 J Clin Aesthet Dermatol. 2020;13(3):17–19
J Clin Aesthet Dermatol. 2020;13(3):17–19
by Joana Cruz Matos Calvão da Silva, MD; Ricardo Vieira, Prof, MD; and Maria Manuel Brites, MD
Drs. Calvão da Silva, Vieira, and Brites are with the Dermatology Department at Coimbra University Hospital in Coimbra, Portugal.
FUNDING: No funding was provided for this study.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: Hailey-Hailey disease (HHD), or chronic benign familial pemphigus, is a rare inherited acantholytic dermatosis, characterized by chronic, recurrent vesicles, erosions, and maceration in intertriginous sites. We present a case of a male patient with longstanding HHD who presented with an acute exacerbation characterized by the worsening of pre-existing lesions but also with the appearance of new large, tense bullae on an erythematous base in the areas of the groin (i.e., inguinal region), trunk, and arms, associated with intense pruritus. Blood work revealed eosinophilia. Histopathology and direct immunofluorescence were compatible with the diagnosis of bullous pemphigoid (BP). Indirect immunofluorescence showed positivity for autoantibodies to BP antigen 180. We started oral methylprednisolone, oral antihistamines, and local care with potassium permanganate baths, a potent corticosteroid, and fusidic acid, with resulting improvement of the lesions. The case was further complicated by the occurrence of eczema herpeticum, which was successfully treated with acyclovir. At the time of discharge from the hospital, the patient was medicated with a low dose of oral steroid and oral doxycycline. During a later examination, the lesions had totally disappeared, but the skin had some residual hyperpigmented patches and excoriated papules. This case was a diagnostic challenge due to the simultaneous occurrence of three distinct bullous diseases with different etiopathogeneses. To our knowledge, there are no other reports of the coexistence of HHD and BP in the literature.
KEYWORDS: Hailey-Hailey, bullous pemphigoid, doxycycline, eczema herpeticum
Hailey-Hailey disease (HHD), or familial benign chronic pemphigus, is a rare autosomal-dominant acantholytic dermatosis. It is hypothesized to result from a genetic defect in the calcium pump protein coded by the ATPase calcium-transporting type 2C member 1 gene (ATP2C1) on chromosome 3.1 Bullous pemphigoid (BP), the most common autoimmune bullous disease, preferentially involves elderly patients and is significantly associated with neurological disorders.2 Biopsies, direct immunofluorescence, indirect immunofluorescence, and serum autoantibodies are required to establish a diagnosis of BP.3 Eczema herpeticum, also known as Kaposi varicelliform eruption, is a secondary herpes simplex virus infection that occurs with a variety of skin disorders, most commonly in the setting of atopic dermatitis, but also alongside other dermatoses, including HHD.4 Although it can be life threatening, eczema herpeticum can be easily and effectively treated with antiviral agents if detected early.5
Here, we present a rare case in which the coexistence of HHD and BP, complicated by eczema herpeticum, was observed.
Case Presentation
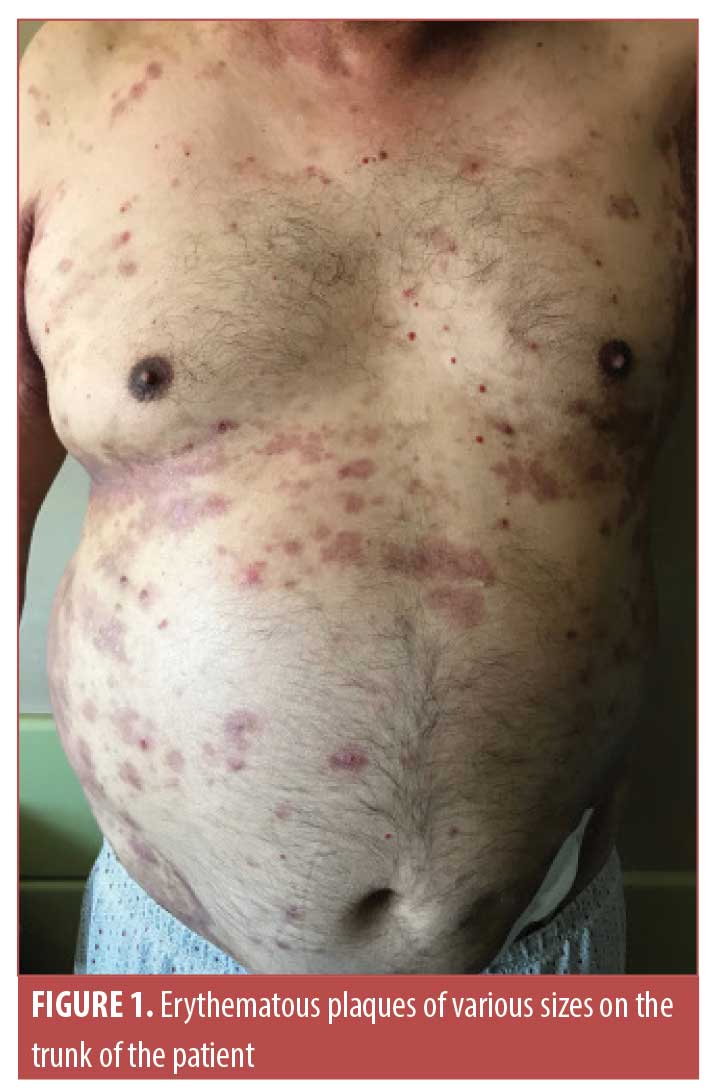
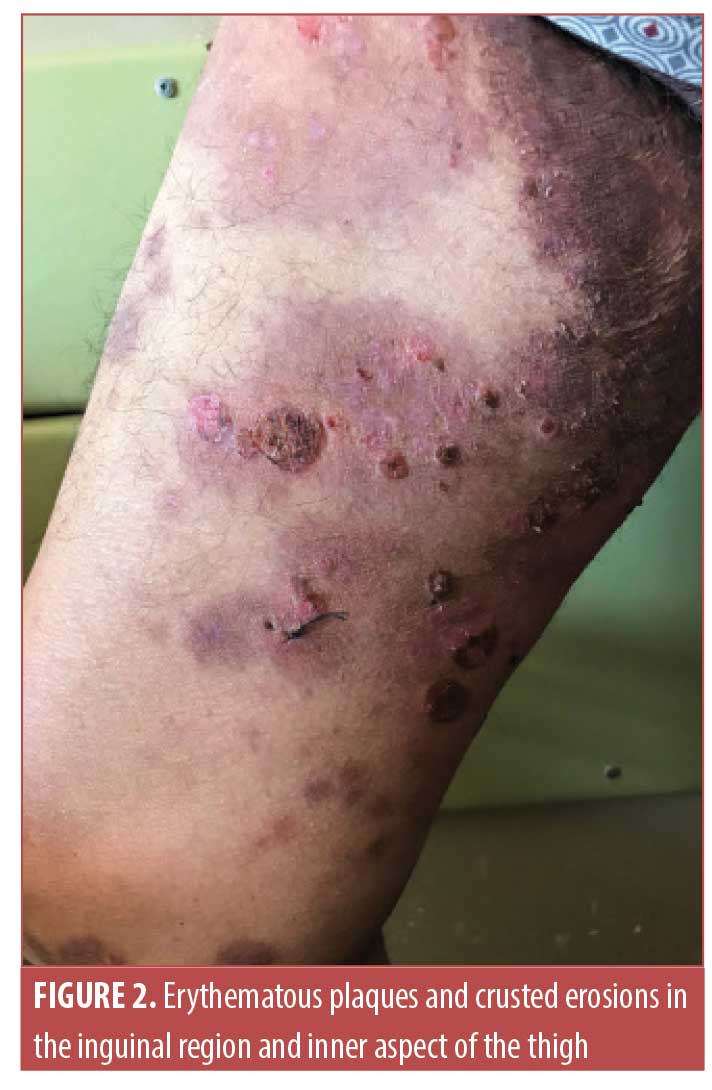
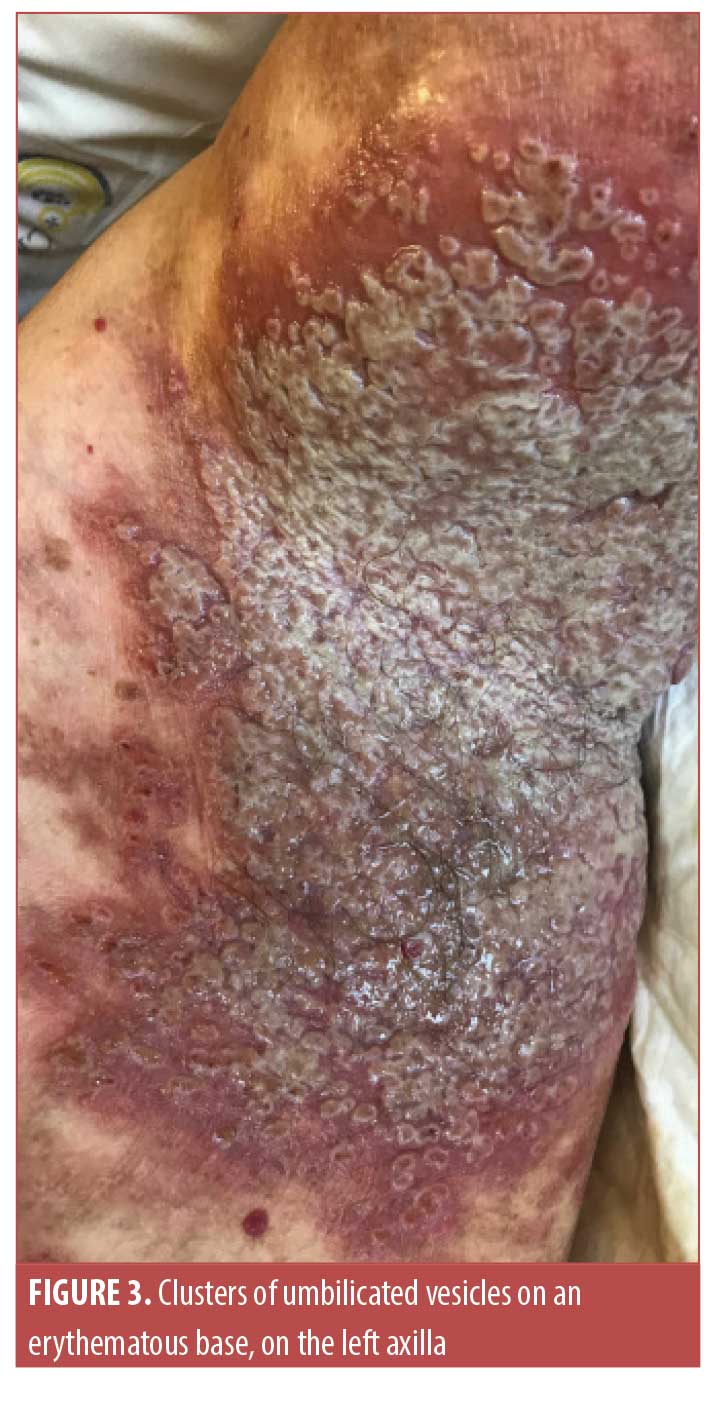
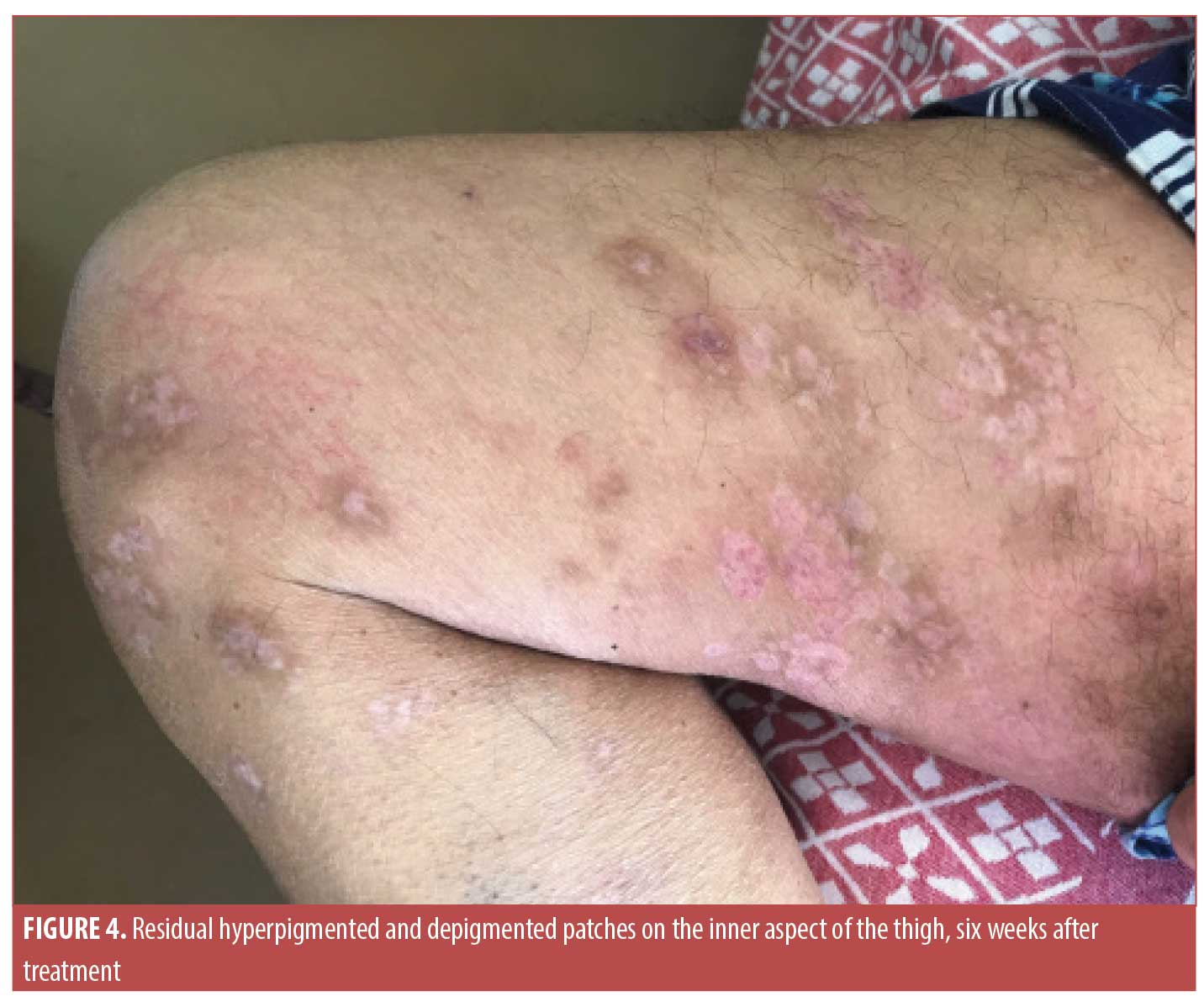
A 62-year-old male patient with longstanding HHD (30 years of evolution), confirmed by histopathology, presented with an acute exacerbation characterized by worsening of pre-existing axillary and inguinal lesions, malodorous drainage, and the appearance of large tense bullae on an erythematous base, localized especially in the inguinal region in a symmetric distribution. Erythematous plaques of various sizes were also found on the arms, trunk, and legs, and were associated with intense pruritus (Figures 1 and 2). In the past, the patient had undergone CO2 laser ablation, with clinical benefit, and had been treated with oral minocycline, topical corticosteroids, and antifungals during some flare-ups of the disease. At presentation, the patient was undergoing treatment with naltrexone 6mg daily and doxycycline 100mg daily. Blood work revealed eosinophilia (20%) with no other changes. Since this episode had some peculiarities, the patient was admitted to our ward; a biopsy of one of the bullae and a perilesional punch biopsy for direct immunofluorescence were performed. Histopathology revealed the presence of a subepidermal blister containing fibrin and inflammatory cells as well as dermal edema with a subepidermal inflammatory infiltrate rich in eosinophils. Direct immunofluorescence revealed immunoglobulin G deposition along the dermoepidermal junction. Indirect immunofluorescence showed positivity for autoantibodies to BP antigen 180 (BP180) (title>200 U/mL) but negative results for anti-BP230 and anti-desmoglein 1 and 3. These findings are typical of BP. The patient was started on oral methylprednisolone (24mg daily), oral antihistamines, and local care with potassium permanganate baths, a potent topical corticosteroid, topical fusidic acid, and a topical antifungal, which led to improvement of the lesions. However, on the eighth day of admission, he also developed an eruption of painful clusters of umbilicated vesicles on the left axilla (Figure 3). Clinically, these lesions where highly suggestive of eczema herpeticum and he was given intravenous acyclovir with resulting improvement within a few days. We slowly tapered the dose of oral steroids, and, at the time of hospital discharge, the patient had no bullae or lesions of eczema herpeticum, with significant improvement of axillary and inguinal lesions. He maintained a low dose of oral methylprednisolone for a few days and restarted doxycycline. Roughly 45 days later, the patient came in for follow-up, showing an almost complete clearance of the lesions, with only some residual hyperpigmented patches and excoriated papules (Figure 4).
Discussion
HHD was first described by the Hailey brothers in 1939. It usually appears in the third or fourth decade of life, although it can occur at any age.5–7 It is a chronic autosomal-dominant disorder with incomplete penetrance, and approximately two-thirds of affected patients have family history of this disorder.8 The pathogenic gene of HHD was reported to be the ATP2C1 gene, which regulates Ca2+/Mn2+ homeostasis.1 The resulting calcium dysregulation affects epidermal desmosomes and leads to suprabasilar acantholysis (i.e., epidermal blistering). Clinically, this results in erosions and vesicles, often coalescing into weeping and crusting plaques that are frequently localized in symmetrical intertriginous areas, such as the axillae, groin, and inframammary folds.9 This disease is characterized by recurrent exacerbations, tending to be chronic and recalcitrant to treatment. Therapeutic options include topical and oral antibiotics and corticosteroids, oral retinoids, and other immunosuppressants, as well as botulinum toxin injections (which reduce sweating in the intertriginous areas, thereby reducing the risk of secondary infection and exacerbations), surgical methods, such as excision and dermabrasion, and CO2 laser.5,9–11 Naltrexone has been recently tested, with variable success, although the ideal dose has not been elucidated.11 Doxycycline suppresses epidermal proliferation and microorganism-mediated exacerbations through its antibiotic potential, and has anti-inflammatory properties and anticollagenase activity via the inhibition of matrix metalloproteinases, which are normally upregulated in the event of dermal destruction. Due to these properties and its safe profile, doxycycline has been used with success in both BP and HHD.12–14
BP is the most common autoimmune subepidermal blistering disease in Western countries and typically affects the elderly. BP is immunologically characterized by tissue-bound and circulating autoantibodies directed against either BP180 (or BPAG2) or BP antigen 230 (BP230 or BPAG1e), or even both, which are components of hemidesmosomes involved in dermal–epidermal cohesion. Clinically, BP is an intensely pruritic, erythematous eruption with widespread tense blister formation.2 There are numerous reports of patients with BP in association with other disorders (i.e., neurologic, psychiatric, hematologic, pulmonary, and cardiovascular).3 The association of BP with other immune-mediated dermatoses, such as pemphigus vulgaris, psoriasis, and lichen planus, has also been described;2,3 however, coexistence with other bullous dermatoses, as in our case, is unusual.
In the treatment of BP, although the advent of systemic steroid therapy has significantly improved its prognosis, the use of steroids is limited by their side effects. Based on the growing understanding of the pathogenesis of autoimmune bullous diseases, novel treatment targets have emerged, some of which are currently being evaluated in clinical trials, such as rituximab (NCT00525616), ixekizumab (NCT03099538), and bertilimumab (NCT02226146).15 A Phase II clinical trial (NCT03286582) is currently comparing the topical effects of clobetasol 0.05% ointment with AC-203 1% ointment, a topical formulation of an oral modulator of inflammasome and interleukin-1beta pathways. The primary endpoint is the incidence of adverse effects during the treatment period. No results from this trial are currently available.
While the occurrence of eczema herpeticum in the setting of skin disorders, particularly HHD, is commonly reported in the literature, the simultaneous occurrence of three distinct bullous diseases with different etiopathogeneses (i.e., genetical, autoimmune, and infectious) is unusual, and was a diagnostic challenge in our reported case. One possible explanation for the presentation of these three bullous diseases seems to be that the presence of a bullous disease facilitates the onset of a second bullous disease in the same patient due to a possible individual predisposition to blistering.3
Conclusion
To our knowledge, this is the first case report describing the copresentation of HHD and BP. David et al15 in 1989 published a case of a patient with longstanding localized BP and the recent finding of a HHD-like histologic pattern, suggesting the possibility of coexistence of these two bullous diseases, but concluded that the HHD-like pattern more likely represented an incidental microscopic finding or a solitary acantholytic lesion.15 Since then, there have been no reports of an association between these two diseases in the literature. Our patient was successfully treated with oral methylprednisolone, oral antihistamines, potassium permanganate baths, corticosteroids, and fusidic acid, followed by acyclovir for eczema herpeticum and a low dose of an oral steroid and oral doxycycline upon discharge.
References
- Deng H, Xiao H. The role of the ATP2C1 gene in Hailey-Hailey disease. Cell Mol Life Sci. 2017;74(20):3687–3696.
- Bernard P, Antonicelli F. Bullous pemphigoid: a review of its diagnosis, associations and treatment. Am J Clin Dermatol. 2017;18(4):513–528.
- Ruocco E, Wolf R, Caccavale S, et al. Bullous pemphigoid: associations and management guidelines: facts and controversies. Clin Dermatol. 2013;31(4):400–412.
- Arnold M, Vu M, Grimshaw E, Wilkerson M. Hailey-Hailey disease complicated by herpes simplex viral infection. SKIN The Journal of Cutaneous Medicine. 2017;1(2):100–103.
- Lee GH, Kim YM, Lee SY, et al. A case of eczema herpeticum with Hailey-Hailey disease. Ann Dermatol. 2009;21(3):311–314.
- de Aquino Paulo Filho T, deFreitas YKR, da Nóbrega MTA, et al. Hailey-Hailey disease associated with herpetic eczema-the value of the Tzanck smear test. Dermatol Pract Concept. 2014;4(4):29–31.
- Zhao QF, Hasegawa T, Komiyama E, Ikeda S. Hailey-Hailey disease: a review of clinical features in 26 cases with special reference to the secondary infections and their control. Dermatologica Sin. 2017;35(1):7–11.
- Geisinger T and Delaney A. Hailey-Hailey Syndrome. J Am Acad Dermatol. 2011;64(2)S1:AB96.
- Arora H, Bray FN, Cervantes J, Falto Aizpurua LA. Management of familial benign chronic pemphigus. Clin Cosmet Investig Dermatol. 2016;9:281–290.
- Kothapalli A, Caccetta T. Botulinum toxin type A for the first-line treatment of Hailey-Hailey disease. Australas J Dermatol. 2019;60(1):73–74.
- Cao S, Lilly E, Chen ST. Variable response to naltrexone in patients With Hailey-Hailey disease. JAMA Dermatol. 2018:154(3):362–363.
- Le Saché-De Peufeilhoux L, Raynaud E, Bouchardeau A, et al. Familial benign chronic pemphigus and doxycycline: a review of 6 cases. J Eur Acad Dermatology Venereol. 2014;28(3):370–373.
- Grantham HJ, Stocken DD, Reynolds NJ. Doxycycline: a first-line treatment for bullous pemphigoid? Lancet. 2017;389(10079):1586–1588.
- Flores-Terry M, Cortina-de la Calle MP, López-Nieto M, et al. Enfermedad de Hailey-Hailey, adecuada respuesta a doxiciclina. Actas Dermosifiliogr. 2016;107(6):537–539.
- Izumi K, Bieber K, Ludwig RJ. Current clinical trials in pemphigus and pemphigoid. Front Immunol. 2019;10(May):1–11.
- Mehregan DA, Umbert IJ, Peters MS. Histologic findings of Hailey-Hailey disease in a patient with bullous pemphigoid. J Am Acad Dermatol. 1989;21(5):1107–1112.

