 by Joseph F. Fowler, MD; James Q. Del Rosso, DO; Refika I. Pakunlu, PhD; and Srinivas Sidgiddi, MD
by Joseph F. Fowler, MD; James Q. Del Rosso, DO; Refika I. Pakunlu, PhD; and Srinivas Sidgiddi, MD
Dr. Fowler is Co-Founder of Dermatology Specialists Research in Louisville, Kentucky, and Clinical Professor of Dermatology, University of Louisville School of Medicine, Louisville, Kentucky. Dr. Del Rosso is Research Director at JDR Dermatology Research in Las Vegas, Nevada; Adjunct Clinical Professor of Dermatology at Touro University Nevada, Las Vegas; and with Dermatology and Cutaneous Surgery at Thomas Dermatology in Las Vegas, Nevada. Dr. Pakunlu is Associate Director of Chemistry, Manufacturing, and Controls, and Dr. Sidgiddi is Director of Clinical Development at Promius Pharma, a subsidiary of Dr. Reddy’s Laboratories, in Princeton, New Jersey.
Funding: This study was funded by Promius Pharma, a subsidiary of Dr. Reddy’s Laboratories (DRL Publication No. 802).
Disclosures: The authors have no financial conflicts relevant to the content of this article.
Abstract: Objective. To assess patient-reported satisfaction, efficacy, and tolerability associated with the use of betamethasone dipropionate spray 0.05% when applied twice daily in subjects with moderate plaque psoriasis.
Design. This was an open-label, multicenter study involving 45 patients with moderate plaque psoriasis, with the aim of evaluating patient-reported outcomes with betamethasone dipropionate spray 0.05%. Patients treated all affected areas twice daily with betamethasone dipropionate (BD) spray 0.05% for 28 days per label instructions.
Measurements. Outcome measures included the Treatment Satisfaction Questionnaire for Medication (TSQM), Dermatology Life Quality Index (DLQI), Investigators Global Assessment (IGA), and Total Sign Score (TSS). In addition, the lesions were photographed at baseline (Day 1) and on Day 8, Day 14, and Day 29.
Results. The results indicated that BD spray 0.05% treatment is associated with improved quality of life. BD spray 0.05% also led to improved IGA and TSS values and a reduction in the percentage of body surface area affected.
Conclusion. In subjects with moderate plaque psoriasis, BD spray 0.05% demonstrated good levels of patient satisfaction and quality of life measures, in combination with improvements in the global assessment of disease and the level of itching experienced by subjects.
Keywords: Betamethasone dipropionate, patient satisfaction, Treatment Satisfaction Questionnaire for Medication, Dermatology Life Quality Index, Investigators Global Assessment, Total Sign Score
J Clin Aesthet Dermatol. 2017;10(11):13–18
Introduction
Psoriasis is a chronic, immune-mediated inflammatory skin disease that affects approximately two percent of the United States population.1–3 The most common type is plaque psoriasis, which occurs in about 90 percent of those with the disease.4 Plaque psoriasis is characterized by silvery white plaques covering inflamed lesions, most commonly on and around the knees and elbows. Although regarded as a disease with a genetic component, psoriasis can be triggered by various external stimulants such as stress, bacterial infection, and certain medications.4 Plaque psoriasis treatment choice depends on the extent of body surface involvement, lesion location, previous treatment, and patient preference. Treatment includes topical formulations, systemic therapy, and ultraviolet light.
Guidelines for the treatment of psoriasis advocate the use of topical corticosteroids, typically as first-line treatment for mild-to-moderate plaque psoriasis or as part of a regimen of treatment for those with more extensive disease.5 Physicians generally choose topical treatment for those patients with smaller body surface area (BSA) involvement. This helps to reduce systemic exposure to corticosteroids, which have the potential to cause hypothalamic-pituitary-adrenal (HPA) axis suppression and other systemic side effects,6 including immunosuppression that can lead to opportunistic infections. When properly used, topical corticosteroids have a lower potential to cause systemic side effects, but can cause local reactions, such as burning, stinging, atrophy, and telangiectasia.
Plaque psoriasis can have a profound social, occupational, and psychological impact, especially when lesions are highly visible on the face, neck, and limbs.7 However, patient adherence with topical corticosteroids has been found to be only around 40 percent.8 While patient quality of life (QoL) is clearly impacted by psoriasis, it can also be affected either positively or negatively by its treatment. Topical treatments can cause local reactions that worsen the appearance of affected skin and do not always provide the results a patient anticipated.
Patients want to see rapid improvement, and expect results much more quickly than their physicians. Many patients will discontinue treatment for “lack of efficacy” when, in reality, they stop therapy because the results didn’t occur quickly enough.9–11 Patients with pruritus (~85% of patients12), burning sensations, or flaky skin are most likely to seek relief quickly from their symptoms, and products that do not ease these symptoms soon enough may be discontinued.9 Patients will stop therapy if they think the treatment is ineffective or not aggressive enough, or if they simply lose faith in its value.8
Factors known to affect adherence include the ease of use and the acceptability of the product (smell, texture, staining); patients also fear side effects of topical corticosteroid use.13,14 For any product in this difficult arena, the opinion of the end user is of vital importance.
Betamethasone dipropionate (BD) spray 0.05% is a medium-potency topical corticosteroid recently introduced for the treatment of mild-to-moderate plaque psoriasis.15
The objective of this study was to evaluate patient QoL, treatment satisfaction, efficacy, and tolerability after treatment with BD spray 0.05% using the Dermatology Life Quality Index (DLQI), a validated, 10-item questionnaire16; the Treatment Satisfaction Questionnaire for Medication (TSQM), version 2.017; the Investigators Global Assessment (IGA); the Total Sign Score (TSS); and visual assessment using photographs of lesions taken at baseline (Day 1) and on Days 8, 14, and 29.
Methods
Study oversight. Written informed consent was obtained from all subjects and the study was conducted in compliance with current International Council for Harmonisation guidelines for Good Clinical Practice, United States Food and Drug Administration regulations, and the Declaration of Helsinki.7–9,11
Study design. In this 28-day multicenter, multidose, open-label study, patients with moderate plaque psoriasis aged 18 years or older applied BD spray 0.05% twice daily to all affected areas of the body, excluding the face, scalp, groin, axillae, and other intertriginous areas. Subject assessments took place at screening, baseline (Day 1), and on Days 8, 14, and 29 after a washout period for topical and systemic products.
Subjects. A total of 45 male and female adult patients with moderate plaque psoriasis affecting a BSA of at least three percent were enrolled. At each site, approximately half of the patients had 3- to 10-percent BSA involvement, and half had more than 10-percent BSA involvement. Patients applied BD spray 0.05% twice daily for 28 days per the label.
Outcome measures. The primary efficacy endpoints were changes in clinical response (IGA, TSS, BSA) and QoL (DLQI) from Day 1 to Day 14. An additional co-primary endpoint was treatment satisfaction score (TSQM) at Day 14.
Secondary endpoints were changes in clinical response (IGA, TSS, BSA) and QoL (DLQI) from Days 1 to 8 and from Days 1 to 29. Additional secondary endpoints were treatment satisfaction scores (TSQM) at Days 8 and 29. At each visit, the investigator also rated the severity of pruritus at target lesions on a scale of 0 (none) to 3 (severe).
In addition, three post-hoc analyses on changes in IGA and TSS were completed. These included 1) the proportion of patients with IGA score of 0 (3-grade reduction from baseline) or 1 (2-grade reduction from baseline) at Day 14; 2) the proportion of patients with IGA score of 1 (2-grade reduction from baseline) at Day 14; and 3) the proportion of patients with TSS reduction of at least 50 percent at Day 14.
The DLQI is a validated, 10-item questionnaire that assesses dermatology-specific quality of life,16,18 and yields a total score of 0 (best QoL) to 30 (worst QoL).19 Thus, a decrease in DLQI score indicates improvement. The TSQM version 2.017 is an 11-item instrument measuring four dimensions of treatment satisfaction (Effectiveness, Side Effects, Convenience, and Overall Satisfaction) that yields a total score ranging from 0 (extremely dissatisfied) to 100 (extremely satisfied).
Each subject had two target lesions selected at baseline that were representative of the individual’s psoriasis. These were used for TSS assessments. TSS is a numeric score ranging from 0 to 9, calculated by summing the scores for erythema, scaling, and plaque elevation, which are each graded on a four-point scale (0=clear; 3=severe). Photographs were taken of these lesions at baseline (Day 1) and at visits on Days 8, 14, and 29.
Results
The mean age of subjects enrolled in this study was 50.7 years (47% female, 53% male). Subject enrollment was categorized into two groups based on percentage BSA involvement. Subject demographics are presented in Table 1.
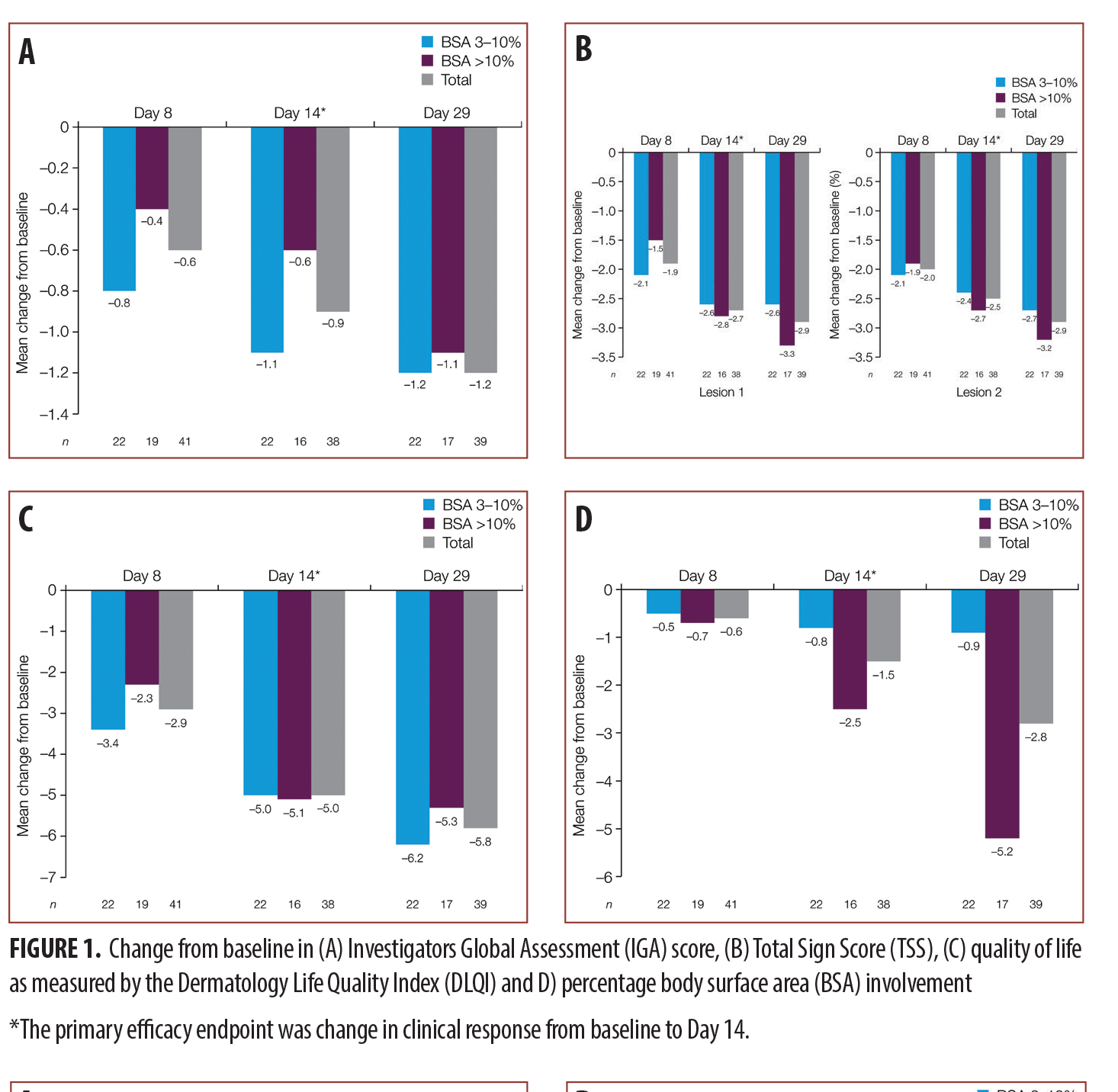
The primary endpoint was the change in IGA, TSS, DLQI, TSQM, and percent BSA at Day 14. The results indicate an approximately one-grade reduction in IGA from baseline to Day 14, while TSS showed an improvement in the range of 2.5 to 2.7 grades. DLQI also showed an improvement of five points on a scale of 30, while BSA showed a 1.5% decrease. The results for all parameters were similar between the groups with low and high BSA involvement. These data are summarized in Figure 1.
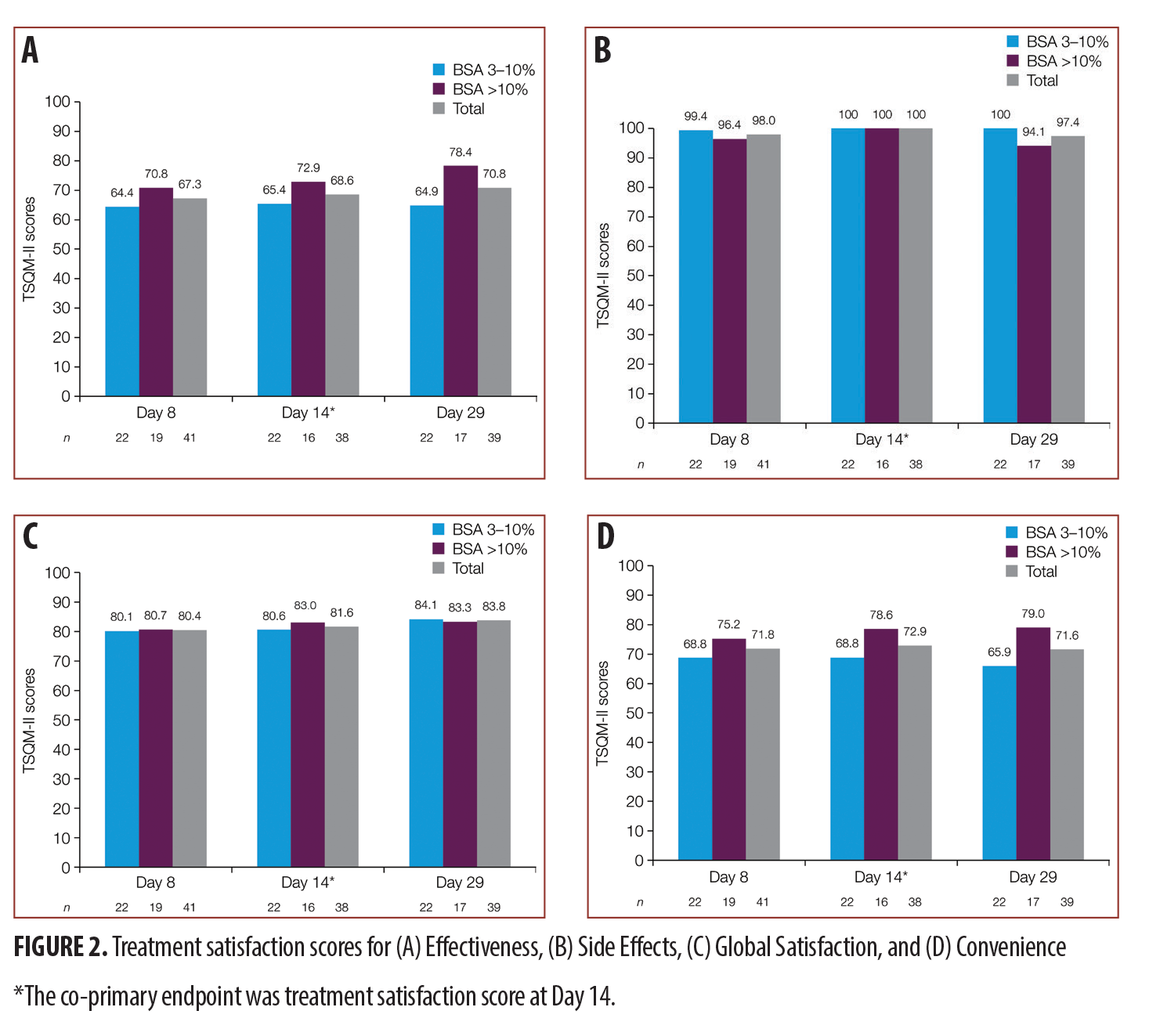
Treatment satisfaction scores at Day 14 for TSQM were Convenience, 81.6/100; Side Effects, 100/100; Effectiveness, 68.6/100; and Global Satisfaction, 72.9/100. Higher scores indicate greater satisfaction. The results indicate that subjects were generally more than “satisfied” with the ability of the medication to prevent or treat their condition, the way the medication relieved their symptoms, and the amount of time it took for the medication to start working. For the group with more than 10-percent BSA, mean scores were higher and there was a trend for increasing satisfaction with time. TSQM scores are presented in Figure 1.
The secondary endpoints of the study were the IGA, TSS, change in percent BSA, and DLQI scores at Days 8 and 29. Overall, the results showed a slightly lower performance on Day 8 as compared to Day 14, and a higher performance on Day 29, indicating that treatment effects continued to improve for all tested parameters. The overall improvement in IGA score was 1.2 grades at Day 29, while the TSS scores showed a reduction of 2.9 in the severity of the lesions. The change in percent BSA was a 2.8 percent reduction, and the DLQI scores decreased by 5.8. The IGA scores were similar between the patients with lower and higher BSA involvement, while TSS showed a better performance in the group with higher BSA involvement. Interestingly, DLQI score improvement was greater in patients with lesser BSA involvement. Detailed scores for each of these parameters are provided in Figure 1.
Treatment satisfaction scores indicate patients’ perception of BD spray 0.05% in terms of Convenience, 80.4 and 83.8 for Day 8 and Day 29, respectively; Side Effects, 98 and 97.4; Effectiveness, 67.3 and 70.8; and Overall Satisfaction, 71.8 and 71.6. The best performance for BD spray 0.05% again seems to be with respect to the Side Effects parameter, where the scores indicate maximum satisfaction for all groups, followed by Convenience, then Effectiveness. The results indicate that the satisfaction on all parameters was similar between the two groups with low and high BSA involvement, except Overall Satisfaction, which seemed to be numerically better in the higher BSA group at both Day 8 and Day 29. The detailed TSQM scores for both Day 8 and Day 29 are presented in Figure 2.
Pruritus severity was evaluated on Days 8, 14, and 29, and the results indicate progressive improvement from baseline to Day 29. The improvement was similar across the two BSA groups at all time points. The detailed results are presented in Table 2.
In the post-hoc analyses, 13.3 percent of all subjects (6 of 45) achieved an IGA score of 0 or 1 (16.7% and 9.5% in the 3–10% and >10% BSA groups, respectively) and 8.9 percent of all subjects (4 of 45) achieved an IGA score of 1 at Day 14 (8.3% and 9.5%, respectively).
TSS at Day 14 showed that Lesion 1 scores were reduced by at least 50 percent in 42.2 percent of all subjects (19 of 45) and Lesion 2 scores were reduced by at least 50 percent in 37.8 percent of all subjects (17 of 45).
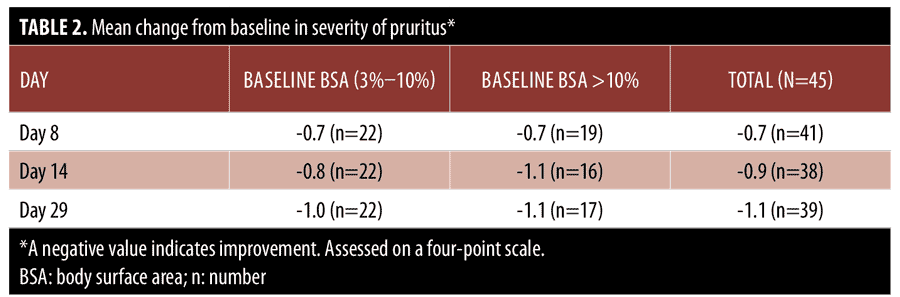
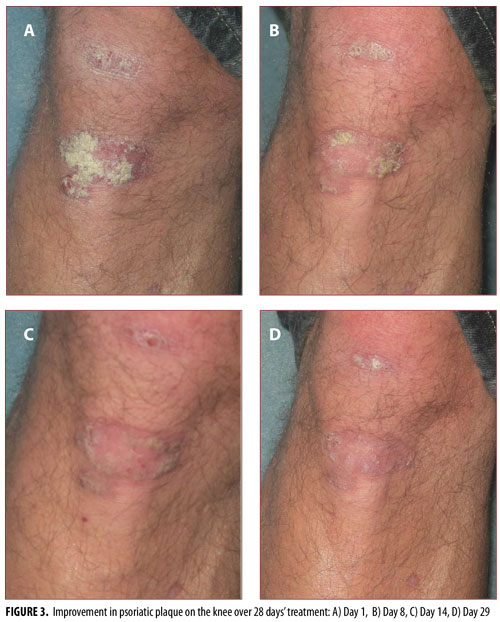
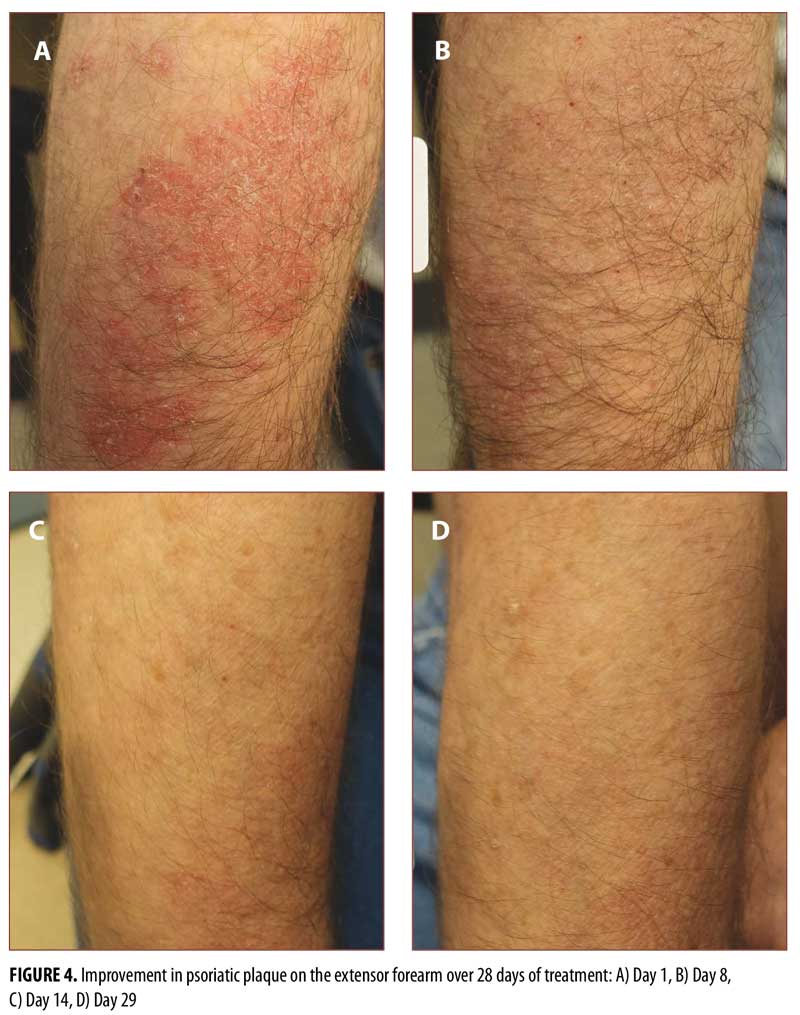
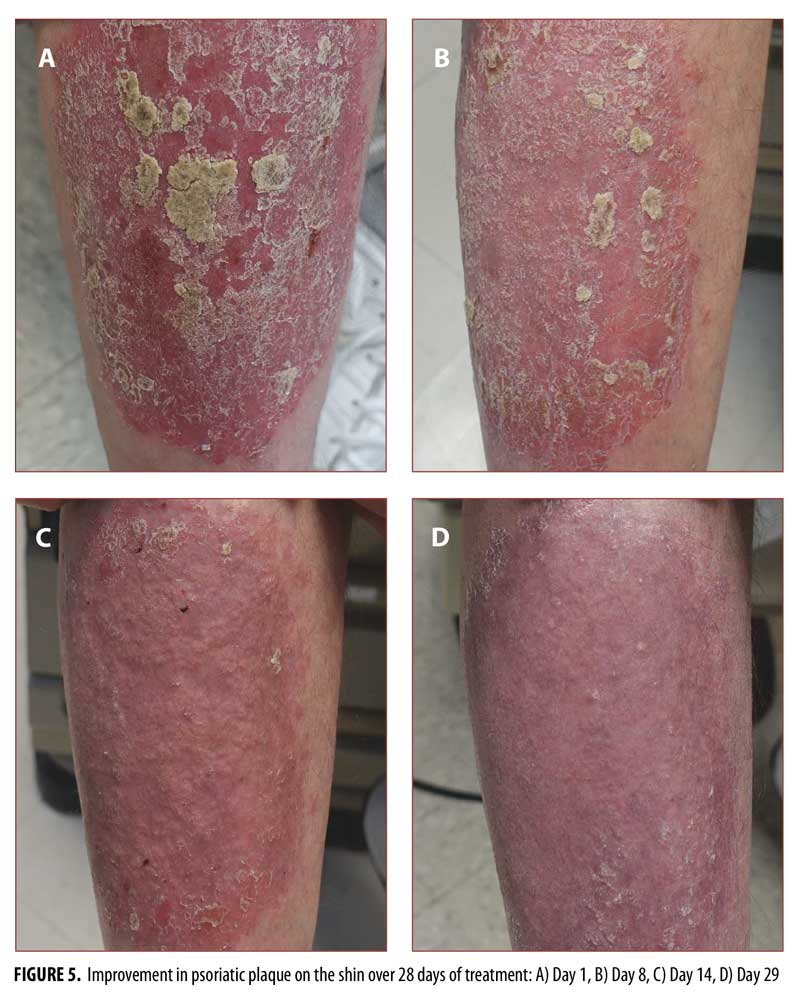
In psoriasis treatment, hairy body areas with creases are often considered to be difficult to treat.20,21 Standard photographs of lesions on the knee (Figure 3), hairy extensor forearm (Figure 4), and shin (Figure 5) were taken at Days 1, 8, 14, and 29, showing improvement from Days 8 to 29 in all treated areas.
Safety results. Overall, BD spray 0.05% was well tolerated. Five subjects reported a total of seven adverse events (AEs; 4 with 3–10% BSA involved and 1 with >10% BSA involved). The AEs seen in the study were as expected for this population and this class of drug. One AE of moderate severity (hypertension) was deemed to be possibly related to use of the study product. Two serious AEs were reported by one subject during the course of the study but were not judged to be connected with the study medication.
Discussion
Twice-daily treatment with BD spray 0.05% showed a progressive improvement for the three clinical assessments of IGA, TSS, and percent BSA, as well as for the QoL assessment (DLQI). The overall improvement in mean scores for the IGA, TSS, and percent BSA variables was better in the higher (i.e., >10%) BSA group. The average decrease in IGA of approximately one grade from baseline to Day 14 indicated that most subjects’ IGA grade changed from “moderate” to “mild” after two weeks of treatment. Post-hoc analyses showed that 13.3 percent of all patients (6 of 45) achieved an IGA score of 0 or 1 at Day 14. Two phase III registration trials (total n=660) conducted with BD spray 0.05% demonstrated an IGA success of 20.3 percent (pooled analysis) of all subjects achieving an IGA score of 0 or 1 at Day 15, and 38.7 percent achieving treatment success at Day 29.22 The IGA success noted in this trial was less than that seen in the phase III program, possibly due to the small size of the study population in the current study.
Central to the success of a topical corticosteroid for plaque psoriasis is its use for 14 to 28 days, twice a day, consistently. The chance of this happening in an individual patient is increased if the product prescribed is pleasant to use and shows relatively rapid improvement in skin condition. In this study, subjects reported good satisfaction with the study product across all four domains of the TSQM version 2.0 questionnaire at Day 14 (primary endpoint), and on Days 8 and 29 (secondary endpoints). Subjects were more than satisfied with the time it took for their symptoms to start improving.
The severity of pruritus decreased steadily from baseline to Days 8, 14, and 29 (secondary endpoints) for the combined BSA groups, with an approximate one-grade maximal improvement for all BSA groups at Days 14 and 29. These results show that patients had some relief of itching over the four-week treatment period.
In general, extending the treatment from two weeks to four weeks resulted in greater improvement in all primary and secondary efficacy variables (IGA, TSS, %BSA), and this benefit was seen more significantly in the group with greater than 10-percent BSA involvement.
BD spray 0.05% was well tolerated. The type, incidence, and severity of the AEs reported during the study are consistent with those anticipated based on the drug class.
All of the enrolled subjects in this study completed the full four-week course of treatment. Though the full completion might be attributed to the subjects being part of a clinical trial, as this in itself increases the adherence rate in subjects, it could also be considered a testament to the acceptability of the product to its end users.
Conclusion
This 28-day study of BD spray 0.05% in subjects with moderate plaque psoriasis demonstrated good levels of patient satisfaction and QoL measures, in combination with an improvement in the global assessment of disease and the level of itching experienced by subjects.
References
- Gudjonsson JE, Johnston A, Sigmundsdottir H, Valdimarsson H. Immunopathogenic mechanisms in psoriasis. Clin Exp Immunol. 2004;135(1):1–8.
- Nesle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361(5):496–509.
- Raychaudhuri SP. A cutting-edge overview: psoriatic disease. Clin Rev Allergy Immunol. 2013;44(2):109–113.
- Sarac G, Koca TT, Baglan T. A brief summary of clinical types of psoriasis. North Clin Istanb. 2016;3(1):79–82.
- American Academy of Dermatology Work Group, Menter A, Korman NJ, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 6. Guidelines of care for the treatment of psoriasis and psoriatic arthritis: case-based presentations and evidence-based conclusions. J Am Acad Dermatol. 2011;65(1): 137–174.
- Swartz SL, Dluhy RG. Corticosteroids: clinical pharmacology and therapeutic use. 1978;16(3):238–255.
- Nguyen CM, Beroukhim K, Danesh MJ, et al. The psychosocial impact of acne, vitiligo, and psoriasis: a review. Clin Cosmet Investig Dermatol. 2016;9:383–392.
- Richards HL, Fortune DG, Griffiths CE. Adherence to treatment in patients with psoriasis. J Eur Acad Dermatol Venereol. 2006;20(4):370–379.
- Hougier FG, Cook-Bolden FE, Rodriguez D, Berlin JM. Critical considerations of optimizing topical corticosteroid therapy. J Clin Aesthet Dermatol. 2015;8(Suppl 1):S2–S14.
- Burroni AG, Fassino M, Torti A, Visentin E. How do disease perception, treatment features and dermatologist–patient relationship impact on patients assuming topical treatment? An Italian survey. Patient Relat Outcome Meas. 2015;6:9–17.
- Atkinson MJ, Kumar R, Cappelleri JC, Hass SL. Hierarchical construct validity of the treatment satisfaction questionnaire for medication (TSQM version II) among outpatient pharmacy consumers. Value Health. 2005;8(Suppl 1):
S9–S24. - Prignano F, Ricceri F, Pescitelli L, Lotti T. Itch in psoriasis: epidemiology, clinical aspects and treatment options. Clin Cosmet Investig Dermatol. 2009;2:9–13.
- Dawn A, Yosipovitch G. Treating itch in psoriasis. Dermatol Nurs. 2006;18(3):227–233.
- Brown KK, Rehmus WE, Kimball AB. Determining the relative importance of patient motivations for nonadherence to topical corticosteroid therapy in psoriasis. J Am Acad Dermatol. 2006:55(4):607–613.
- Sernivo™ Prescribing Information. Promius Pharma, Princeton, NJ. Available at: http://sernivo.com/documents/sernivo-pi.pdf. Accessed April 19, 2017.
- Cardiff University Department of Dermatology. Dermatology Quality of Life Index (DLQI). Available at: http://sites.cardiff.ac.uk/dermatology/quality-of-life/dermatology-quality-of-life-index-dlqi/. Accessed April 19, 2017.
- QuintilesIMS™. Treatment Satisfaction Questionnaire for Medication (TSQM). Available at: http://www.quintiles.com/landing-pages/treatment-satisfaction-questionnaire-for-medication-tsqm. Accessed April 19, 2017.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI): a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–216.
- Murray CS, Rees JL. How robust are the Dermatology Life Quality Index and other self-reported subjective symptom scores when exposed to a range of experimental biases?. Acta Derm Venereol. 2010;90(1):34–38.
- Wozel G. Psoriasis treatment in difficult locations: scalp, nails, and intertriginous areas. Clin Dermatol. 2008;26(5):448–459.
- Zeichner JA. Use of topical coal tar foam for the treatment of psoriasis in difficult-to-treat areas. J Clin Aesthet Dermatol. 2010 Sep;3(9):37–40.
- Stein Gold L, Jackson JM, Knuckles ML, et al. Improvement in extensive moderate plaque psoriasis with a novel emollient spray formulation of betamethasone dipropionate 0.05. J Drugs Dermatol. 2016;15(3):334?342.

