J Clin Aesthet Dermatol. 2025;18(8):34–40.
by Yvonne Nong, MD; Jeffrey Sugarman, MD, PhD; Jean Philippe York, PhD; Ofra Levy-Hacham, PhD; Dawnica Nadora, MS; Rinat Mizrahi, BSc; Aidan Galati, BSc; Richard L. Gallo, MD, PhD; and Raja K. Sivamani, MD, MS, AP
Dr. Nong is with Integrative Skin Science and Research, the Pacific Skin Institute, and the Department of Dermatology at the University of California, Davis in Sacramento, California. Dr. Sugarman is with the University of California, San Francisco in San Francisco, California. Dr. York is with Galderma Laboratories LP in Dallas, Texas. Dr. Levy-Hacham and Ms. Mizrahi are with Sol-Gel Technologies in Ness Ziona, Israel. Ms. Nadora is with Integrative Skin Science and Research in Sacramento, California. Mr. Galati is with Integrative Skin Science and Research and the Pacific Skin Institute in Sacramento, California. Dr. Gallo is with the Department of Dermatology at the University of California, San Diego in San Diego, California. Dr. Sivamani is with Integrative Skin Science and Research, the Pacific Skin Institute, the Department of Dermatology at the University of California, Davis in Sacramento, California, and the College of Medicine at California Northstate University in Elk Grove, California.
FUNDING: Funding for the study was provided by Sol-Gel Technologies. Funding for the preparation of the manuscript was provided by Galderma Laboratories LP.
DISCLOSURES: The authors declare no conflicts of interest relevant to the content of this article.
Abstract: Objective: We sought to evaluate changes in skin microbiome biodiversity and correlation with rosacea improvement of microencapsulated benzoyl peroxide (E-BPO) versus vehicle cream in patients with rosacea in a 12-week crossover study with a no-treatment period of four weeks (Week 16). Methods: This was a randomized, double-blind, single-center, crossover, vehicle-controlled evaluation of E-BPO on the skin microbiome in rosacea. Thirty-one participants had facial rosacea with global severity of 3 or 4 on the Investigator’s Global Assessment (IGA) scale. Participants were randomly assigned to two groups. The E-BPO/vehicle group applied E-BPO for eight weeks, then vehicle for four weeks. The vehicle/E-BPO group applied vehicle for eight weeks, then E-BPO for four weeks. Clinical assessments were performed using IGA, inflammatory rosacea scale, and erythema scale. Determination of change in skin microbiome was based on facial swab sampling. Results: Shifts in the microbiome correlated with improvements in IGA, inflammatory rosacea, and erythema. At Week 8, similar bacterial species diversity profiles were observed among all participants. After crossover of the vehicle/E-BPO group at eight weeks to E-BPO, the relative abundance of Staphylococcus epidermidis was markedly lowered, and the relative abundance of Cutibacterium acnes was slightly increased. In the E-BPO/vehicle group, the relative abundance of S. epidermidis and C. acnes at Weeks 12 and 16 remained at the level observed at Week 8. Limitations: The study had a short duration, which may not fully capture the long-term effects and durability of E-BPO in real-world clinical practice. Conclusion: Even after withdrawal at 16 weeks, efficacy and shifts in the skin microbiome were maintained over the duration of the study period with demonstrated clinical improvement and a well-tolerated safety profile. Keywords: Topicals, rosacea, microencapsulation, benzoyl peroxide, microbiome
Introduction
Rosacea is a chronic inflammatory skin disorder characterized by facial erythema, papules, and pustules, affecting approximately 16 million adults in the United States and resulting in substantial patient burden.1 Although the exact pathogenesis of rosacea remains unknown, skin-environmental interactions involving physical, chemical, and microbial factors are believed to play an important role. Imbalances in cutaneous organisms, including Staphylococcus epidermidis, Cutibacterium acnes, Demodex folliculorum, and Bacillus oleronius, have been attributed to rosacea.2 An altered immune response to environmental triggers is also considered a contributing factor,2 with elevated levels of S. epidermidis detected in rosacea pustules and changes in the relative abundance of S. epidermidis, C. acnes, and Corynebacterium kroppenstedtii observed in relation to rosacea severity.3–6
Despite rosacea primarily occurring in sebaceous gland-rich skin, significant alterations in skin barrier integrity have been observed in affected individuals.7 Demodex mites, which can be either primary or secondary to rosacea, stimulate immune responses that trigger angiogenesis and inflammation.8,9 Demodex mites harbor their own microbiome, including B. oleronius, that induces proliferation of peripheral blood mononuclear cells in patients with rosacea and stimulates the production of cathelicidin, matrix metalloproteinase-9, tumor necrosis factor, and interleukin-8 in healthy participants.3,10,11
Currently, United States Food and Drug Administration-approved treatments for rosacea include microencapsulated benzoyl peroxide (E-BPO 5% cream), metronidazole gel, azelaic acid gel and foam, ivermectin cream, minocycline foam, oral doxycycline, brimonidine gel, and oxymetazoline cream.12 E-BPO is a novel treatment for rosacea. Nonencapsulated BPO, which can cause local skin irritation, erythema, burning, and stinging after application, has largely been abandoned due to poor tolerability.13,14 Phase III trials have demonstrated the efficacy and safety of E-BPO.15 The mechanism of action of BPO in rosacea is unknown, but its antimicrobial properties may contribute to its efficacy, in addition to its keratolytic properties.
In a randomized vehicle-controlled clinical trial (NCT05675501), E-BPO significantly reduced the relative abundance of S. epidermidis and increased C. acnes, indicating a positive impact on the skin microbiome.16 This report further explores the efficacy of E-BPO in treating patients with rosacea and its effects on the skin microbiome in this same clinical trial, focusing on the correlation between clinical improvements and shifts in microbiome diversity.
Methods
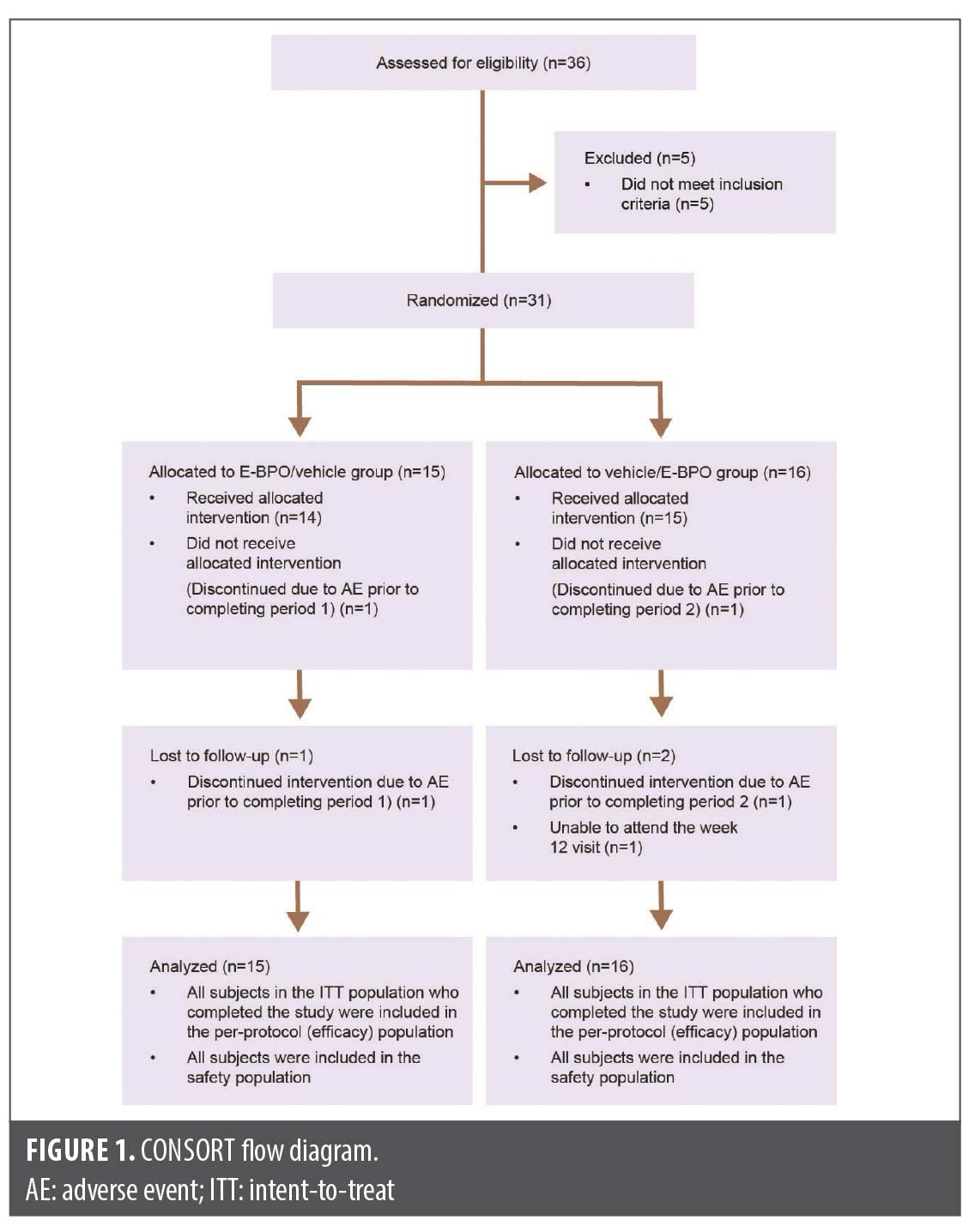
This was an eight-week, randomized, double-blind, crossover, vehicle-controlled trial conducted between February 2020 and July 2021 (Figure 1).16 The study included 31 participants with moderate to severe facial rosacea who were randomized 1:1 to receive either E-BPO for the first eight weeks followed by vehicle for four weeks, or vehicle for the first eight weeks followed by E-BPO for four weeks. Both groups underwent a four-week treatment withdrawal and observation period. The study was approved by an institutional review board and conducted in compliance with ethical guidelines, with all participants providing written informed consent.
Eligible participants were required to have facial rosacea with a global severity score of 3 (moderate) or 4 (severe) on the Investigator’s Global Assessment (IGA) scale. The study aimed to enroll at least 30 participants aged 18 years and older, allowing for a 10-percent dropout rate to ensure at least 24 participants completed the study. Exploratory efficacy endpoints included correlations between IGA, inflammatory rosacea scale, and erythema scale with shifts in microbiome diversity and individual bacteria.
Clinical assessments were conducted at baseline and at Weeks 1, 2, 4, 8, 12, and 16. IGA was scored on a five-point scale from 0 (clear) to 4 (severe), while inflammatory rosacea and erythema were graded on four-point scales from 0 (none) to 3 (severe). Erythema was graded on a four-point scale ranging from 0 (none) to 3 (severe).
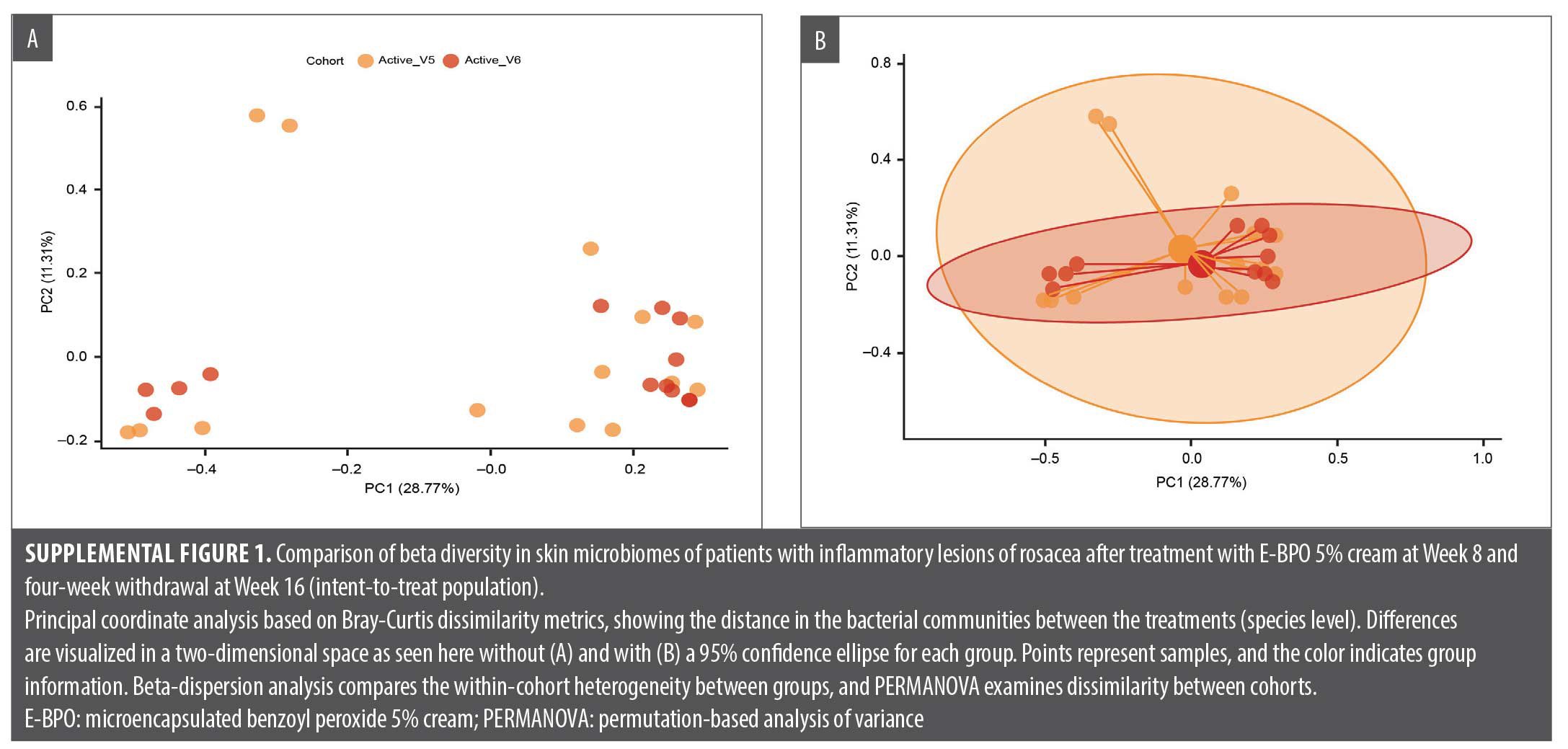
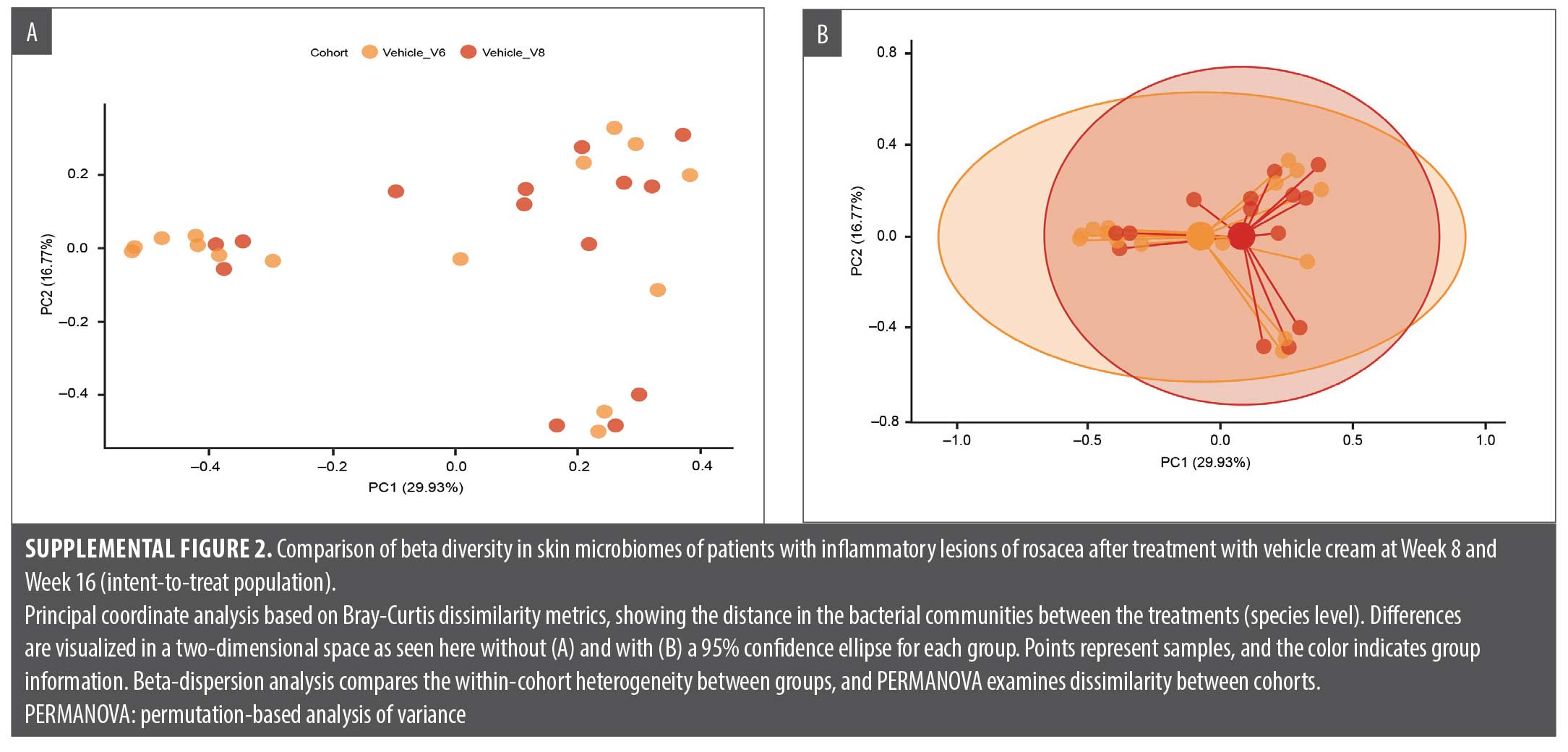
Correlation of efficacy endpoints with microbiome diversity analysis included relative abundance at the bacterial genus and species levels, alpha diversity box plots, and beta diversity principal coordinates analysis (PCoA). Linear discriminant analysis of effect size to identify bacterial MetaCyc metabolic pathways enriched in different treatment groups was calculated. Changes in skin microbiome diversity were assessed compared with baseline both after the second treatment period (Week 12) and after treatment withdrawal and a follow-up period of four weeks (Week 16).
Safety was assessed through adverse event (AE) reporting, including local and systemic events; the investigator’s cutaneous safety assessment ratings (dryness and scaling); the participants’ local tolerability assessment ratings (itching and burning/stinging) on a four-point scale ranging from 0 (none) to 3 (severe); physical examinations; and vital signs. Results of the safety analysis were reported previously.16
Results
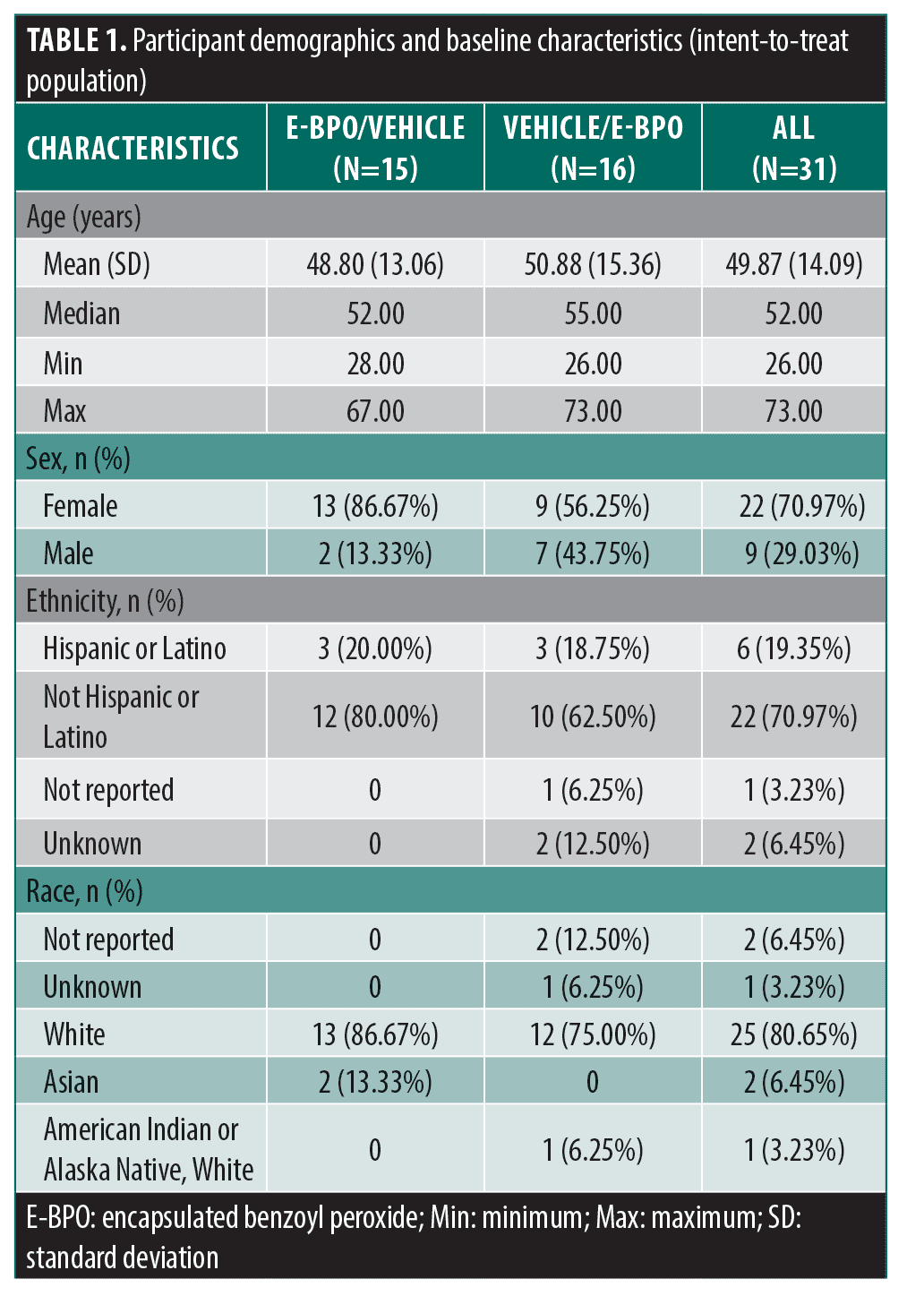
Participants. A total of 31 participants were randomized, with 15 subjects in the E-BPO/vehicle group and 16 in the vehicle/ E-BPO group (Table 1). Demographics were comparable between the two groups. Investigators examined the impact of E-BPO in the vehicle/E-BPO group and the durability of effect after the participants stopped treatment.
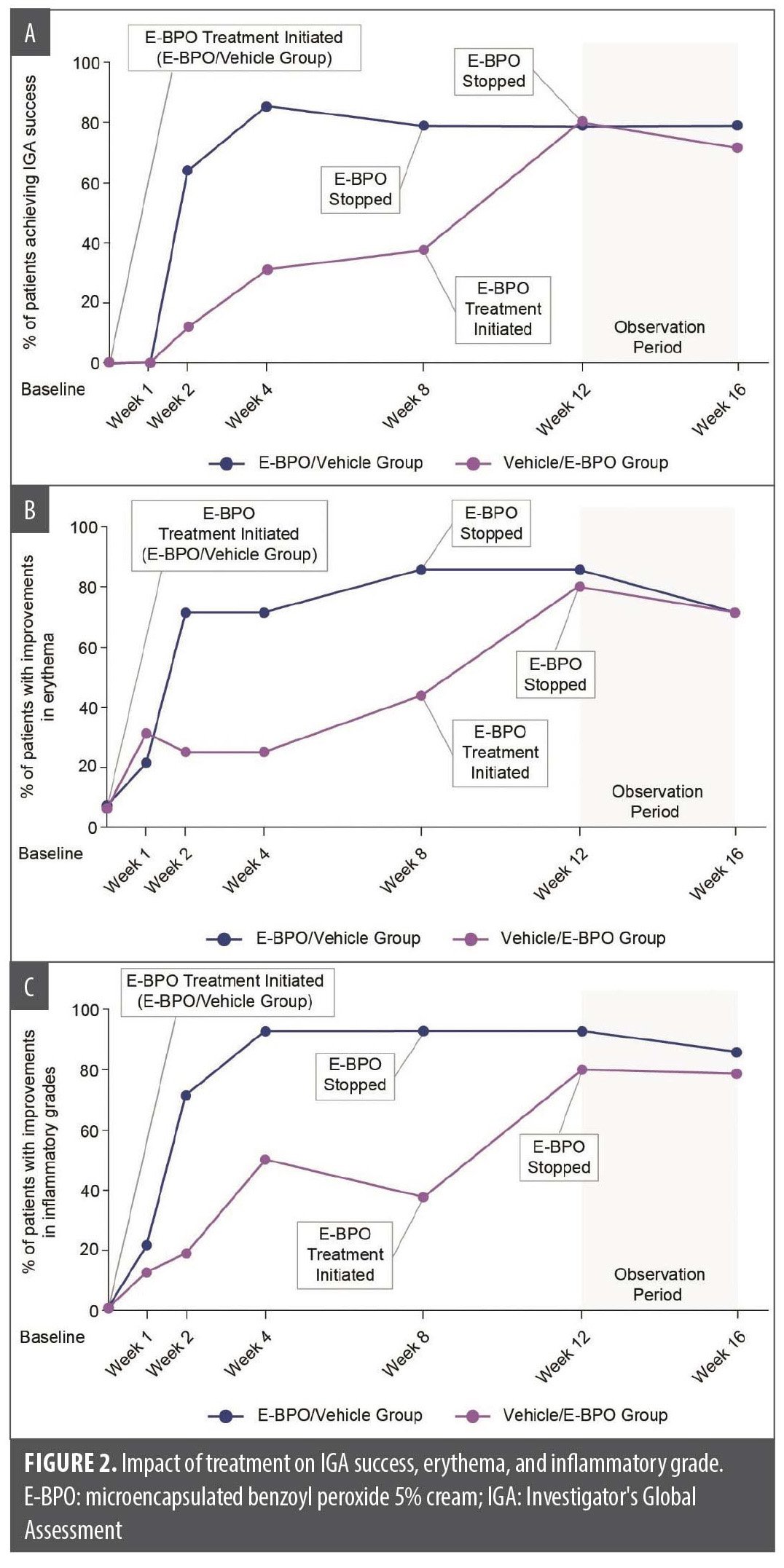
Efficacy and skin microbiome. IGA results indicated that a higher percentage of participants achieved “clear” or “almost clear” scores with E-BPO compared with vehicle at each postbaseline visit in Period 1 (Figure 2A). After switching treatments, the percentage of participants with IGA success remained high in both groups. Facial erythema (Figure 2B) and inflammatory grades (Figure 2C) also improved more significantly with E-BPO compared with vehicle from baseline to Week 16. These improvements were maintained during the follow-up period, indicating the durability of E-BPO.
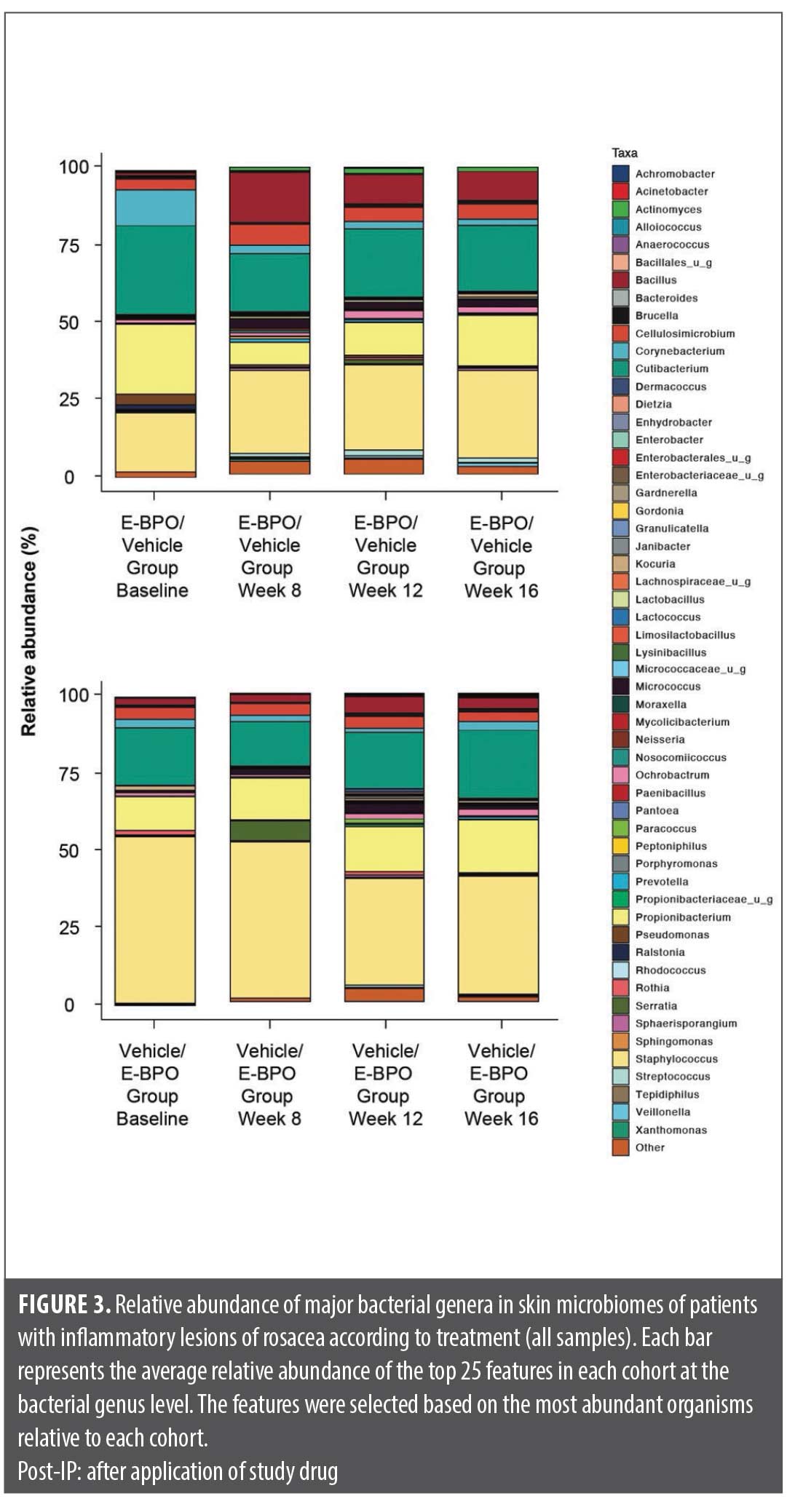
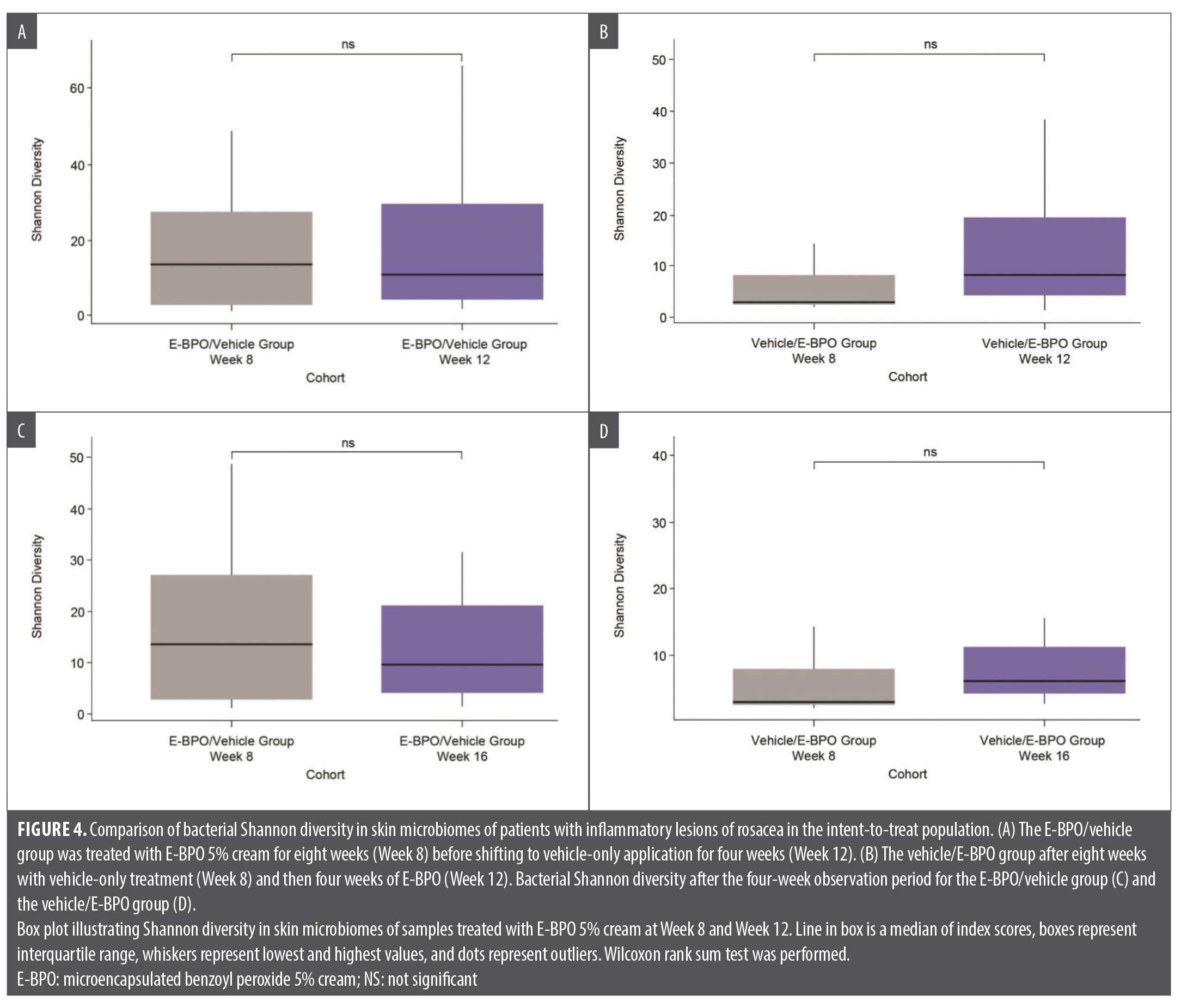
Exploratory analyses showed that the most noticeable changes in skin microbiome diversity occurred in the vehicle/E-BPO group. After participants crossed over from eight weeks of treatment with vehicle to treatment with E-BPO, the relative abundance of S. epidermidis markedly decreased, and the relative abundance of C. acnes slightly increased (Figure 3). Microbiome diversity analyses, including Shannon diversity and PCoA, did not reveal significant differences within the E-BPO/vehicle group at Week 8 or Week 12 (Figure 4A). However, an increase in diversity was observed in the vehicle/E-BPO group after switching to E-BPO, although it was not statistically significant (Figure 4B). The same analysis showed no significant difference between Week 8 and Week 16 within the E-BPO/vehicle group (Figure 4C) or the vehicle/E-BPO group (Figure 4D).
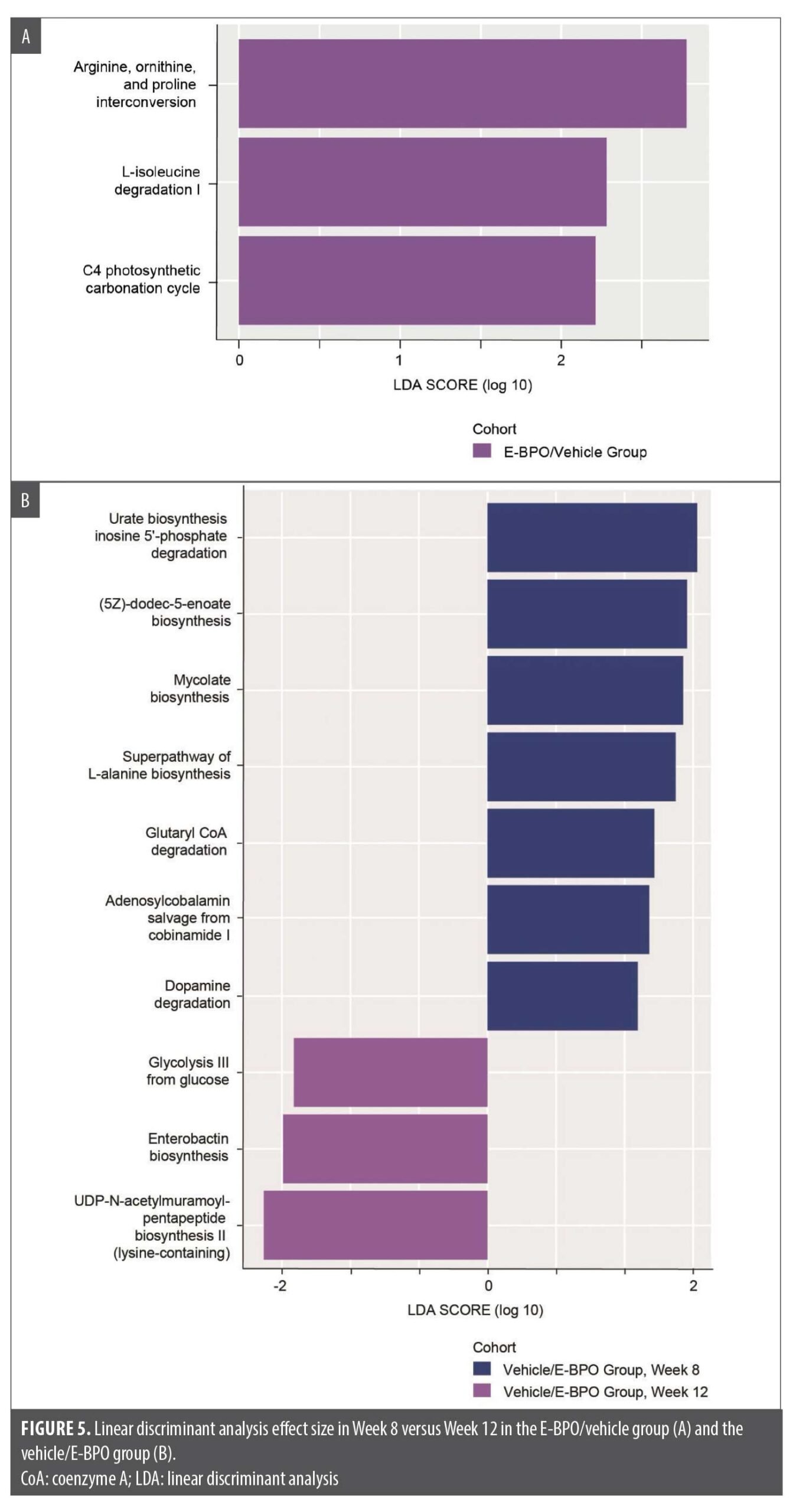
Functional gene presence analysis. At Week 12 compared with Week 8, after four weeks of E-BPO treatment, the E-BPO/vehicle group showed microbes expressing genes of the isoleucine degradation pathway after treatment (Figure 5).
Safety. Safety and tolerability were similar to that reported in the Phase III studies, in which five participants (0.9%) discontinued the study drug due to treatment-related AEs, and 17 participants (3.2%) experienced an AE considered to be related to the study drug. IGA success after 40 weeks of active treatment was 66.5 percent for participants continuing from the Phase III vehicle group (n=172) and 67.6 percent for those who continued Phase III E-BPO (n=363). The study ended in accordance with the protocol.
Discussion
In the analyses of correlation of clinical improvements (IGA, inflammatory rosacea, and erythema) with shifts in the microbiome, the vehicle/E-BPO group had the most noticeable changes in skin microbiome diversity in Week 12 compared with Week 8. The presence of S. epidermidis is thought to contribute to the pathogenesis of rosacea, whereas the loss of C. acnes is associated with rosacea.3–5,17 Following the crossover from eight weeks’ treatment with vehicle to E-BPO, a marked decrease in the relative abundance of S. epidermidis and a slight increase in the relative abundance of C. acnes were observed. Notably, our results suggest that a persistence in the shift of both S. epidermidis and C. acnes abundances exists even after E-BPO withdrawal, which may reflect a potential mechanism for the medication’s durability after withdrawal.
The results of the functional prediction analysis in this study revealed metabolic pathways that were significantly enriched in response to E-BPO, which is consistent with recent research that supports the correlation between circulating amino acids and the presence of rosacea.18 In particular, the E-BPO/vehicle group showed that microbes had a greater presence of genes of the isoleucine degradation pathway after treatment at Week 12 than at Week 8. A study by Liu et al18 analyzed serum amino acid profiles in rosacea patients versus controls and found significant differences. The authors proposed that dysregulated amino acid metabolism may promote neurovascular reactivity in the development of rosacea. These findings offer further insight that the local microbiota may be altering the local amino acid composition of the skin and support a potential systemic metabolic component in rosacea pathogenesis mediated through amino acid signaling pathways. The skin microbiome likely contributes by altering the local amino acid milieu. Further research on these mechanisms may identify novel targets for the treatment of rosacea.
This study did have limitations, including its short duration, which may not fully capture the long-term effects and durability of E-BPO in real-world clinical practice. The chronic and often relapsing nature of rosacea necessitates longer studies to better understand the sustained impact of E-BPO on the skin microbiome and clinical outcomes. Additionally, the study design may not reflect typical patient adherence and treatment patterns outside of a controlled trial setting.
Conclusion
The data from the exploratory endpoints presented in this study show that shifts in the microbiome associated with E-BPO treatment are correlated to improvements in IGA, inflammatory rosacea, and erythema, and the durability of these results continues after treatment has ended.
References
- Harper J, Del Rosso JQ, Ferrusi IL. Cross-sectional survey of the burden of illness of rosacea by erythema severity. J Drugs Dermatol. 2018;17(2):150–158.
- Daou H, Paradiso M, Hennessy K, Seminario-Vidal L. Rosacea and the microbiome: a systematic review. Dermatol Ther (Heidelb). 2021;11(1):1–12.
- Holmes AD. Potential role of microorganisms in the pathogenesis of rosacea. J Am Acad Dermatol. 2013;69(6):1025–1032.
- Whitfeld M, Gunasingam N, Leow LJ, et al. Staphylococcus epidermidis: a possible role in the pustules of rosacea. J Am Acad Dermatol. 2011;64(1):49–52.
- Woo YR, Lee SH, Cho SH, et al. Characterization and analysis of the skin microbiota in rosacea: impact of systemic antibiotics. J Clin Med. 2020;9(1):185.
- Rainer BM, Thompson KG, Antonescu C, et al. Characterization and analysis of the skin microbiota in rosacea: a case-control study. Am J Clin Dermatol. 2020;21(1):139–147.
- Medgyesi B, Dajnoki Z, Béke G, et al. Rosacea is characterized by a profoundly diminished skin barrier. J Invest Dermatol. 2020;140(10):1938–1950.
- Lacey N, Russell-Hallinan A, Zouboulis CC, Powell FC. Demodex mites modulate sebocyte immune reaction: possible role in the pathogenesis of rosacea. Br J Dermatol. 2018;179(2):420–430.
- Bucki R, Leszczyńska K, Namiot A, Sokołowski W. Cathelicidin LL-37: a multitask antimicrobial peptide. Arch Immunol Ther Exp (Warsz). 2010;58(1):15–25.
- Two AM, Wu W, Gallo RL, Hata TR. Rosacea: part I. Introduction, categorization, histology, pathogenesis, and risk factors. J Am Acad Dermatol. 2015;72(5):749–758.
- Lacey N, Delaney S, Kavanagh K, Powell FC. Mite-related bacterial antigens stimulate inflammatory cells in rosacea. Br J Dermatol. 2007;157(3):474–481.
- Desai SR, Baldwin H, Del Rosso JQ, et al. Microencapsulated benzoyl peroxide for rosacea in context: a review of the current treatment landscape. Drugs. 2024;84(3):275–284.
- Montes LF, Cordero AA, Kriner J, et al. Topical treatment of acne rosacea with benzoyl peroxide acetone gel. Cutis. 1983;32(2):185–190.
- Green LJ, Lain E, Prunty T, Rhoades R. Enhancing topical pharmacotherapy for acne and rosacea: vehicle choices and outcomes. J Clin Aesthet Dermatol. 2022;15(5):36–40.
- Bhatia N, Lain E, Baldwin H, et al. Encapsulated benzoyl peroxide (E-BPO): a novel formulation of BPO for long-term management of rosacea. SKIN. 2022;6(2);s14.
- Nong Y, Sugarman J, York JP, et al. Effect of topical microencapsulated benzoyl peroxide on the skin microbiome in rosacea: a randomized, double-blind, crossover, vehicle-controlled clinical trial. J Clin Aesthet Dermatol. 2024;17(8):19–26.
- Wang R, Farhat M, Na J, et al. Bacterial and fungal microbiome characterization in patients with rosacea and healthy controls. Br J Dermatol. 2020;183(6):1112–1114.
- Liu T, Xiao W, Chen M, et al. Aberrant amino acid metabolism promotes neurovascular reactivity in rosacea. JCI Insight. 2022;7(22):e161870.