a high prevalence of CAM utilization by dermatology patients, understanding CAM efficacy and potential side effects is relevant for dermatologic surgeons.
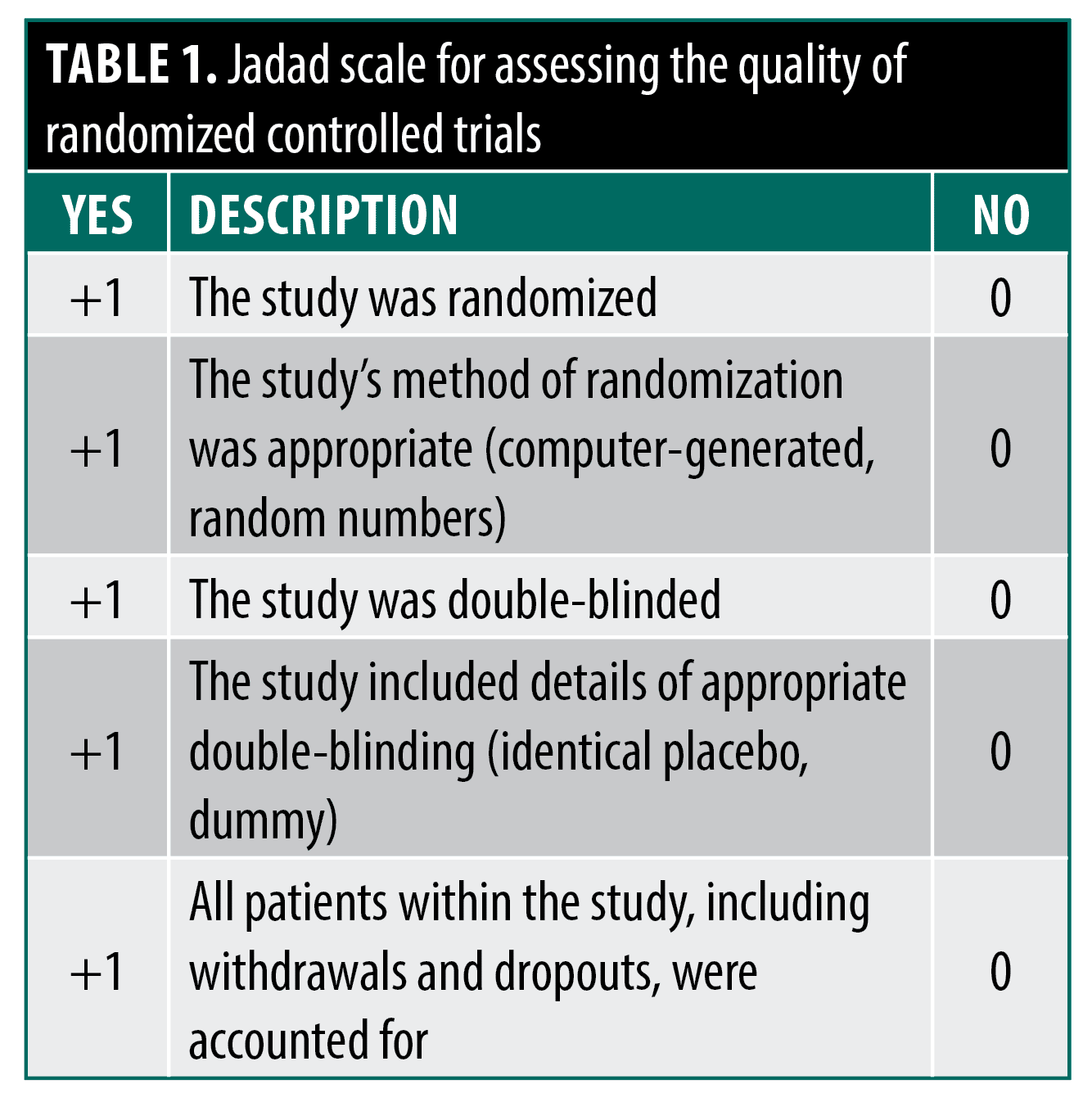
This review also explores CAM agents utilized for cosmetic dermatology applications like photoaging. “Cosmeceutical” and “nutraceutical” usage is widespread, contributing to a $4 billion cosmetic botanicals market in 2002, despite limited randomized controlled trial (RCT) data supporting manufacturer claims.5,6 Dermatologists play an important counseling role for patients using these modalities. Additionally, this review highlights CAM agents with dermatologic side effect risks to aid diagnosis and treatment. When reasonable, the Jadad Scale will assess the quality of RCT evidence on dermatologic CAM therapies to guide practitioners on potential efficacy (Table 1).7 Heightened scrutiny of studies should help practitioners differentiate between scientifically proven CAM agents and those that merely claim efficacy.
Skin Cancer—Prevention and Primary Treatment
Polyphenols and green tea catechins in skin cancer prevention.The literature on CAM therapies for non-melanoma skin cancer (NMSC) and melanoma primarily focuses on polyphenols like green tea catechins’ effects on preventing precancerous events.8 Numerous in vitro and animal studies elucidate the proposed protective mechanisms, including anti-inflammatory (decreased COX-2, PGE, cytokines), antioxidant (decreased reactive oxygen species, increased antioxidant enzymes), and DNA repair activity (decreased pyrimidine dimers via NER), especially epigallocatechin-3-gallate (EGCG).9–23 Human studies show similar photoprotective effects like reduced erythema and reduced markers of oxidative stress markers. However, these human studies suffer from small sample sizes, lack of vehicle controls, and potential investigator bias.19,24–28
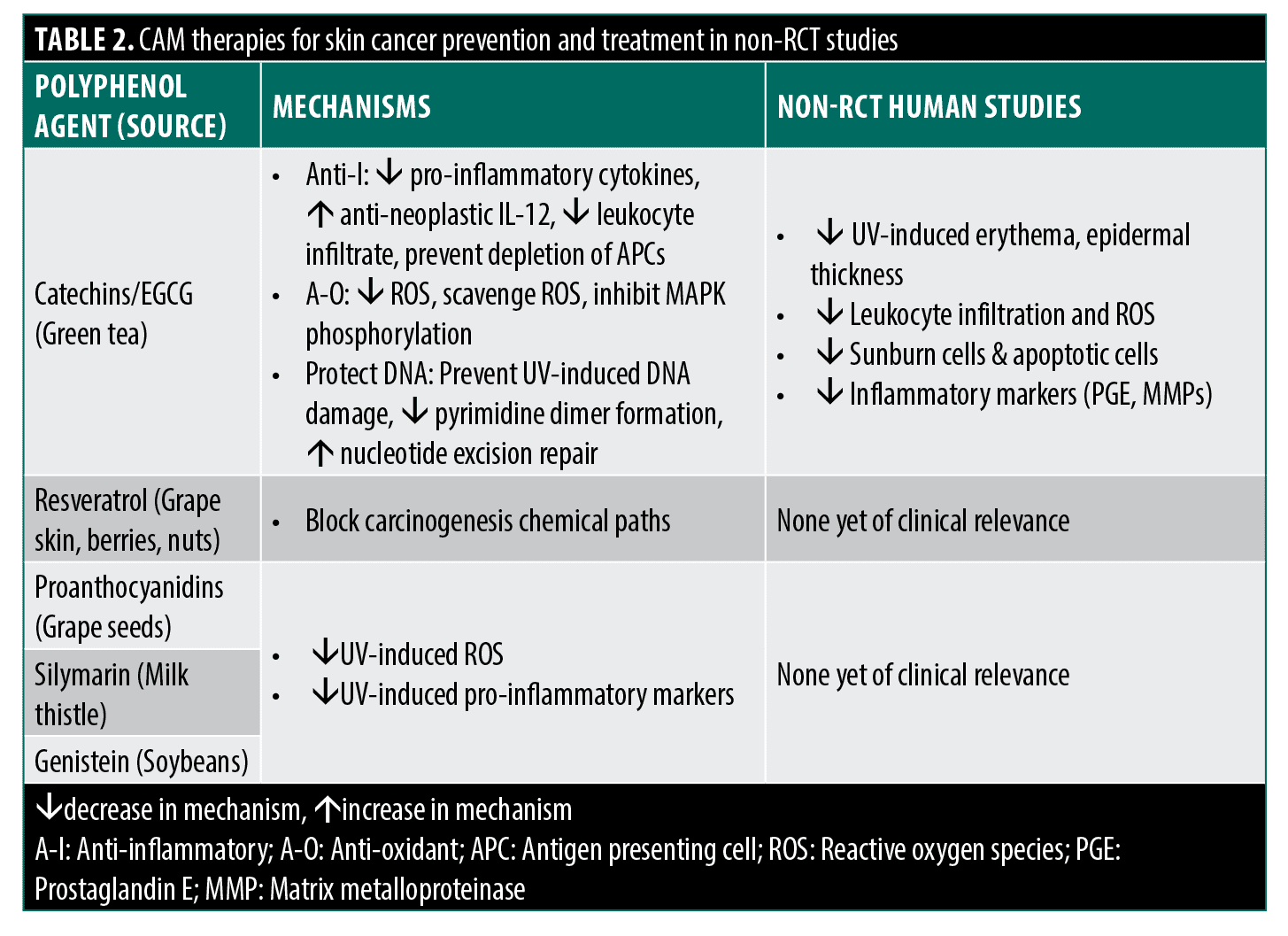
Other human studies have deployed proper controls to study the UV-protective effects of lotion containing green tea extract (GTE), but have also found more mixed results. Mnich et al25 compared the topical application of lotion with and without 0.4% GTE in 18 subjects and found an inhibition of p53 expression and decreased keratinocyte apoptosis in GTE-treated skin. However, this study found no difference in erythema reduction or thymidine dimer formation when comparing lotions with and without GTE. It should also be noted that, while decreased p53 expression and apoptosis may be a sign of photoprotection, these same processes are also critical in halting neoplasm development, such that GTE may potentially have the opposite effect than intended. Li et al28 deployed a series of lotions with a range of higher GTE concentrations (2–5%) compared to lotion-only controls and concluded that GTE-containing lotions decreased UV-induced erythema, epidermal thickness, and MMP expression. However, no statistics were offered for erythema or MMP measurements, and epidermal thickness was only modestly decreased in some GTE formulations and still substantially greater than in non-UV-treated skin. Perhaps most critically, there was no dose-response seen in any of the measurements, with efficacy found to be inexplicably higher at lower or moderate GTE concentrations and discordant between outcomes.
One critical aspect to note for both animal and human studies is that ECGC or GTE treatments were generally applied immediately before an acute UV exposure, and there has been little assessment of the extent of lingering protective effects if UV exposure occurs at a considerable length of time after application or if UV exposure is less intense, but prolonged. Examination of this more chronic photoprotective effect is essential as it would more realistically model how green tea or its constituent catechins would be used practically by patients as a CAM therapy. While promising, few rigorous RCTs examine clinical endpoints; only one RCT studied topical green tea extract (sinecatechins) for basal cell carcinoma.29 More RCT-level evidence on clinical outcomes is needed before recommending catechins for skin cancer prevention.
Other polyphenols for cancer prevention. Resveratrol, a polyphenol found in high concentration in grape skin, berries, nuts, and other plant sources, has been studied at great length in vitro and in vivo as a potent antioxidant and anti-inflammatory agent with wide-ranging applications including cardiovascular disease, aging, and cancer.30,31 Studies have shown inhibition of carcinogenesis at multiple stages in vitro and inhibition of skin cancer initiation and growth in rodent models with promising results. Nonetheless, no human studies or RCTs have been performed.30 Similar effects have been shown with the polyphenol classes proanthocyanidins, silymarin, and the flavonoid genistein, respectively found primarily in grape seeds, milk thistle, and soybeans, with in vitro and animal studies reporting some effects decreasing UV-induced H2O2 and ROS production and inhibition of a variety of UV-induced pro-inflammatory mediators. However, these findings have yet to be shown in human studies in a clinically relevant way.8 Like green tea catechins, these other polyphenols have shown great promise in non-clinical studies and should prompt further investigation in RCT-level studies for effect on skin cancer prevention.
Escharotic agents. Escharotic or caustic agents are promoted as an alternative treatment for many skin lesions including NMSC with advocates claiming the products effectively destroy diseased skin leaving healthy skin in place.32 Bloodroot (Sanguinaria candensis) and zinc chloride have traditionally been the most commonly used substances, where topical application leads to tissue necrosis and eschar formation. Some in vitro data suggest that bloodroot may also possess additional anti-microbial, anti-inflammatory, and antioxidant activity.33 These escharotics, in particular zinc chloride, were initially popularized by Frederic Mohs for use as an in situ fixative agent as part of Mohs surgery but were always deployed along with surgical excision, never alone. They have since been proven less useful than post-excision fixation of fresh tissue given that both methods show equivalent cure rates, but the in situ technique is more painful, destroys healthy tissue, and requires a 24-hour exposure period before excision.32
These agents are sometimes promoted as the folk remedy “black and yellow salve” or sold online as a mixture of multiple different escharotic and non-escharotic CAM agents and are typically marketed as effective alternative cures with misleading wording and lack of appropriate disclaimers.33,34 There are no credible controlled studies supporting the use of escharotic agents as an alternative or complementary treatment for NMSC, melanoma, or other skin neoplasms. Despite this fact, several case reports in the literature not only illustrate their persistent use by patients but also highlight the risks of their use. These risks include non-selective destruction of healthy skin, resultant severe scarring, and the high potential for cure failure and recurrence in the scarred skin or in an adjacent site requiring a much more extensive subsequent excision.35 While some animal study data suggests that there may still be a useful complementary role of escharotic agents before Mohs excision of melanoma to promote immune stimulation,36 no clinical studies yet exist to establish this efficacy, and dermatologists should be aware of the risks of these agents and strongly advise patients against their use as NMSC therapy.
Phytotherapeutics as NMSC and melanoma treatment. Glycoalkaloids (solasodine glycosides) initially derived from the Sodom apple plant (Solanum sodomaeum) and also found in eggplant have been shown in vitro to cause cancer cell-specific apoptosis and have been under investigation as a topical treatment for NMSC.37 Early open studies of its application on keratoses and NMS, in addition to a recent case report involving Bowen’s disease, have suggested efficacy in cancer treatment.38,39 Punjabi et al40 performed one RCT in an attempt to confirm these observations, comparing BCC treatment with a keratolytic salicylic acid/urea cream with and without solasodine glycosides. The authors concluded effectiveness based on higher reported treatment success at eight weeks. However, there was no description of criteria for the primary endpoint of treatment success, and the treatment group suffered heavily from unexplained withdrawals, with these withdrawn patients inappropriately scored as treatment successes. Furthermore, even setting aside that roughly half of patients did not make it to the recurrence assessment at the one-year follow-up, recurrence rates were still higher than 20 percent, indicating this treatment is far from effective.
A myriad of other phytotherapeutic agents have been purported as remedies for different types of cancer that are too numerous to describe in detail. Oleander (Nerium oleander) and its constituent cardiac glycosides oleandrin, oleandrigenin, and others have demonstrated several potential anti-cancer mechanisms in vitro including inhibition of fibroblast growth factor-2 release via Na+/K+-ATPase pump inhibition,41 inhibition of NF-κβ,42 tumor cell cytotoxicity through increases in ROS,43 and enhancement of apoptotic activity to limit tumor growth and enhance sensitivity to radiotherapy.44,45 A commercial preparation of oleander extract (Anvirzel) is under investigation for cancer prevention and therapy and has been proven safe in a Phase I trial46 but has yet to demonstrate efficacy in a Phase II trial or other RCT-level human study.
Other phytotherapeutics of note that have demonstrated efficacy specifically for skin cancer in animal models include berberine (isoquinoline alkaloids) derived from Goldenseal (Hydrastis canadensis) amongst other plants, sesquiterpenes in ginger (Zingiber officinale), extracts from willow trees (Salix caprea), and isoflavones from red clover (Trifolium pretense). All of these phytotherapeutics have demonstrated an inhibition of photocarcinogenesis in vivo, largely through anti-oxidant and selective apoptosis mechanisms,33 and some have been investigated in their roles in combatting obesity and metabolism,47 but none have thus far been tested in RCT-level human studies involving NMSC or melanoma.
CAMs in Post-Surgical Wound Treatment
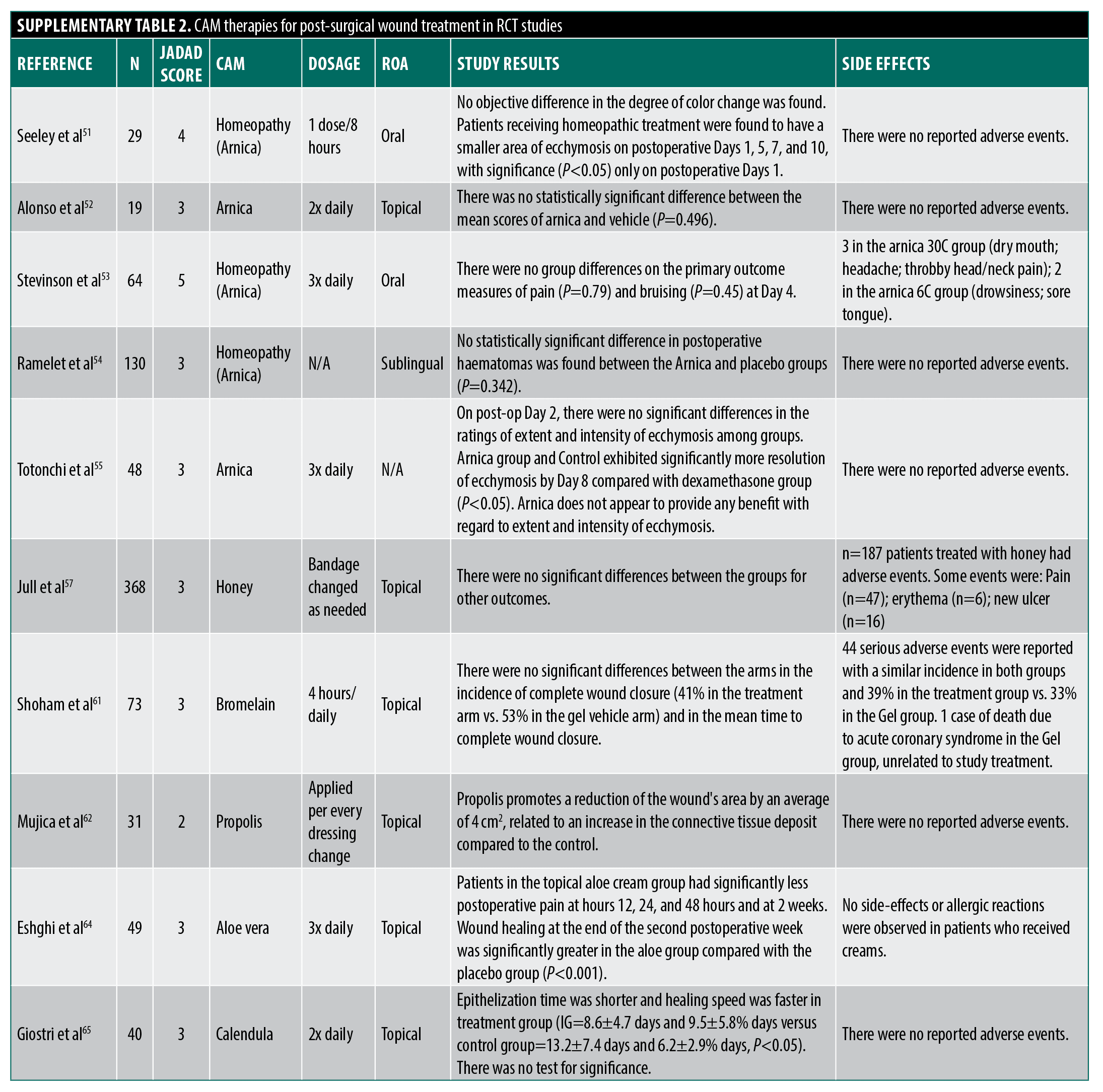
Several agents have been purported as helpful in the perioperative period as wound healing agents. Arnica montana promotes wound healing via anti-inflammatory effects (sesquiterpene inhibition of NFκB).48 However, only homeopathic arnica preparations avoid toxicity concerns like hypertension and nephrotoxicity related to higher concentrations experienced when ingested.3,49 Pilot studies in the 1970s suggested bruising improvements with homeopathic arnica but had small samples, a lack of acceptable statistical analysis, and unclear blinding protocols.50 A recent manufacturer-funded study claims benefits for post-operative ecchymosis but lacks proper randomization, blinding, and consistent endpoints.51 More rigorous studies show no arnica effect on bruising, pain, or swelling across various surgeries,52–55 yielding a Jadad score of 3.6.
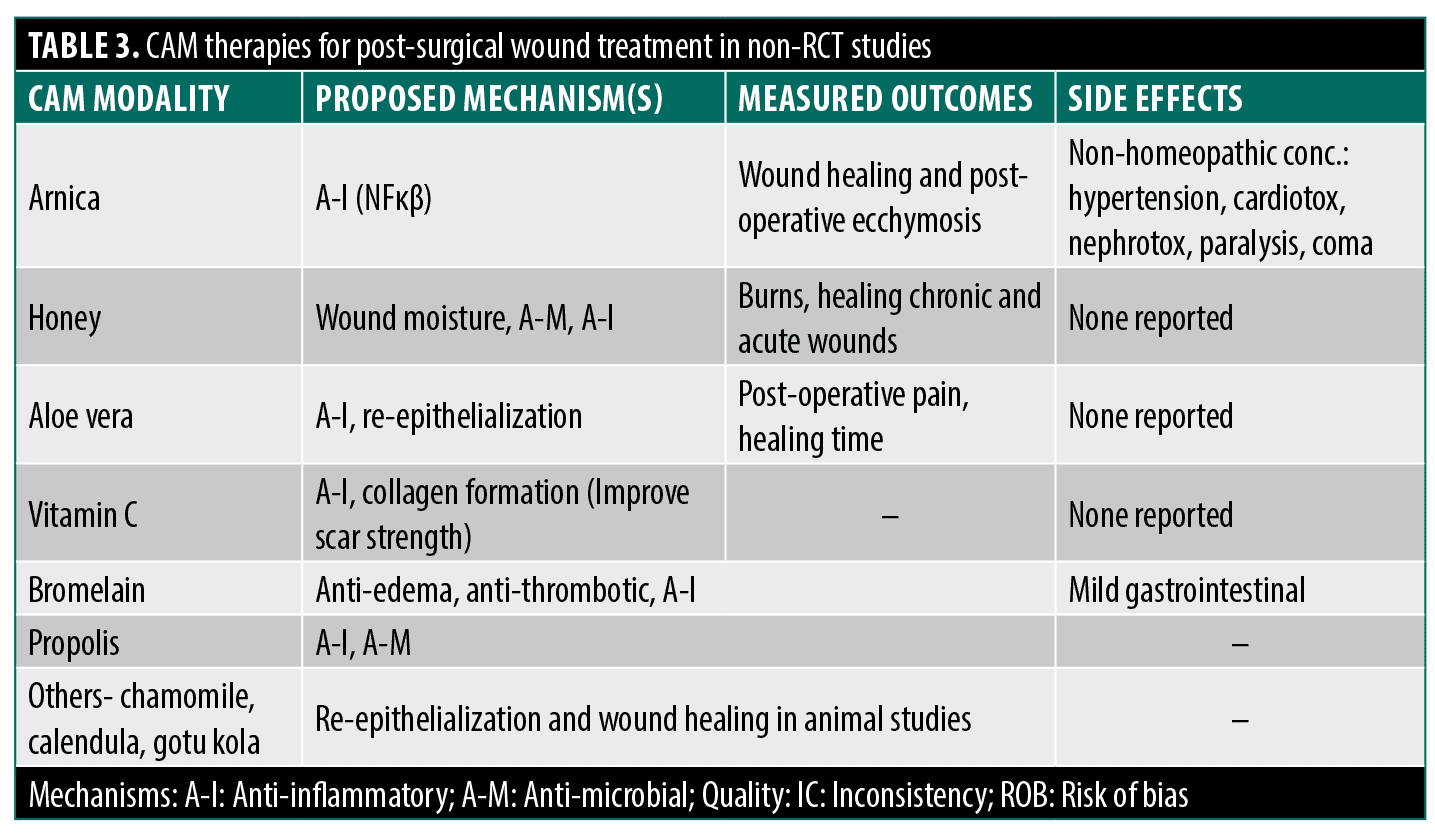
Other proposed CAM agents include honey (promoting a moist and decolonized environment) but Cochrane reviews found no efficacy for acute non-burn wounds.56,57 Curcumin shows promise in vitro/animal models but has only limited human RCTs.58 Though with limited accessible human RCT data, Broeolain shows insignificant findings while propolis shows a significant reduction of the wound’s surface area.3,59–62 However, Vitamin C was found to improve scar strength post-surgery.63 Aloe vera showed potential benefits (decreased postoperative pain and faster wound healing compared to control) post-hemorrhoidectomy but with randomization concerns.64 Formulations of chamomile and gotu kola demonstrate re-epithelialization effects in animals but lack rigorous human studies.3 Calendula had one study that presented a faster healing time than the control, though there was no statistical significance.65
Scar Treatment
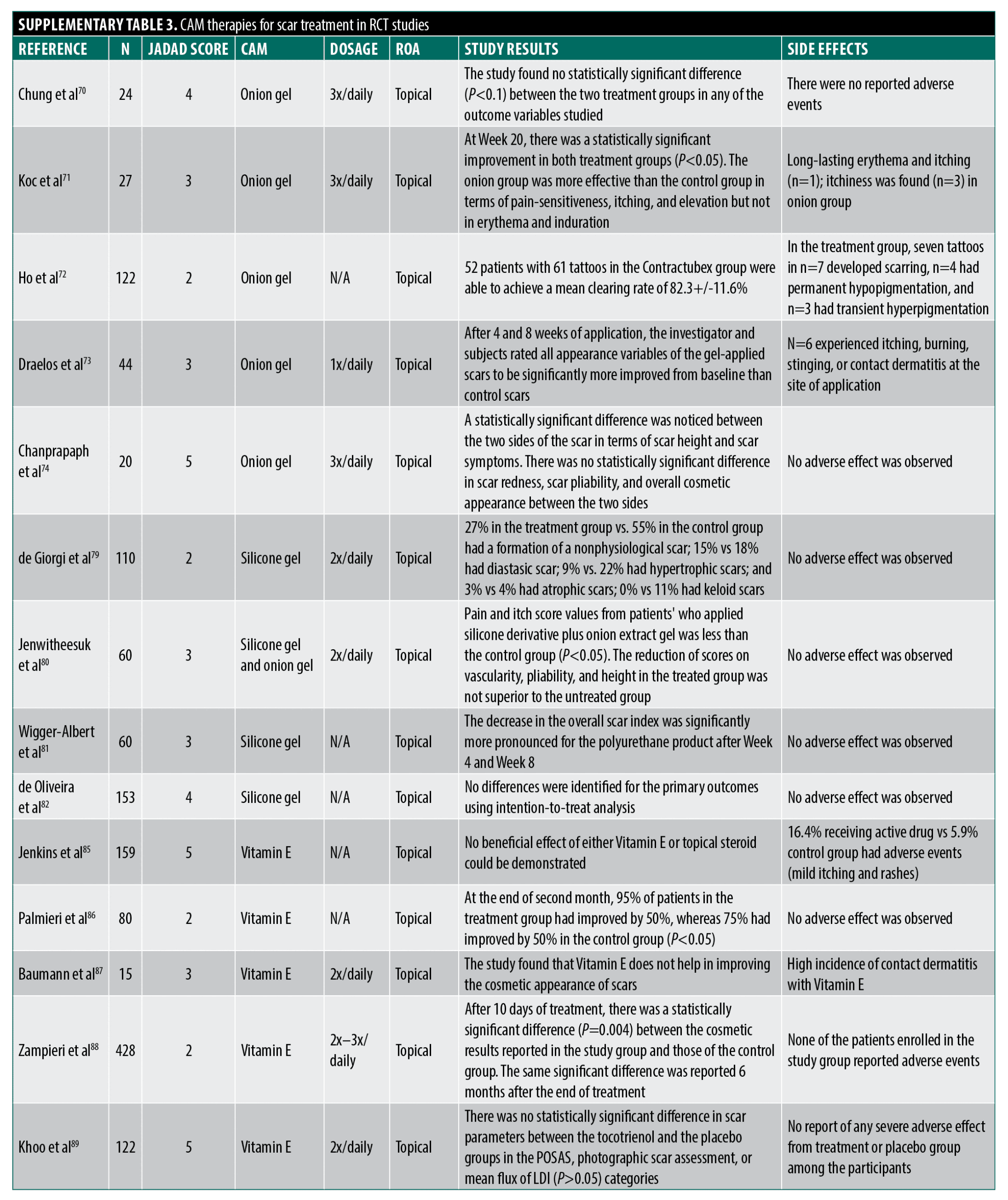
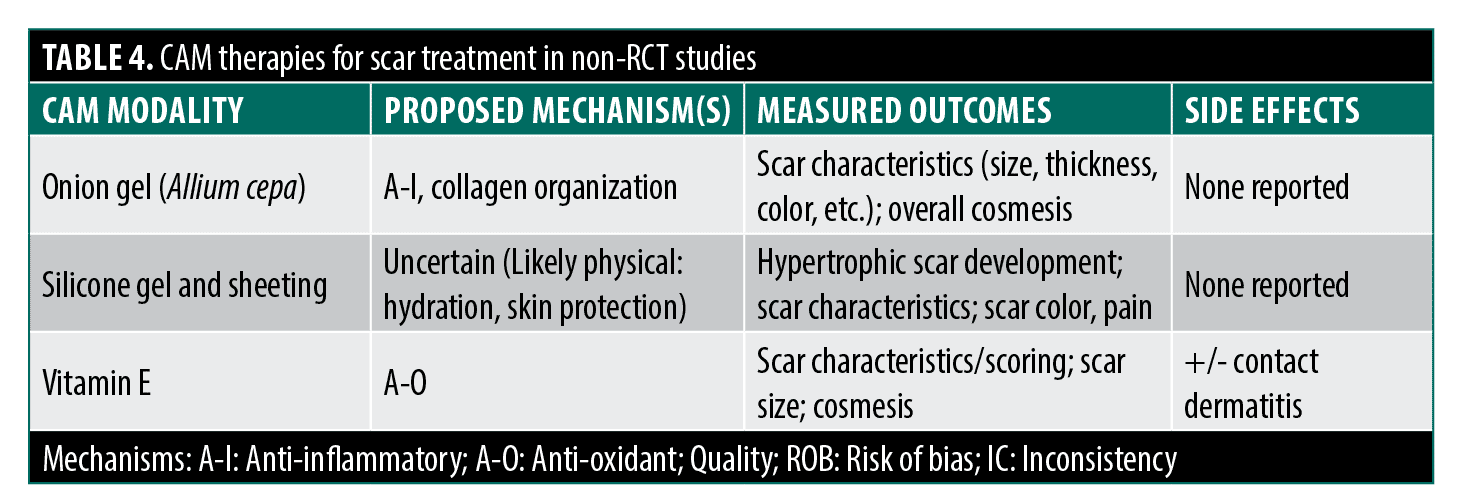
Onion gel. Hypertrophic scars and keloids following surgery or trauma have prompted interest in CAM-based scar treatments due to limited conventional options.66 Onion extract (Allium cepa, Mederma) has been studied for potential anti-inflammatory and collagen regulation benefits.67 However, RCTs show mixed results with dubious methodological limitations.
Current human studies found no significant effects of onion gel on scar erythema and pruritus,68 overall improvement and scar characteristics,69 and scar appearance.70 Jackson et al68 lacked randomization/blinding, Clarke et al69 had placebo uncertainty/baseline differences, and Chung et al70 studied non-hypertrophic scars.
Published studies with positive findings suffer from methodological flaws: Koc et al71 found additive onion gel benefits with triamcinolone injections but lacked topical control/blinding/statistics. Ho et al72 reported reduced scarring post-laser tattoo removal without a proper description of blinding and methods. Draelos et al73 showed intra-group scar improvement in a manufacturer-funded split-scar study lacking a critical placebo control. In a well-designed study, Chanprapaph et al74 found modest benefits in height/symptoms in a split-scar cesarean study but lacked confirmation of adequate patient blinding. The lack of vehicle-placebo controls in many studies is a major limitation, as scar hydration affects healing. With inconsistent results and significant methodological issues like lack of blinding/randomization/controls (Jadad score 3.4), evidence for onion extract improving scar cosmesis remains inconclusive at best.
Silicone gel or sheeting. Silicone gel sheets/ointments (over 60 polydimethylsiloxane products) are widely used for scar prevention/treatment, though the mechanism is unclear, potentially modifying evaporative water loss and fibroblast collagen deposition rather than a chemical effect.67,75–78 A 2008 Cochrane review of 15 RCTs found silicone sheets did not prevent overall scar development compared to no treatment, but reduced hypertrophic scarring in high-risk patients (95% CI 0.21-0.98).78 For scar treatment, silicone sheets reduced thickness but not size, appearance, pain, or itch compared to controls. Results were mixed for color/elasticity, with no benefit over non-silicone dressings or lasers, and inferiority to triamcinolone injections for keloids.78 Subsequent studies had issues too: inadequate masking/controls,79 unclear randomization/outcome assessment,80 and manufacturer affiliation.81 The lack of benefit over non-silicone dressings suggests the occlusive effect, not silicone itself, may drive positive outcomes.78,81–83 Despite inconclusive overall efficacy and design limitations (average Jadad 3), silicone remains recommended for hypertrophic scar management, with stronger evidence in high-risk patients for reducing scar thickness.75,84
Vitamin E. Topical vitamin E (tocopherol) has been suggested to treat scarring, with proposed anti-oxidant mechanisms, acting to reduce the formation of ROS and spread of peroxidation of cellular membrane lipids that impede healing and potentially altering the production of collagen and glycosaminoglycans.75 A few RCTs have been performed investigating these effects. Jenkins et al85 followed patients who underwent reconstructive graft surgery for burns for one year receiving treatment with topical triamcinolone diacetate (Aristocort), topical Vitamin E or carrier cream finding no difference in range of motion, scar thickness, graft size change, and overall cosmetic appearance between groups with outcomes helpfully grouped by burn location (hands, axillary, neck). With the caveat that outcome assessment methods were not thoroughly described, this study was large and well-designed with outcomes measured out to one year. Adverse reactions consisting of mild itching and maculopapular eruptions occurred slightly more frequently in the two treatment groups compared to control. Palmieri et al86 tested the complementary application of topical E to silicone plates to treat established scars for two months and concluded enhanced efficacy over the silicone alone based on data showing a higher percentage of patients with a greater than 50 percent improvement on a subjective scar scale. Little weight should be placed on this study, however, as no randomization was described, no placebo control gel was used, no blinding was described, and no numerical data with standard deviations of the scoring was reported. In a different small RCT, Baumann et al87 examined post-Mohs scarring in a split-scar treatment design, comparing an ointment with and without added Vitamin E. The authors found no improvement in blinded subjective assessment of cosmesis but did note a substantial percentage of contact dermatitis reactions on the Vitamin E sites. The methodology of this study is seriously flawed, however, as scar sides were inappropriately randomized (patient-chosen), no information was provided on outcome measurements of improvement, and no statistics were provided. Furthermore, the formulation and concentration of Vitamin E applied, taken from caplets intended for oral use, is known to cause contact reactions, with other formulations that are more typically topically applied and do not readily cause contact dermatitis not tested. In a larger study, Zampieri et al88 compared topical application of a commercial Vitamin E gel or petrolatum control for 15 days before and 30 days after inguinal incision surgery in children with authors concluding improved efficacy of the Vitamin E treatment based on minor, but significant differences in keloid formation and subjective cosmesis. There was no mention of the method of randomization in this study or description of baseline characteristics for comparison between groups, and investigators were not blinded. Furthermore, the control topical may have not been well-matched (was not a treatment gel vehicle), and the primary outcome relied on parental (subjective) evaluation and classification of the degree of scar cosmesis and keloid formation, clinical descriptors that would have been more appropriately evaluated by an investigating physician. Khoo et al89 recently performed a large (n=122), well-designed study comparing the treatment of new (<2 weeks) surgical scars with 5% Vitamin E cream or a well-matched placebo and found no inter-group differences throughout the 16-week study in blinded patient and investigator scoring, visual analog scale scoring of photographs by independent investigators or live Doppler imaging of blood flow. While no intention-to-treat analysis was performed, drop-out rates were almost identical between groups. Further of note, the authors did not record any adverse events related to contact dermatitis. Overall, the average Jadad score for the quality of evidence that Vitamin E promotes scar treatment or prevention is 3 for reasons already mentioned.
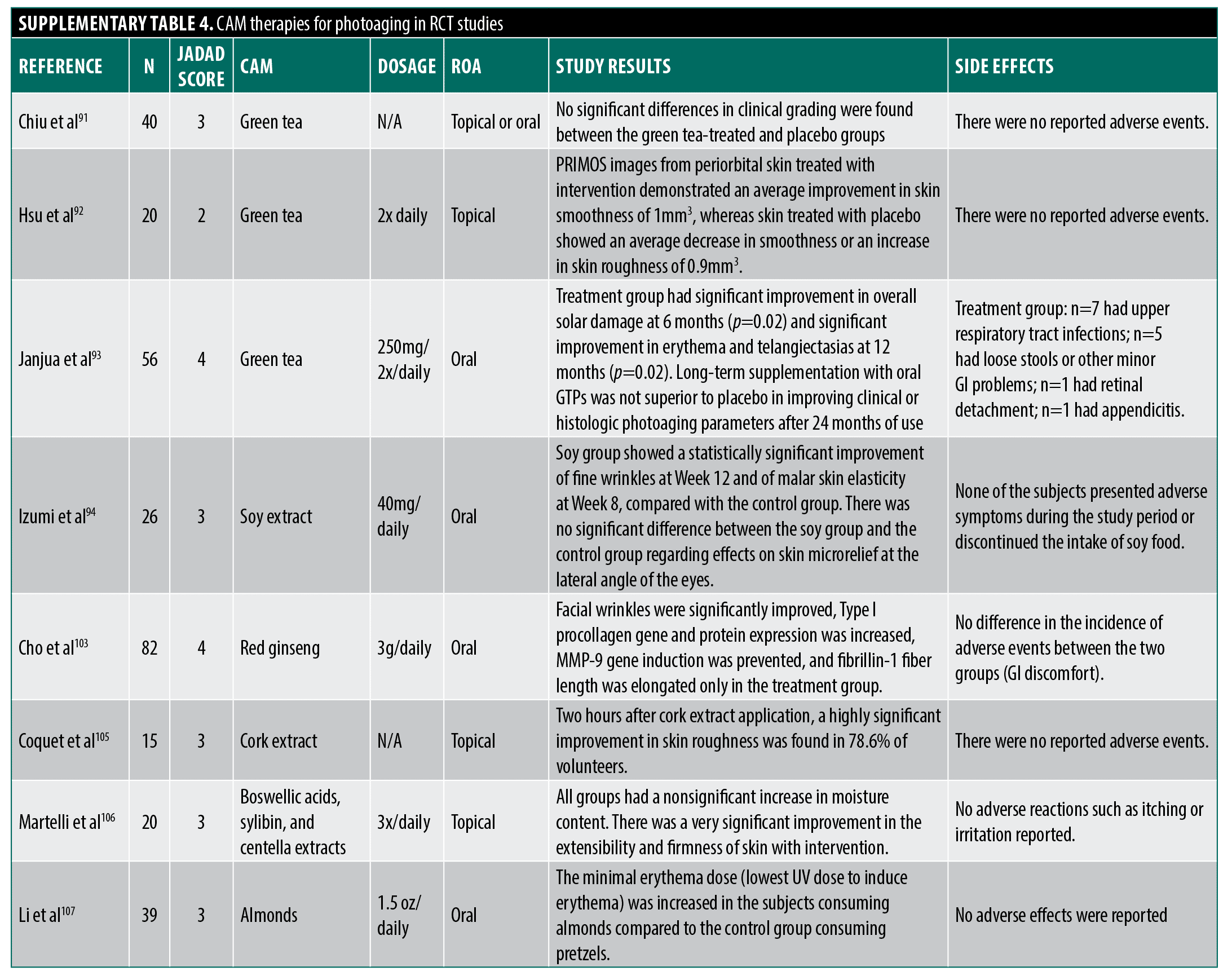
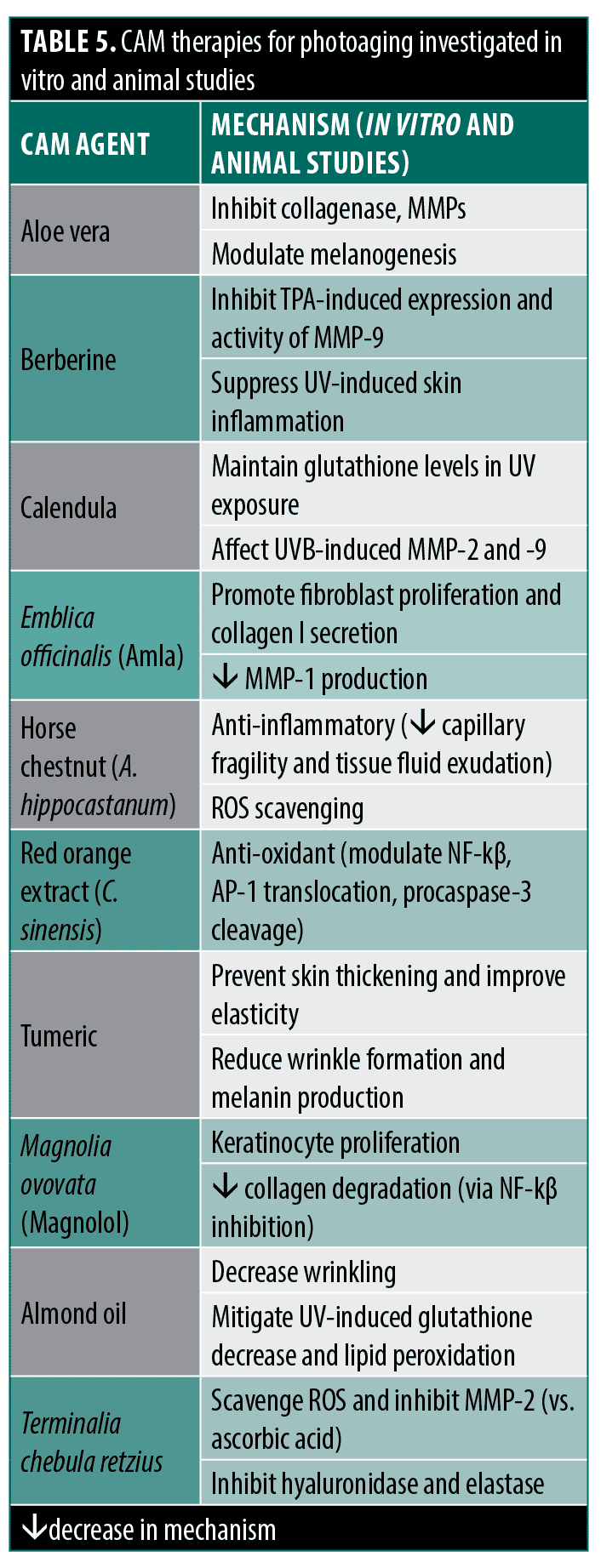
Photoaging. Patient demand for photoaging CAM products generated over $2 billion in sales in 2000, driving research on potential therapies.90 Polyphenols like green tea catechins have been widely studied due to proposed anti-inflammatory and antioxidant mechanisms (anti-aging effects). However, few rigorous RCTs show clinical efficacy. In one well-designed study, Chiu et al91 compared patients using a combined 10% topical and 300mg oral GTE supplements to placebo. Investigators found no improvement after eight weeks in several patient and physician-assessed subjective photoaging-related skin characteristics (wrinkling, pigmentation) and no difference in several histologic parameters (epidermal thickness, collagen content), but did find an interesting significant difference in skin elastin content. Hsu et al92 conducted a left-right trial with a commercial green tea cream that also contained, amongst several other botanical agents, extracts from mangosteen (Garcinia mangostana, constituent anti-oxidant xanthones) and pomegranate (constituent polyphenols). Study authors concluded positive efficacy compared to placebo based on subjective patient preference evaluations and trending differences in computer image-analyzed periorbital skin topography characteristics. However, the results were not net positive, which contradicts this conclusion, as topography improvement was not significant, and blinded physician-assessed wrinkle grades showed no difference. Furthermore, this study featured significant methodological issues as, in addition to potential bias related to funding from the treatment cream manufacturer, the cream contained some potential botanical effectors and no information was provided to show placebo equivalence in appearance, feel, or smell to ensure proper patient blinding. Finally, Janjua et al93 conducted a well-designed, prolonged two-year study comparing oral green tea supplements to placebo with evaluations including blinded physicians’ assessment of solar damage, erythema, and telangiectasias at multiple time points and study-endpoint histological characteristics. While the green tea group showed significant improvements at a few study time points, the authors ultimately concluded it was ineffective as these improvements were not sustained throughout the study or at the two-year endpoint. Given that most studies find no improvement in clinically evident measures of photoaging with green tea supplements and the major methodological problems of the few studies that do show improvement (risk of bias, publication bias, improper blinding details), the assigned averaged Jadad score for green tea supplements protection against photoaging is three.
Other agents studied with inconclusive findings include soy isoflavones,94 black soybean anthocyanins,95,96 resveratrol, grape seed proanthocyanidins, and genistein.97 Though Witch hazel has promising in vitro findings,98,99 human studies have thus far been restricted to non-RCT level studies examining reduction in UV-induced erythema following immediate application of Hamamelis-containing lotion with investigators finding slight efficacy in chromometric erythema reduction, significantly improved over matched controls only in a few of the tested vehicles and UV exposure conditions.100,101
Ginseng (Panax ginseng) and its constituent ginsenosides or saponins have wide-ranging anti-inflammatory, antioxidant, and immunomodulatory effects in vitro.102 Cho et al103 tested the effects of an oral preparation of red ginseng extract combined with extract from two other herbs, Torilus fructus and Corni fructus in a six-month trial. The authors concluded efficacy over placebo based on greater improvements from baseline in the treatment group in a few viscometer-measured wrinkle parameters and an improvement over baseline in content of procollagen I and fibrillin-1 fiber length derived from biopsies. However, inter-group comparisons were not made and the other endpoints tested (most wrinkle parameters and elasticity, for example) showed no change from the intra-group baseline, leaving conclusions suspect.
Other RCTs on peony root,104 cork extract,105 and milk thistle, gotu kola,106 and almonds107 had significant methodological limitations, jeopardizing study findings. While many other CAM agents exhibit significant anti-inflammatory and antioxidant mechanisms for anti-aging effects in vitro and animal studies,108–122 rigorous human RCT evidence remains limited.
Cutaneous side effects of dermatologic CAM
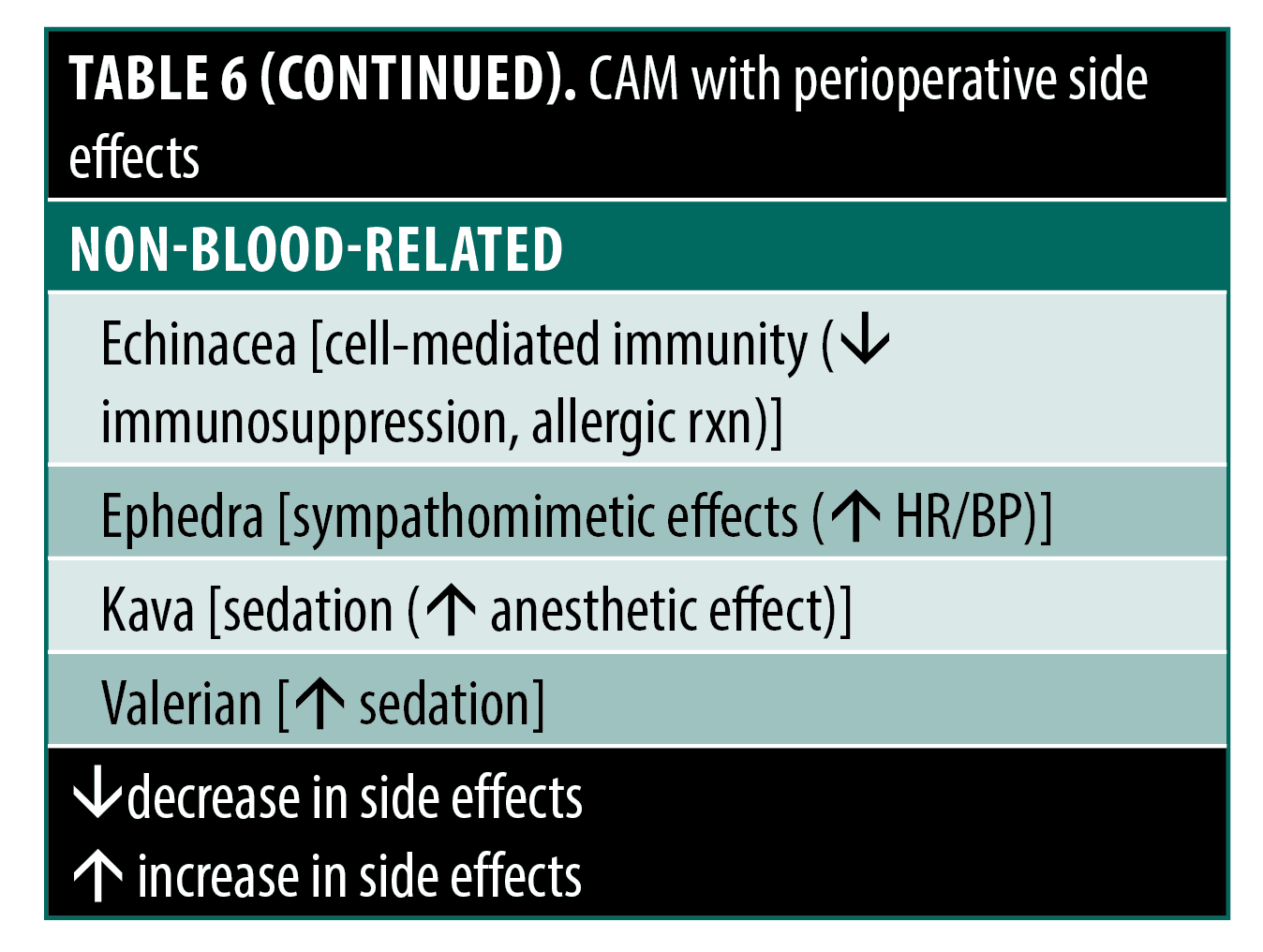
Though most side effects assessed from mentioned RCTs and other studies in this review are already noted, here we present side effect profiles on specific CAM agents, more generally for dermatologic surgery. These agents could provide some reason for concern during use in the perioperative period, especially those that influence clotting and coagulation through effects on platelets or interactions with warfarin. These CAMs, their possible effect on perioperative interest, and a general mechanism are summarized in Table 4.3,123,124 In general, information on the effect of these CAM agents on clotting or coagulation comes from in vitro or animal studies and isolated case reports, not RCTs. Thus, it is difficult for the physician to make recommendations regarding these CAMs as there is limited supporting information regarding direct mechanistic causality, dosage, concomitant confounding lifestyle or disease factors, or any other identified characteristics of patients that may be at risk for coagulation-related events if they use these agents.

Conclusion
The prevalence of CAMs in dermatology reflects consumer demand for integrative medicine approaches. While some studies suggest a promising efficacy of CAMs, significant design flaws, and methodological issues, such as improper blinding, small sample sizes, and subjective measures, undermine the credibility of these findings. Authors have tended to present results in a way that overstates CAM efficacy. Nonetheless, these studies provide a foundation for further rigorous investigation through well-designed RCTs comparing CAMs to conventional therapies. Until such trials are conducted, dermatologists should critically evaluate published CAM studies for bias and potential adverse effects. By doing so, clinicians can responsibly guide patients interested in exploring CAM products while optimizing overall outcomes.
Supplemental Figures
References
- World Health Organization, eds. WHO global report on traditional and complementary medicine 2019. Geneva; 2019.
- Norred CL. Complementary and alternative medicine use by surgical patients. AORN J. 2002;76(6):1013–1021.
- Reddy KK, Grossman L, Rogers GS. Common complementary and alternative therapies with potential use in dermatologic surgery: risks and benefits. J Am Acad Dermatol. 2011;68(4):e127–e135.
- Dinehart SM, Alstadt K. Use of alternative therapies by patients undergoing surgery for nonmelanoma skin cancer. Dermatol Surg. 2002;28(6):443–446.
- Thornfeldt C. Cosmeceuticals containing herbs: fact, fiction, and future. Dermatol Surg. 2005;31(7 Pt 2):873–880; discussion 880.
- Newburger AE. Cosmeceuticals: myths and misconceptions. Clin Dermatol. 2009;27(5):446–452.
- Jadad A, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996;17(1):1–12.
- Nichols JA, Katiyar SK. Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Arch Dermatol Res. 2010;302(2):71–83.
- Yang CS, Wang X. Green tea and cancer prevention. Nutr Cancer. 2010;62(7):931–937.
- Katiyar SK. Green tea prevents non-melanoma skin cancer by enhancing DNA repair. Arch Bioch Biophys. 2010;508(2):152–158.
- Meeran SM, Akhtar S, Katiyar SK. Inhibition of UVB-induced skin tumor development by drinking green tea polyphenols is mediated through DNA repair and subsequent inhibition of inflammation. J Invest Dermatol. 2009;129(5):1258–1270.
- Katiyar SK, Challa A, McCormick TS, et al. Prevention of UVB-induced immunosuppression in mice by the green tea polyphenol (-)-epigallocatechin-3-gallate may be associated with alterations in IL-10 and IL-12 production. Carcinogenesis. 1999;20(11):2117–2124.
- Katiyar SK, Mukhtar H, Ia MHC. Green tea polyphenol (-)-epigallocatechin-3-gallate treatment to mouse skin prevents UVB-induced infiltration of leukocytes, depletion of antigen-presenting cells, and oxidative stress. J Leukoc Biol. 2001;69(5):719–726.
- Katiyar SK, Afaq F, Azizuddin K, et al. Inhibition of UVB-induced oxidative stress-mediated phosphorylation of mitogen-activated protein kinase signaling pathways in cultured human epidermal keratinocytes by green tea polyphenol (-)-epigallocatechin-3-gallate. Toxicol Appl Pharmacol. 2001;176(2):110–117.
- Wei H, Zhang X, Zhao JF, et al. Scavenging of hydrogen peroxide and inhibition of ultraviolet light-induced oxidative DNA damage by aqueous extracts from green and black teas. Free Radic Biol Med. 1999;26(11–12):1427–1435.
- Huang CC, Fang JY, Wu WB, et al. Protective effects of (-)-epicatechin-3-gallate on UVA-induced damage in HaCaT keratinocytes. Arch Dermatol Res. 2005;296(10):473–481.
- Huang CC, Wu WB, Fang JY, et al. (-)-Epicatechin-3-gallate, a green tea polyphenol is a potent agent against UVB-induced damage in HaCaT keratinocytes. Molecules. 2007;12(8):1845–1858.
- Vayalil PK, Elmets CA, Katiyar SK. Treatment of green tea polyphenols in hydrophilic cream prevents UVB-induced oxidation of lipids and proteins, depletion of antioxidant enzymes and phosphorylation of MAPK proteins in SKH-1 hairless mouse skin. Carcinogenesis. 2003;24(5):927–936.
- Katiyar SK, Matsui MS, Elmets CA. Polyphenolic antioxidant (- )-epigal locatechin-3-gallate from green tea reduces uvb-induced inflammatory responses and infiltration of leukocytes in human skin. Photochem Photobiol.1999;69(2):148–153.
- Morley N, Clifford T, Salter L, et al. The green tea polyphenol (-)-epigallocatechin gallate and green tea can protect human cellular DNA from ultraviolet and visible radiation-induced damage. Photodermatol Photoimmunol Photomed. 2005;21(1):15–22.
- Chatterjee ML, Agarwal R, Mukhtar H. Ultraviolet B radiation-induced DNA Lesions in mouse epidermis: An assessment using a novel32p-postlabelling technique. Biochem Biophys Res Commun. 1996;229(2):590–595.
- Meeran SM, Mantena SK, Elmets CA, et al. (-)-Epigallocatechin-3-gallate prevents photocarcinogenesis in mice through interleukin-12-dependent DNA repair. Cancer Res. 2006;66(10):5512–5520.
- Schwarz A, Maeda A, Kernebeck K, et al. Prevention of UV radiation-induced immunosuppression by IL-12 is dependent on DNA repair. J Exp Med. 2005;201(2):173–179.
- Elmets CA, Singh D, Tubesing K, et al. Cutaneous photoprotection from ultraviolet injury by green tea polyphenols. J Am Acad Dermatol. 2001;44(3):425–432.
- Mnich CD, Hoek KS, Virkki LV, et al. Green tea extract reduces induction of p53 and apoptosis in UVB-irradiated human skin independent of transcriptional controls. Exp Dermatol. 2009;18(1):69–77.
- Katiyar SK, Afaq F, Perez A, et al. Green tea polyphenol (–) -epigallocatechin-3-gallate treatment of human skin inhibits ultraviolet radiation-induced oxidative stress. Carcinogenesis 2001;22:287–294.
- Katiyar SK, Perez A, Mukhtar H. Green tea polyphenol treatment to human skin prevents formation of ultraviolet light b-induced pyrimidine dimers in DNA. Clin Cancer Res. 2000;6(10):3864–3869.
- Li Y-H, Wu Y, Wei H-C, et al. Protective effects of green tea extracts on photoaging and photommunosuppression. Skin Res Technol. 2009;15(3):338–345.
- Kessels J, Voeten L, Nelemans P, et al. Topical sinecatechins, 10%, ointment for superficial basal cell carcinoma: A randomized clinical trial. JAMA Dermatol. 2017;153(10):1061–1063.
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: The in vivo evidence. Nat Rev Drug Discov. 2006;5(6):493–506.
- Shukla Y, Singh R. Resveratrol and cellular mechanisms of cancer prevention. Ann N Y Acad Sci. 2011;1215:1–8.
- McDaniel S, Goldman GD. Consequences of using escharotic agents as primary treatment for nonmelanoma skin cancer. Arch Dermatol. 2002;138(12):1593–1596.
- Jellinek N, Maloney ME. Escharotic and other botanical agents for the treatment of skin cancer: a review. J Am Acad Dermatol. 2005;53(3):487–495.
- Osswald SS, Elston DM, Farley MF, et al. Self-treatment of a basal cell carcinoma with “black and yellow salve”. J Am Acad Dermatol. 2005;53(3):509–511.
- Elston DM. Escharotic agents, Fred Mohs, and Harry Hoxsey. J Am Acad Dermatol. 2005;53(3):523–525.
- Kalish RS, Wood JA, Siegel DM, et al. Experimental rationale for treatment of high-risk human melanoma with zinc chloride fixative paste: increased resistance to tumor challenge in murine melanoma. Dermatol Surg. 1998;24(9):1021–1025.
- Ren W, Tang DG. Extract of solanum muricatum (Pepino/CSG) inhibits tumor growth by inducing apoptosis. Anticancer Res. 1999;19(1A):403–408.
- Cham B, Daunter B, Evans R. Topical treatment of malignant and premalignant skin lesions by very low concentrations of a standard mixture (BEC) of solasodine glycosides. Cancer Lett. 1991;59(3):183–192.
- Goldberg LH, Landau JM, Moody MN, et al. Treatment of Bowen’s disease on the penis with low concentration of a standard mixture of solasodine glycosides and liquid nitrogen. Dermatol Surg. 2011;37(6):858–861.
- Punjabi S, Cook LJ, Kersey P, et al. Solasodine glycoalkaloids: a novel topical therapy for basal cell carcinoma. A double-blind, randomized, placebo-controlled, parallel group, multicenter study. Int J Dermatol. 2008;47(1):78–82.
- Smith JA, Madden T, Vijjeswarapu M, et al. Inhibition of export of fibroblast growth factor-2 (FGF-2) from the prostate cancer cell lines PC3 and DU145 by Anvirzel and its cardiac glycoside component, oleandrin. Biochem Pharmacol. 2001;62(4):469–472.
- Afaq F, Saleem M, Aziz MH, et al. Inhibition of 12-O-tetradecanoylphorbol-13-acetate-induced tumor promotion markers in CD-1 mouse skin by oleandrin. Toxicol Appl Pharmacol. 2004;195(3):361–369.
- Newman RA, Yang P, Hittelman WN, et al. Oleandrin-mediated oxidative stress in human melanoma cells. J Exp Ther Oncol. 2006;5(3):167–181.
- Siddiqui BS, Khatoon N, Begum S, et al. Flavonoid and cardenolide glycosides and a pentacyclic triterpene from the leaves of Nerium oleander and evaluation of cytotoxicity. Phytochemistry. 2012;77:238–244.
- Nasu S, Milas L, Kawabe S, et al. Enhancement of radiotherapy by oleandrin is a caspase-3 dependent process. Cancer Lett. 2002;185(2):145–151.
- Mekhail T, Kaur H, Ganapathi R, et al. Phase 1 trial of Anvirzel in patients with refractory solid tumors. Invest New Drugs. 2006;24(5):423–427.
- Taghizadeh M, Farzin N, Taheri S, et al. The effect of dietary supplements containing green tea, capsaicin and ginger extracts on weight loss and metabolic profiles in overweight women: a randomized double-blind placebo-controlled clinical trial. Ann Nutr Metab. 2017;70(4):277–285.
- Ekenäs C, Zebrowska A, Schuler B, et al. Screening for anti-inflammatory activity of 12 arnica (asteraceae) species assessed by inhibition of NF-kB and release of human neutrophil elastase. Planta Med. 2008;74(15):1789–1794.
- Simonart T, Kabagabo C, De Maertelaer V. Homoeopathic remedies in dermatology: a systematic review of controlled clinical trials. Br J Dermatol. 2011;165(4):897–905.
- Ernst E, Pittler M. Efficacy of homeopathic arnica. Arch Surg. 1998;133(11):1187–1190.
- Seeley BM, Denton AB, Ahn MS, et al. Effect of homeopathic arnica montana on bruising in face-lifts. Arch Facial Plast Surg. 2006;8(1):54–59.
- Alonso D, Lazarus MC, Baumann L. Effects of topical arnica gel on post-laser treatment bruises. Dermatol Surg. 2002;28(8):686–688.
- Stevinson C, Devaraj VS, Fountain-Barber A, et al. Homeopathic arnica for prevention of pain and bruising: randomized placebo-controlled trial in hand surgery. J R Soc Med. 2003;96(2):60–65.
- Ramelet A, Buchheim G, Lorenz P, et al. Homoeopathic arnica in postoperative haematomas: A double-blind study. Dermatology. 2000;201(4):347–348.
- Totonchi A, Guyuron B. A randomized, controlled comparison between arnica and steroids in the management of postrhinoplasty ecchymosis and edema. Plast Reconstr Surg. 2007;120(1):271–274.
- Sharp A. Beneficial effects of honey dressings in wound management. Nurs Stand. 2009;24(7):66–74.
- Jull A, Walker N, Parag V, et al. Randomized clinical trial of honey-impregnated dressings for venous leg ulcers. Br J Surg. 2008;95(2):175–182.
- Gupta SC, Prasad S, Kim JH, et al. Multitargeting by curcumin as revealed by molecular interaction studies. Nat Prod Rep. 2011;28(12):1937–1955.
- Maurer H. Bromelain: Biochemistry, pharmacology and medical use. Cell Mol Life Sci. 2001;58(9):1234–1245.
- Baumann LS. Less-known botanical cosmeceuticals. Dermatol Ther. 2007;20(5):330–342.
- Shoham Y, Shapira E, Haik J, et al. Bromelain-based enzymatic debridement of chronic wounds: results of a multicentre randomized controlled trial. Wound Repair Regen. 2021;29(6):899–907.
- Mujica V, Orrego R, Fuentealba R, et al. Propolis as an adjuvant in the healing of human diabetic foot wounds receiving care in the diagnostic and treatment centre from the regional hospital of Talca. J Diabetes Res. 2019;2019:2507578.
- Ellinger S, Stehle P. Efficacy of vitamin supplementation in situations with wound healing disorders: results from clinical intervention studies. Curr Opin Clin Nutr Metab Care. 2009;12(6):588–595.
- Eshghi F, Hosseinimehr SJ, Rahmani N, et al. Effects of aloe vera cream on posthemorrhoidectomy pain and wound healing: results of a randomized, blind, placebo-control study. J Alter Complement Med. 2010;16(6):647–650.
- Giostri GS, Novak EM, Buzzi M, et al. Treatment of acute wounds in hand with Calendula officinalis L.: A randomized trial. Tissue Barriers. 2022;10(3):1994822.
- Alster TS, Tanzi EL. Hypertrophic scars and keloids: etiology and management. Am J Clin Dermatol. 2003;4(4):235–243.
- Berman B, Perez OA, Konda S, et al. A review of the biologic effects, clinical efficacy, and safety of silicone elastomer sheeting for hypertrophic and keloid scar treatment and management. Dermatol Surg. 2007;33(11):1291–1302; discussion 1302–1303.
- Jackson BA, Shelton AJ. Pilot study evaluating topical onion extract as treatment for postsurgical scars. Dermatol Surg. 1999;25(4):267–269.
- Clarke L, Baker B, Trahan C, et al. A prospective double-blinded study of Mederma skin care vs. placebo for post-traumatic scar reduction. Cosmet Dermatol. 1999;19–26.
- Chung VQ, Kelley L, Marra D, et al. Onion extract gel versus petrolatum emollient on new surgical scars: A prospective double-blinded study. Dermatol Surg. 2006;32(2):193–197.
- Koc E, Arca E, Surucu B, et al. An open, randomized, controlled, comparative study of the combined effect of intralesional triamcinolone acetonide and onion extract gel and intralesional triamcinolone acetonide alone in the treatment of hypertrophic scars and keloids. Dermatol Surg. 2008;34(11):1507–1514.
- Ho WS, Ying SY, Chan PC, et al. Use of onion extract, heparin, allantoin gel in prevention of scarring in chinese patients having laser removal of tattoos: A prospective randomized controlled trial. Dermatol Surg. 2006;32(7):891–896.
- Draelos ZD, Baumann L, Fleischer AB Jr, et al. A new proprietary onion extract gel improves the appearance of new scars. J Clin Aesthet Dermatol. 2012;5(6):18–24.
- Chanprapaph K, Tanrattanakorn S, Wattanakrai P, et al. Effectiveness of onion extract gel on surgical scars in asians. Dermatol Res Pract. 2012;2012:212945.
- Zurada JM, Kriegel D, Davis IC. Topical treatments for hypertrophic scars. J Am Acad Dermatol. 2006;55(6):1024–1031.
- Fulton JE Jr. Silicone gel sheeting for the prevention and management of evolving hypertrophic and keloid scars. Dermatol Surg. 1995;21(11):947–951.
- Ahn ST, Monafo WW, Mustoe TA. Topical silicone gel: a new treatment for hypertrophic scars. Surgery. 1989;106(4):781–786; discussion 786-787.
- O’Brien L, Pandit A. Silicon gel sheeting for preventing and treating hypertrophic and keloid scars. Cochrane Database Syst Rev. 2006;(1):CD003826.
- de Giorgi V, Sestini S, Mannone F, et al. The use of silicone gel in the treatment of fresh surgical scars: a randomized study. Clin Exp Dermatol. 2009;34:688–693.
- Jenwitheesuk K, Surakunprapha P, Jenwitheesuk K, et al. Role of silicone derivative plus onion extract gel in presternal hypertrophic scar protection: a prospective randomized, double blinded, controlled trial. Int Wound J. 2012;9(4):397–402.
- Wigger-Albert W, Kuhlmann M, Wilhelm D, et al. Efficacy of a polyurethane dressing versus a soft silicone sheet on hypertrophic scars. J Wound Care. 2009;18(5):208,210–214.
- de Oliveira GV, Nunes TA, Magna LA, et al. Silicone versus nonsilicone gel dressings: a controlled trial. Dermatol Surg. 2001;27(8):721–726.
- Wiseman J, Simons M, Kimble R, et al. Effectiveness of topical silicone gel and pressure garment therapy for burn scar prevention and management in children 12-months postburn: A parallel group randomized controlled trial. Clin Rehabil. 2021;35(8):1126–1141.
- Foo CW, Tristani-Firouzi P. Topical modalities for treatment and prevention of postsurgical hypertrophic scars. Facial Plast Surg Clin North Am. 2011;19(3):551–557.
- Jenkins M, Alexander JW, MacMillan BG, et al. Failure of topical steroids and vitamin E to reduce postoperative scar formation following reconstructive surgery. J Burn Care Rehabil. 1986;7(4):309–312.
- Palmieri B, Gozzi G, Palmieri G. Vitamin E added silicone gel sheets for treatment of hypertrophic scars and keloids. Int J Dermatol. 1995;34(7):506–509.
- Baumann LS, Spencer J. The effects of topical vitamin E on the cosmetic appearance of scars. Dermatol Surg.1999;25(4):311–315.
- Zampieri N, Zuin V, Burro R, et al. A prospective study in children: Pre- and post-surgery use of vitamin E in surgical incisions. J Plast Reconstr Aesthet Surg. 2010;63(9):1474–1478.
- Khoo TL, Halim AS, Zakaria Z, et al. A prospective, randomised, double-blinded trial to study the efficacy of topical tocotrienol in the prevention of hypertrophic scars. J Plast Reconstr Aesthet Surg. 2011;64(6):e137–145.
- Hunt KJ, Hung SK, Ernst E. Botanical extracts as anti-aging preparations for the skin: a systematic review. Drugs Aging. 2010;27(12):973–985.
- Chiu AE, Chan JL, Kern DG, et al. Double-blinded, placebo-controlled trial of green tea extracts in the clinical and histologic appearance of photoaging skin. Dermatol Surg. 2005;31:855–860.
- Hsu J, Skover G, Goldman MP. Evaluating the efficacy in improving facial photodamage with a mixture of topical antioxidants. J Drugs Dermatol. 2007;6(11):1141–1148.
- Janjua R, Munoz C, Gorell E, et al. A two-year, double-blind, randomized placebo-controlled trial of oral green tea polyphenols on the long-term clinical and histologic appearance of photoaging skin. Dermatol Surg. 2009;35(7):1057–1065.
- Izumi T, Saito M, Obata A, et al. Oral intake of soy isoflavone aglycone improves the aged skin of adult women. J Nutr Sci Vitaminol (Tokyo). 2007;53(1):57–62.
- Tsoyi K, Park HB, Kim YM, et al. Protective effect of anthocyanins from black soybean seed coats on UVB-induced apoptotic cell death in vitro and in vivo. J Agric Food Chem. 2008;56(22):10600–10605.
- Greul AK, Grundmann JU, Heinrich F, et al. Photoprotection of UV-irradiated human skin: an antioxidative combination of vitamins E and C, carotenoids, selenium and proanthocyanidins. Skin Pharmacol Appl Skin Physiol. 2002;15(5):307–315.
- Afaq F, Mukhtar H. Botanical antioxidants in the prevention of photocarcinogenesis and photoaging. Exp Dermatol. 2006;15(9):678–684.
- Masaki H, Sakaki S, Atsumi T, et al. Active-oxygen scavenging activity of plant extracts. Biol Pharm Bull. 1995;18(1):162–166.
- Deters A, Dauer A, Schnetz E, et al. High molecular compounds (polysaccharides and proanthocyanidins) from Hamamelis virginiana bark: influence on human skin keratinocyte proliferation and differentiation and influence on irritated skin. Phytochemistry. 2001;58(6):949–958.
- Hughes-Formella BJ, Bohnsack K, Rippke F, et al. Anti-inflammatory effect of hamamelis lotion in a UVB erythema test. Dermatology. 1998;196(3):316–322.
- Hughes-Formella BJ, Filbry A, Gassmueller J, et al. Anti-inflammatory efficacy of topical preparations with 10% hamamelis distillate in a UV erythema test. Skin Pharmacol Appl Skin Physiol. 2002;15(2):125–132.
- Attele AS, Wu JA, Yuan CS: Ginseng pharmacology: Multiple constituents and multiple actions. Biochem Pharmacol. 1999;58(11):1685–1693.
- Cho S, Won CH, Lee DH, et al. Red ginseng root extract mixed with Torilus fructus and Corni fructus improves facial wrinkles and increases type I procollagen synthesis in human skin: A randomized, double-blind, placebo-controlled study. J Med Food. 2009;12(6):1252–1259.
- Lee S, Lim JM, Jin MH, et al. Partially purified paeoniflorin exerts protective effects on UV-induced DNA damage and reduces facial wrinkles in human skin. J Cosmet Sci. 2006;57(1):57–64.
- Coquet C, Bauza E, Oberto G, et al. Quercus suber cork extract displays a tensor and smoothing effect on human skin: An in vivo study. Drugs Exp Clin Res. 2005;31(3):89–99.
- Martelli L, Berardesca E, Martelli M. Topical formulation of a new plant extract complex with refirming properties. Clinical and non-invasive evaluation in a double-blind trial. Int J Cosmet Sci. 2000;22(3):201–206.
- Li JN, Henning SM, Thames G, et al. Almond consumption increased UVB resistance in healthy asian women. J Cosmet Dermatol. 2021;20(9):2975–2980.
- Barrantes E, Guinea M. Inhibition of collagenase and metalloproteinases by aloins and aloe gel. Life Sci. 2003;72(7):843–850.
- Jones K, Hughes J, Hong M, et al. Modulation of melanogenesis by aloesin: A competitive inhibitor of tyrosinase. Pigment Cell Res. 2002;15(5):335–340.
- Kim S, Kim Y, Kim JE, et al. Berberine inhibits TPA-induced MMP-9 and IL-6 expression in normal human keratinocytes. Phytomedicine. 2008;15(5):340–347.
- Kim S, Chung JH. Berberine prevents UV-induced MMP-1 and reduction of type I procollagen expression in human dermal fibroblasts. Phytomedicine. 2008;15(9):749–753.
- Fonseca YM, Catini CD, Vicentini FT, et al. Protective effect of calendula officinalis extract against UVB-induced oxidative stress in skin: evaluation of reduced glutathione levels and matrix metalloproteinase secretion. J Ethnopharmacol. 2010;127(3):596–601.
- Chanvorachote P, Pongrakhananon V, Luanpitpong S, et al. Type I pro‐collagen promoting and anti‐collagenase activities of Phyllanthus emblica extract in mouse fibroblasts. J Cosmet Sci. 2009;60(4):395–403.
- Fujii T, Wakaizumi M, Ikami T, et al. Amla (Emblica officinalis Gaertn.) extract promotes procollagen production and inhibits matrix metalloproteinase-1 in human skin fibroblasts. J Ethnopharmacol. 2008;119(1):53–57.
- Fujimura T, Tsukahara K, Moriwaki S, et al. A horse chestnut extract, which induces contraction forces in fibroblasts, is a potent anti-aging ingredient. J Cosmet Sci. 2007;57(5):369–376.
- Guillaume M, Padioleau F. Veinotonic effect, vascular protection, antiinflammatory and free radical scavenging properties of horse chestnut extract. Arzneimittelforschung. 1994;44(1):25–35.
- Cimino F, Cristani M, Saija A, et al. Protective effects of a red orange extract on UVB-induced damage in human keratinocytes. Biofactors. 2007;30(2):129–138.
- Sumiyoshi M, Kimura Y. Effects of a turmeric extract (Curcuma longa) on chronic ultraviolet B irradiation-induced skin damage in melanin-possessing hairless mice. Phytomedicine. 2009;16(12):1137–1143.
- Tanaka K, Hasegawa J, Asamitsu K, et al. Magnolia ovovata extract and its active component magnolol prevent skin photoaging via inhibition of nuclear factor kappaB. Eur J Pharmacol. 2007;565(1–3):212–219.
- Sultana Y, Kohli K, Athar M, et al. Effect of pre-treatment of almond oil on ultraviolet B-induced cutaneous photoaging in mice. J Cosmet Dermatol. 2007;6(1):14–19.
- Manosroi A, Jantrawut P, Akihisa T, et al. In vitro anti-aging activities of Terminalia chebula gall extract. Pharm Biol. 2010;48(4):469–481.
- Kim SJ, Sancheti SA, Sancheti SS, et al. Effect of 1,2,3,4,6-penta-O-galloyl-β-D-glucose on elastase and hyaluronidase activities and its Type II collagen expression. Acta Pol Pharm. 2010;67(2):145–150.
- Heck A, Dewitt B, Lukes A. Potential interactions between alternative therapies and warfarin. Am J Health Syst Pharm. 2000;57(13):1221–1227; quiz 1228–1230.
- Ang-Lee MK, Moss J, Yuan CS. Herbal medicines and perioperative care. JAMA. 2001;286(2):208–216.