J Clin Aesthet Dermatol. 2019;12(12):21–24
 by Naveen Kumar, PhD; Pramod Kumar, MS, MCH; Satheesha Nayak B, PhD; Ashwini Aithal P, PhD; and Anitha Guru, MSc
by Naveen Kumar, PhD; Pramod Kumar, MS, MCH; Satheesha Nayak B, PhD; Ashwini Aithal P, PhD; and Anitha Guru, MSc
Drs. N. Kumar, Nayak, Aithal, and Ms. Guru are with the Department of Anatomy at Melaka Manipal Medical College, Manipal Campus, at the Manipal Academy of Higher Education in Manipal, Karnataka, India. Dr. P. Kumar is with King Fahad Central Hospital in Jazan, Saudi Arabia.
FUNDING: No funding was provided for this study.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. Stretching force on the skin leads to the widening of scars and/or hypertrophy. The uneven distribution profile of collagen and elastic fibers in the human dermis (evaluated based on their topographic areas) might determine the direction of incision for a more pleasing aesthetic result.
Materials and methods. Full-thickness skin samples were collected in the horizontal and vertical directions from 15 areas of 32 human cadavers. The histological preparation of the skin samples was performed using special Verhoeff–van Gieson staining. Image analysis in the TissueQuant software was performed using photomicrographs. Quantitative fraction measures for collagen and elastic fibers were completed. From the data obtained, various ratios between collagen in the horizontal (CH) and vertical (CV) directions and elastic fibers in the horizontal (EH) and vertical (EV) directions were established and expressed as CH:CV and EH:EV.
Results. In the areas where CV:CH was less than 1 (low ratio of significance), the collagen content was significantly higher in the horizontal direction than the vertical direction (CH>CV). However, this finding is reversed (CV>CH) in areas where CV:CH is greater than 1 (high ratio of significance). Similarly, in areas where EV:EH is less than 1 (low ratio of significance), the elastic fiber content was significantly higher in the horizontal direction than the vertical direction (EH>EV), whereas such is reversed (EV>EH) where EV:EH is greater than 1 (high ratio of significance).
Conclusion. The evaluation of ratios of dermal collagen and elastic fibers in different directions together with the data of asymmetric distribution provide a useful guideline for aesthetic surgeons looking to place elective incisions in the direction that will ensure an improved aesthetically pleasing result.
KEYWORDS: Collagen, elastic, ratio, scar, skin lines
Wound closure is generally facilitated by stretching of the adjacent skin. Though the exact mechanism of tissue adaptation to stretch is still not precise, the role of collagen and elastic fibers, according to their orientation and morphology in stretched and nonstretched skin, are being correlated with wound healing-related complications. A research study reported an increased parallel alignment of collagen fibers in stretched skin and stretched scar compared to unstretched skin and nonstretched scar.1 A similar pattern of arrangement was also observed for elastic fibers after the stretch event.1 Excessive wound contraction and postgrafting scarring usually occur as a result of a lack of organized elastic fiber network as was observed in the dermal component of artificial skin substitutes.2
The excessive laydown of collagen and stretching of the scar is high in more elastic skin, such as in children and younger subjects.3 On the other hand, stretching due to obesity (i.e., fat deposition under the skin) is opposed by the underlying elastic fibers. Thus, the skin becomes loose as the result of the loss of fat from subcutaneous tissue. It might therefore be indirectly inferred that the skin exerts a stretching force on the scar so that its behavior and appearance are altered in proportion to the power, which might vary depending upon the elastic fiber content and inherent properties of elastic tissue.4
Our previous research study on the quantitative fraction analysis of dermal collagen and elastic fibers in directions that are perpendicular to one another in various regions revealed multiple conclusions. An asymmetrical content of dermal collagen and elastic fibers in different orientations in the head and neck region indicates an anatomical basis for an explanation to earlier experience that suggests scars placed in a particular direction yield better aesthetic results. To achieve an effective cosmetic intervention and to minimize the complications of scars on the face, it is not only necessary to employ the appropriate surgical technique but, also, to have a better understanding of the aesthetic units of the face that are accomplished with its complex skin tension lines.5
Collagen content is often more pronounced in one or both directions (horizontal or vertical) as a result of repeated stress due to associated elastic fiber content, physical stretching, or physiological reasons. Based on this context, unequal distribution of dermal collagen and elastic fiber content between two orientations of skin samples from the trunk region of the human body can be evaluated with the perspective of anatomical (stretch due to elastic fiber content), functional (stretch due to joint movement), or physical (stretch due to wound closure under tension) factors.6
The evaluation of the uneven distribution of dermal collagen and elastic fibers in the regions of the extremities is dependent on the topographic region where the joints are involved. The knowledge of the burst force applied on the edges of the skin incision (stretch over skin and soft tissue) due to joint movement and the stretch force applied on the proposed incision due to division of elastic and collagen fibers (determined by the study of quantitative fraction data)7 could provide guidelines for decision-making to achieve a better aesthetic result.
The objective of the present study was to measure the ratios between collagen in the horizontal (CH) and vertical (CV) directions (CH:CV) and elastic fibers in the horizontal (EH) and vertical (EV) directions (EH:EV) for comparing the asymmetric distribution of dermal collagen and elastic fiber content in different areas and different directions in the same area.
Materials and Methods
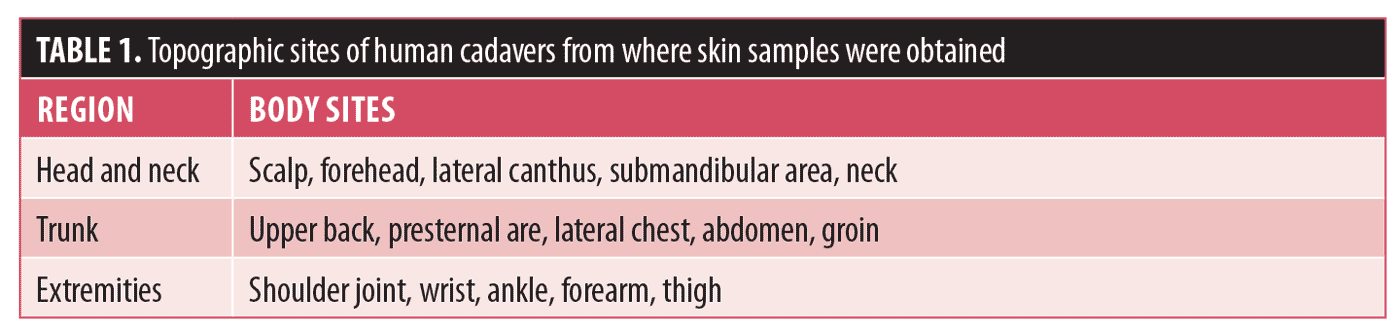
Full-thickness skin samples were collected in the horizontal and vertical directions from 15 areas of 32 human cadavers. The random selection of the sample regions was done with consideration of aesthetic concerns. These areas were categorized into three anatomical regions, i.e., head and neck, trunk, and extremities, with five topographic areas chosen in each region (Table 1). In total, 960 skin samples were examined.
Histological slides were collected from each sample and stained using a Verhoeff–van Gieson (VVG) special staining method for the selective demonstration of collagen and elastic fibers.8 Digital images were acquired at 20× magnification. From each VVG-stained slide, three images were obtained in three different microscopic fields.
Image analysis using the TissueQuant software. Image analysis was performed using the TissueQuant version 1.0 software program. This software measures the area occupied by the colored tissue structures in consideration of the number of pixels assigned to it. This measure corresponds to the quantitative fraction (QF) of the structure. The analysis prerequisites the careful selection of color shades. The total number of pixels corresponds to the area occupied by collagen and elastic fibers calculated as a percentage value by proper calculation.9
The difference in the content of collagen and elastic fibers between two directions were analyzed statistically by paired sample t-test using the Statistical Package for the Social Sciences software program (IBM Corp., Armonk, NY, USA). A p-value of less than 0.05 was considered to be statistically significant.
Ratio value analysis. From the mean values of QF, ratio values were calculated by dividing the vertical value by the horizontal value; these were denoted as the CV:CH ratio (for collagen) and EV:EH ratio (for elastic fibers). The results were expressed in “ratio values,” which imply proportionate changes in the content of vertical tissue with respect to the horizontal direction. For example, in the scalp, the CV:CH ratio is 0.92/1. Hence, the CV:CH ratio value would be denoted as 0.92.
Results
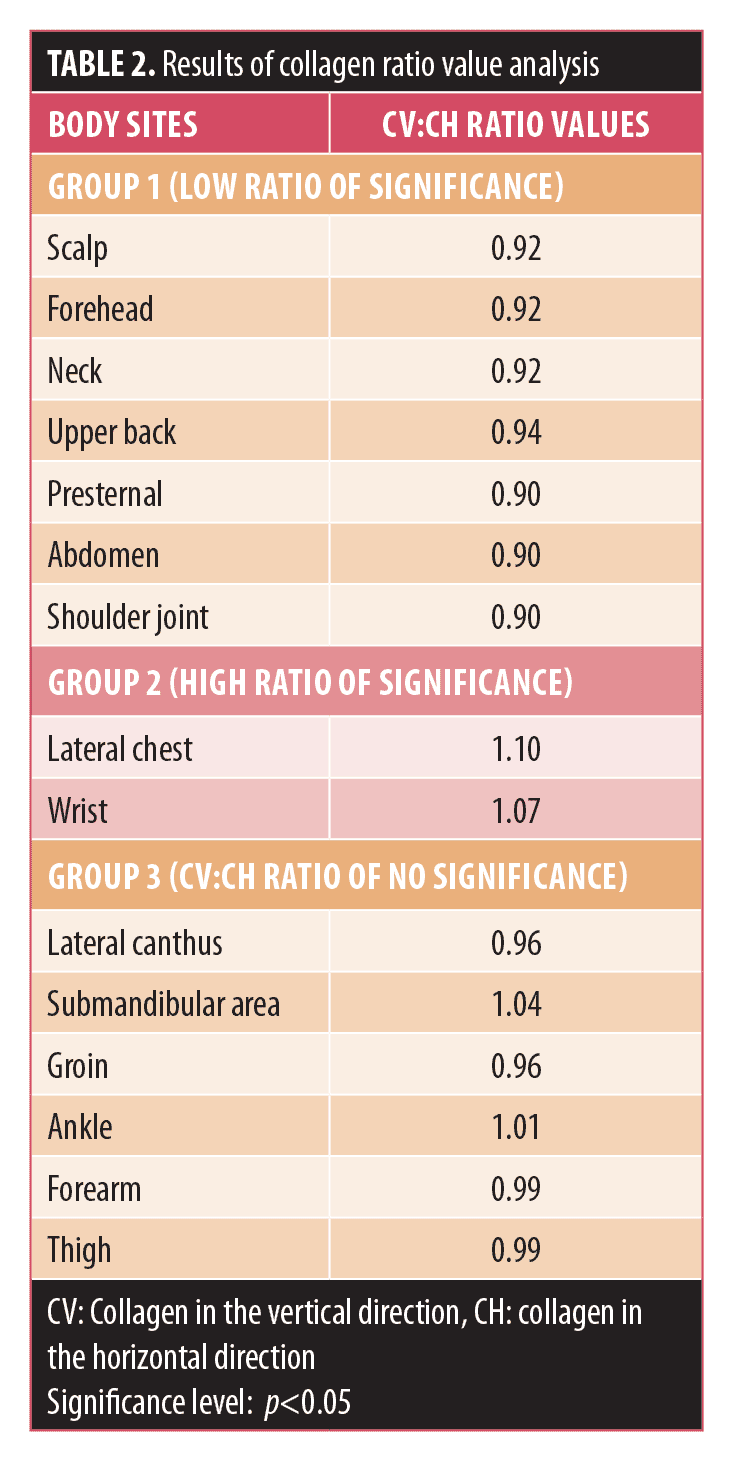
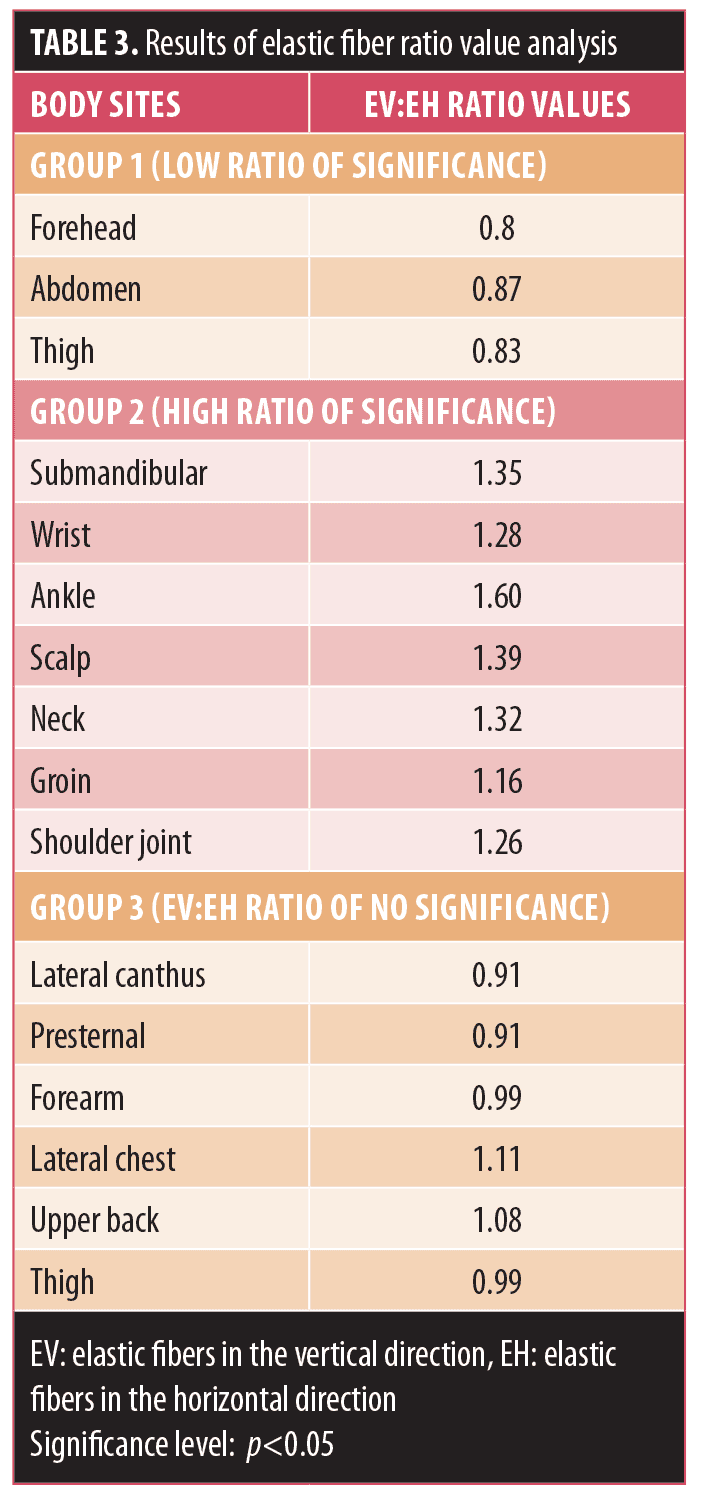
The results of ratio values estimated from the quantitative fractions of dermal collagen and elastic fibers between the horizontal and vertical directions are tabulated in Table 2 (for collagen) and Table 3 (for elastic fibers). The ratio values were classified into different groups based on the level of significance of the difference in the quantitative fractions. Areas with the ratio values corresponding to statistically significant quantitative fractions (p<0.05) were categorized into two groups: Group 1 with a ratio value less than 1 and Group 2 with a ratio value greater than 1. Areas with ratio values from nonsignificant (p>0.05) quantitative fraction analysis were considered as Group 3. This categorization was done individually for collagen (CV:CH) and elastic fibers (EV:EH), respectively. Further information on this stratification follows.
Collagen.
- In Group 1, where CV:CH was less than 1 (low ratio of significance), the collagen content was significantly higher in the horizontal direction than in the vertical one (CH>CV).
- In Group 2, where CV:CH was greater than 1 (high ratio of significance), the collagen content was significantly higher in the vertical direction than in the horizontal one (CV>CH).
- In Group 3, where the CV:CH ratio was nonsignificant, in some of the areas, even though there was no significant difference in the content of collagen between the horizontal and vertical directions, a well-differentiated asymmetric distribution of collagen between the two directions was evident, such as in the lateral canthus and groin (where CH>CV) and in the submandibular area and ankle (where CV>CH).
Elastic fibers.
- In Group 1, where EV:EH was less than 1 (low ratio of significance), the elastic fiber content was significantly higher in the horizontal direction than in the vertical one (EH>EV).
- In Group 2, where EV:EH was greater than 1 (high ratio of significance), the collagen content was significantly higher in the vertical direction than in the horizontal one (EV>EH).
- In Group 3, where the EV:EH ratio was nonsignificant, although there were no statistically significant differences in elastic content between the horizontal and vertical directions, some areas in this group presented an asymmetric distribution of elastic fibers between the two directions, which could be seen distinctly in the lateral canthus and presternal areas (where EH>EV) and on the lateral chest and upper back (where EV>EH).
Discussion
An excessive overproliferation of fibroblasts and collagen production during the prolonged process of inflammation results in unacceptable scars.10 Mechanical tension has been proposed to play a major role in the orientation of the bundles in scar tissue. This factor is one of the major causes in the onset of challenging scar formation that usually occurs at joint regions. However, the morphological difference of collagen in scar tissue and normal skin between joints and control areas has not been established.11 Mechanical stimulus is known to induce changes in collagen in terms of its organization, thickness, type, and the process of synthesis.12 Cell–matrix interactions direct the pattern of collagen fiber in the scar similar to that of the dermis to provide better wound care results.13 In normal skin, thicker bundles and increased space between the bundles are found due to underlying mechanisms of adaptation to stretching.14
Elastic fibers in the dermis also contribute to the structural and functional integration of the skin. Alterations in elastin content and its association have been proven histologically to exist in the formation of scars.15 The influence of mechanical forces on intact tissue and micro/macro deforming forces leading to cellular proliferation and angiogenesis (inflammation) is well-established.16
Collagen ratio value evaluation. In areas (Group 1) with low significance (CV:CH<1) ratio values, there is a less distracting force on the horizontal wound edges during movements compared to the burst force present on the vertical wound edge during the rest period. Clinical experience suggests that the lower the value, the better the long-term result of the horizontally placed scar. In areas (Group 2) with high significant (CV:CH>1) ratios, the force exerted due to circumferential growth in the areas where body growth is less than the effect force produced in the horizontal direction during movements (e.g., lateral bending at the lateral chest or during extension at the wrist). Hence, to provide maximum strength to the skin that has been damaged due to maximum stretch during movement, collagen content in the vertical direction should be more. In other words, more collagen deposition is expected in the vertically placed wound, with more distracting force on the wound edge due to its orientation.
In the areas (Group 3) with no significant ratios, the various forces produced in different directions on the skin due to movement or pull by underlying muscles or during growth were not much different from one another. In this group, areas with lower ratio values (less than or equal to 0.96) clinically show a more acceptable scar if the wound is horizontally placed (probably due to relaxation produced by the underlying muscles). Conversely, areas with relatively higher ratio values (0.99–1.04) show a better scar in the vertical direction. In areas with very high ratio values (1.01; CV>CH), more collagen content probably signifies more pull on the horizontally placed scar by the underlying muscle (as the role of platysma in the submandibular area) and a moderate effect of both burst force and stretch force (as seen in the ankle area).
Elastic ratio value evaluation. In areas (Group 1) with low significant ratio values, frequent movement (if any) in the vertical direction or rotation and slow expansion causes a maximum stretch force in the horizontal direction. Whereas the constant stretching in vertical directions, as we see in the areas (Group 2) wherein high significant ratio values could be appreciated, is probably due to movement or stretching in the vertical direction by embryologic growth pattern and gradual growth after birth, in the areas (Group 3) where no stretching or minimal stretching in both directions is observed, no significant difference is expected.
Limitations of the study. In the present study, the ratio analysis of dermal collagen and elastic fiber contents have been evaluated from limited areas of anatomical regions of the human body, which might not be applicable to other areas of the same region. So, similar evaluations should also be carried out in other areas and assessed accordingly.
Conclusion
The evaluation of the asymmetric distribution of dermal collagen and elastic fibers in different directions and their ratio analysis provide guidelines for the aesthetic surgeons seeking to place elective incisions in the direction that will yield the most aesthetically pleasing result. The results of the study might provide the basis for future retrospective analyses of all elective incisions for a better understanding of scar behavior.
References
- Verhaegen PD, Schoten HJ, Tigchelaar-Gutter W, et al Adaptation of the dermal collagen structure of human skin and scar tissue in response to stretch: an experimental study. Wound Repair Regen. 2012;20(5):658–666.
- Compton CC, Gill JM, Bradford DA, et al. Skin regenerated from cultured epithelial autografts on full-thickness burn wounds from 6 days to 5 years after grafting. Lab Invest. 1989;60(5):600–612.
- Berman B, Viera MH, Amini S, et al. Prevention and management of hypertrophic scars and keloids after burns in children. J Craniofac Surg. 2008;19(4):989–1006.
- Naveen K, Pramod K, Keerthana P, Satheesha NB. A histological study on the distribution of dermal collagen and elastic fibers in different regions of the body. Int J Med Med Sci. 2012;4(8): 171–176.
- Naveen K, Pramod K, Keerthana P, et al. Histomorphometric analysis of dermal collagen and elastic fibers in skin tissues taken perpendicular to each other from head and neck region. Journal of Surgical Academia. 2014;4(1):30–36.
- Naveen K, Pramod K, Satheesha NB, et al. Quantitative fraction evaluation of dermal collagen and elastic fibers in the skin samples obtained in two orientations from the trunk region. Dermatol Res Pract. 2014;2014:251254.
- Naveen K, Pramod K, Keerthana P, et al. Surgical implications of asymmetric distribution of dermal collagen and elastic fibers in two orientations of skin samples from extremities. Plast Surg Int. 2014;2014:364573.
- Bancroft JD, Gamble M. Theory and Practice of Histological Techniques. 5th edition. London; UK: Churchill Livingstone; 2002: 127–156.
- Keerthana I. Colour image analysis for staining intensity quantification—its application to medical research and diagnostic purposes. PhD thesis (chapter 3). Manipal Institute of Technology, Manipal University, Manipal, India; 2012.
- Parlange Mary. New mechanical insights into wound healing and scar tissue formation. Available at: https://www.sciencedaily.com/releases/2007/12/071217092914.htm. Accessed December 30, 2007.
- van Zuijlen PP, Ruurda JJ, van Veen HA, et al. Collagen morphology in human skin and scar tissue: no adaptations in response of mechanical loading at joints. Burns. 2003;29(5):423–431.
- Sawhney RK, Howard J. Slow local movements of collagen fibers by fibroblasts drive the rapid global self-organization of collagen gels. J Cell Biol. 2002;157(6):
1083–1093. - Ehrlich HP, Kummel TM. Regulation of wound healing from a connective tissue perspective. Wound Repair Regen. 1996;4(2):203–210.
- Verhaegen PD, Schoten HJ, Tigchelaar-Gutter W, et al. Adaptation of the dermal collagen structure of human skin and scar tissue in response to stretch: an experimental study. Wound Repair Regen. 2012;20(5):658–666.
- Cohen BE, Geronemus RG, McDaniel DH, Brauer JA. The role of elastic fibers in scar formation and treatment. Dermatol Surg. 2017;43 Suppl 1:S19–S24.
- Urschel JD, Scott PG, Williams HT. The effect of mechanical stress on soft and hard tissue repair; a review. Br J Plast Surg. 1988;41(2):182–186.

