 by Azahara Pérez Davó, PhD, MSc; Maria Teresa Truchuelo, MD, PhD; Maria Vitale, MD; and J. Gonzalez-Castro, MD
by Azahara Pérez Davó, PhD, MSc; Maria Teresa Truchuelo, MD, PhD; Maria Vitale, MD; and J. Gonzalez-Castro, MD
Dr. Perez-Davó is with the Medical Affairs Department at Cantabria Labs in Madrid, Spain. Dr. Truchuelo is with the Vithas Nuestra Señora de América Hospital in Madrid, Spain. Dr. Vitale is with the Medical Department of Cantabria Labs in Madrid, Spain. Dr. Gonzalez-Castro is with IDERMA-Quirón Dexeus Hospital, Methodex, in Barcelona, Spain.
ABSTRACT: Background. Effects of environmental contaminents, such as air pollution and cigarette smoking on skin include increased oxidation, subclinical inflammation, and degradation of the dermal matrix, which can accelerate the skin aging process.
Objective. An open-label, prospective study was conducted to assess the efficacy and tolerability of a topical anti-aging regimen comprising high-concentration retinoids, Deschampsia antarctica extract, and niacinamide in participants living in a heavily polluted (Level III, World Health Organization) city.
Methods. Twenty-two female Caucasian volunteers with Fitzpatrick Skin Types III and IV were treated for 90 days with the topical anti-aging regimen. Subjective clinical assessments using the Rao-Goldman Scoring for Facial Aging, Patient’s Global Assessment (PGA), and Investigator’s Global Assessment (IGA). Additionally, objective instrumental assessments for wrinkles using Visia® (Canfield Scientific, Parsippany, New Jersey) and Visioline® (Courage+Khazaka Electronic GmbH, Cologne, Germany) and viscoelasticity and firmness using Cutometer® (Courage+Khazaka Electronic GmbH ) were completed at baseline, Day 30, and Day 90.
Results. At Day 30, wrinkles in the periocular area significantly improved by 35.7 percent (p=0.003) compared to baseline. At the end of the study (Day 90), a significant improvement in firmness (41.7%) and viscoelasticity (12.8%) were observed. Tolerance for treatment was assessed as “good “ or “very good” in 86.5 percent of the volunteers.
Conclusion. This novel antiaging treatment regimen could potentially serve as an effective and long-term topical treatment option for improving signs of facial aging and protecting the skin from external factors associated with acceleration of the skin aging process, such exposure to UV radiation, air pollution, and cigarette smoke. Larger and longer-term, randomized, controlled clinical trials in more diverse population samples are needed to confirm our results.
KEYWORDS: Antiaging, topical, cosmetic formula, photoaging, pollution, retinol
J Clin Aesthet Dermatol. 2019;12(7):E65–E70
The aging process of human skin is attributed to a variety of internal and external factors.1 Internal, or intrinsic, aging, also called chronoaging, is associated with genetic and hormonal factors. External, or extrinsic, aging is associated with environmental and lifefstyle factors, such as exposure to solar radiation (i.e., ultraviolet [UV] light, visible light, and infrared light), air pollution, and cigarette smoking, as well as stress, poor nutrition, and lack of sleep.1 The clinical signs of aging skin associated with exposure to environmental contaminants include pigmentation changes, telangiectasias, corneal thickness, deep wrinkles, and actinic keratosis, as well as precancerous and cancerous skin lesions.3,4 Intrinsic factors manifest as an accumulation of reactive oxygen species (ROS) and telomere shortening, which result in the formation of small rhytids and dermal atrophy with a loss of skin elasticity.5 The effects of pollution on human skin are being studied in greater detail so that we may better understand its role in the skin aging process and develop more effective preventative measures to reduce its impact on the skin.2
Accumulated damage to the skin from external factors includes a progressive reduction in the skin’s ability to retain water, an increase in the production of ROS, and a loss of structural molecules.4 The production of ROS activates matrix metalloproteinases (MMPs), which, in turn, inhibit collagen synthesis.3 These molecular mechanisms cause inflammation and oxidative stress, which decrease mechanical, protective, and reparative properties of the skin, ultimately accelerating its aging process.5 The aryl hydrocarbon receptor (AhR), which acts as a sensor against environmental contaminants, plays a key role in skin integrity (i.e., barrier function), regulation of pigmentation, response to environmental factors, and skin immunity. The AhR is a transcription factor present in many cell types that regulates gene expression.3 Following ligand binding, AhR translocates to the nucleus and dimerizes with the aryl hydrocarbon receptor nuclear translocator. This enables the complex AhR ligand to bind to deoxyribonucleic acid (DNA) recognition sequences, called dioxin-responsive elements, and eventually initiate gene transcription.3
The stratum corneum is essential to maintaining the barrier function of the skin and protecting the body from external contaminents and invaders, such as UV rays, pollution, viruses, and bacteria. Loricrin, a protein that contributes to the stratum corneum’s protective functioning can be negatively impacted by chronic exposure to air pollution and/or cigarette smoke, reducing the skin’s potective capability.4
According to the World Health Organization’s (WHO) air quality model, over 90 percent of the world’s population resides in areas where air quality levels exceed the organization’s “Ambient Air Quality Guidelines” for annual mean of particulate matter (PM) with a diameter of less than 2.5 micrometres (PM2.5). PM2.5 includes sulfate, nitrates, and black carbon, which can penetrate deep into the lungs and the cardiovascular system.6 Levels of PM2.5 and other air pollutants, such as nitric oxide, ozone, carbon monoxide, and sulfur dioxides, are heavily present in many parts of the world, and can lead to significant health problems.6 Additionally, exposure to indoor contaminants caused by cooking with unclean fuels or inefficient technologies (especially in India and China), burning firewood in confined spaces, and tobacco smoke can also negatively impact the skin, lungs, and cardiovascular system. The pollution problem is growing worldwide, and the number of cities affected is increasing.7,8
A new topical antiaging treatment regimen (Cantabria Labs, Madrid, Spain) combining two patented technologies (RetinSphere Technology® [RST] and EDAFENCE® [EDAT]) and niacinamide has recently been developed to improve the appearance of facial photoaging and reduce the aging effects that long-term exposure to certain environmental hazards can have on human skin.
RST incorporates two topical retinoids: retinol and hydroxypinacolone retinoate. Retinol is known for its instability; thus, in this study, the treatment regimen utilizes a novel delivery system that is designed to improve the tolerance, penetration, and stability of retinol. The hydroxypinacolone retinoate is a retinoic acid ester that binds directly to the retinoic acid receptor, but does not cause the irritation normally observed with retinoic acid.9,10
EDAT comprises an aqueous extract of Deschampsia antarctica (EDA), a polyextremophile plant from Antarctica that is able to tolerate high levels of solar irradiation, salinity, and oxygen, as well as low temperatures and extremely dry environments. EDA has demonstrated positive effects on the premature senescence of skin caused by exposure to H2O2, environmental pollutants (e.g., cadmium, chrome, and arsenic), and tobacco smoke.11 Excessive activation of AhR is produced, at least partially, by the action of dioxin, which causes oxidative stress and consequent DNA damage; EDAT has demonstrated antagonist effects of this receptor on keratinocytes, suggesting its role as a protective agent.12 EDAT has also been linked with the expression of loricrin, a protein that contributes to the protective function of the stratum corneum.12
Nicotinamide is a water-soluble amide isotype of vitamin B3; niacin (nicotinic acid) is the corresponding acid isotype. Nicotinamide has been shown to improve the appearance of wrinkles and lentigines and to increase elasticity in the skin.13 Niacinamide is characterized by its lightening effect due to its ability to block, in a reversible manner, the transfer of melanosomes from melanocytes into keratinocytes.14
The antiaging treatment regimen used in this study comprises two formulations that utilize the RST and EDAT technologies: one formulation contains a high concentration of retinoids (high-concentration formulation) and is designed to treat photoaging, while the other formulation contains a high concentration of moisturizing and soothing ingredients (transition cream) and is designed to help the skin gradually adapt to the high-concentration formula. These two products were developed as anhydrous, semiocclusive formulations to increase the degree of emolientia and hydration and facilitate the diffusion of the active ingredients in the stratum corneum.15
The primary objective of this study was to evaluate the effectiveness of the new antiaging regimen in improving facial photodamage among Caucasian women residing in a region known for its high levels of air pollutants. A secondary objective was to determine the acceptability, cutaneous tolerance, and safety of the regimen when applied under normal conditions for 90 days in the study participants.
Methods
This was an open-label, prospective study designed to assess the efficacy and tolerability of a topical antiaging regimen in female volunteers living in Barcelona, Spain. Barcelona is considered one of the most polluted cities in Europe, currently exceeding the levels of contamination accepted by WHO and those established by the European Union (Level III).8 This is partly attributed to its geography, high traffic density (four times higher than London), and large proportion of diesel-powered vehicles.8
Inclusion criteria for participants were being female, Caucasian with Fitzpatrick’s Skin Types III and IV, aged 45 to 65 years, and demonstrating signs of facial photoaging based on the clincal evaluation of a qualified dermatologist. Exclusion criteria were presence of allergy or hypersensitivity to any of the ingredients in the treatment regimen, current use of any topical or systemic treatment, presence of a chronic or acute illness, and/or pregnancy or breastfeeding. Twenty-two volunteers met the inclusion criteria and were recruited for the study. All participants provided signed informed consent to participate in the study. This study was conducted in ethical compliance of the principles set forth by the Declaration of Helsinki.
Participants were instructed to apply a high-concentration formulation (retinol 0.5%, EDA extract 0.5%, and niacinamide 4%) and a transition cream (retinol 0.02%, EDA extract 0.8%, and niacinamide 3%) to their face, using a specific treatment protocol, for three months (90 days, 12 weeks). The treatment protocol also included the application of a sunscreen every morning (Heliocare 360 fluid cream SPF50, Ferndale Healthcare, Detroit, Michigan, USA).
Instructions for the treatment regimen are shown in Table 1. Every night, subjects applied the designated product to clean, dry facial skin (i.e., forehead, cheeks, nasal areas, and chin). It was necessary to progressively prepare the skin for the introduction of the high-concentration formulation in the following manner:
- Weeks 1 and 2: High-concentration formulation on the first day and transition cream on the following two days, with the sequence repeated until the end of two weeks
- Weeks 3 and 4: High-concentration formulation applied every other day, alternating with the transition cream, repeated until the end of two weeks
- Weeks 5 to 12: High-concentration formulation applied for two days, followed by transition cream for one day, with the sequence repeated until the end of eight weeks
Clinical assessment. The observation period was 90 days (12 weeks), and the subjects were assessed at three timepoints: baseline (T1), Day 30 (T2 [Week 4]) and Day 90 (T3 [Week 12]). Participants were queried at each visit regarding adherence to the treatment protocol.
Medical and dermatological histories for each participant were recorded. Instrumental assessments for wrinkles were measured using Visia® (Canfield Scientific, Parsippany, New Jersey) and Visioline® (Courage+Khazaka Electronic GmbH, Cologne, Germany), and viscoelasticity and firmness were measured using and Cutometer® (Courage+Khazaka Electronic GmbH ). Clinical assessments were measured using the Rao-Goldman Scoring for Facial Aging, Patient’s Global Assessment scale (PGA), and Investigator’s Global Assessment scale (IGA).16a,16b Assessments were performed on the faces of the participants according to the randomization table.
The Rao-Goldman Scoring for Facial Aging is a five-point (1–5) scale assessment that was performed by a single investigator (JGC) to evaluate wrinkles (1=none, 2=shallow but visible, 3=moderately deep, 4=deep, with well-defined edges, and 5=very deep with redundant folds). The IGA and PGA scales were used to measure the perceived overall degree of aging (i.e., wrinkles, spots, loss of elasticity) by the investigator (JGC) and patients, respectively; possible scores ranged from -2 to 3 points, with -2=significant worsening, -1=worsening, 0=none, 1=slight improvement, 2=moderate improvement, and 3=intense improvement. In these subjective questionnaires, aging characteristics were considered “improved” if scores increased by at least 2 points.
The evaluated objective parameters were changes in wrinkles as measured by Visia and Visioline on one half of the face (right or left, according to randomization) and changes in elasticity and firmness as measured by Cutometer in the eye contour area previously delimited at the application of the product area (according to randomization). Visia photographed wrinkles using standard illumination, with specific software assessing dark and light green lines, which represent the most pronounced wrinkles and the finest ones, respectively. Wrinkles were analyzed at T1, T2, and T3 (Baseline, Week 4, and Week 12). Visioline measurements were performed using silicone replicas of the skin surface, as follows: the replica of the skin was illuminated with an oblique light, and the peaks of the replica (representing the wrinkles of the skin) projected shadows that could be measured by a high-resolution camera. Area data (mm2) and number of wrinkles were obtained. These parameters were analyzed at T1, T2, and T3 (Baseline, Week 4, and Week 12). Cutometer recorded two parameters: R0 (firmness) and R6 (elasticity). The measurements were achieved by suction and skin elongation. These parameters were analyzed at T1, T2, and T3 (Baseline, Week 4, and Week 12), and measures were performed after a rest period of 30 minutes in an acclimatized room with temperature and relative humidity (RH) being regulated and controlled (temperature=21±1°C and RH=45±5%).
Participants completed a questionnaire regarding tolerance of the treatment regimen at all three timepoints (T1, T2, and T3); however, acceptability and future use of the product were assessed only at the T3 visit. Patients were asked to report any adverse effects at all three timepoints.
Statistical analysis. The analysis of the data was carried out with the R statistical software program (R Foundation for Statistical Computing, Vienna, Austria). A descriptive analysis of the frequencies of reactions considered as undesirable effects related to investigational product application in the participants and another of frequency of reactions observed by the subjects were performed. To test the differences of the measurements for the different timepoints (i.e., T1, T2, and T3), a test of repeated measures was used in the time factor because each volunteer was evaluated for each of the three times. The appropriate test for this study was a test of repeated measures, with one factor being the Friedman test. To analyze changes in the PGA and IGA scores, a Wilcoxon nonparametric rank test was employed to determine whether the distribution had separated from the median 0. The p-value of the test contrasted if the previous proportion of positive evaluations was higher than 50 percent and consequently higher than the proportion of negative evaluations. This p-value indicated the probability of being wrong when affirming the positive evaluations would be higher than the negative ones, extrapolating to the whole population (independently of those obtained in this sample). The significance indicated the affirmation regarding superiority of the positive evaluations was significant under five percent and indicated that the significance was under one percent.
Results
Twenty-two volunteers 45 to 65 years of age (mean±standard deviation [SD]: 50.45±5.38 years) with Fitzpatrick Skin Types III and IV were enrolled. During the study, two subjects dropped out—one due to inability to attend follow-up visits and the other due to adverse events (mild cutaneous reaction characterized by pruritus, erythema, and flaking of moderate intensity). The remaining 20 volunteers did not report any adverse side effects and were included in the final efficacy data analysis.
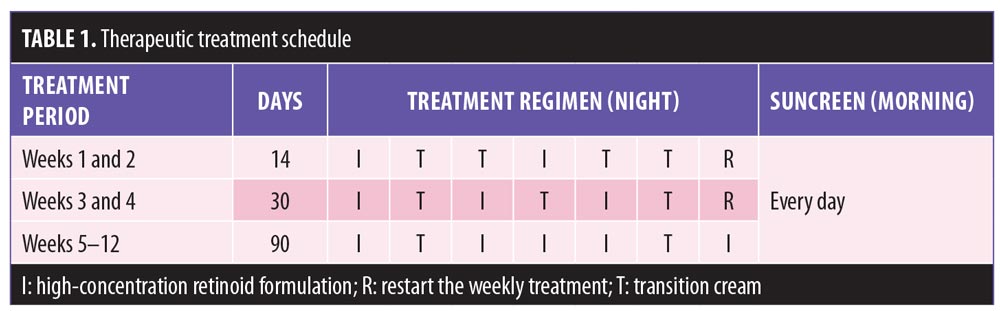
The periocular wrinkle area was evaluated using Visioline. At the T2 visit (Week 4), the periocular wrinkle area decreased significantly by 35.7 percent (p=0.003), compared to baseline. After 90 days of treatment, this area increased by 10.5 percent (p=1.000). To subjectively assess wrinkles, two instruments were used, Visioline and Visia. Results of Visioline indicated a significant decrease of 29.9 percent (p=0.017) in the number of wrinkles after 30 days of treatment (T2, Week 4) (Figures 1 and 2). At 90 days (T3, Week 12) the total decrease in wrinkles, compared to baseline, was 10.7 percent (i.e., the number of wrinkles increased between T2 and T3) and statistically nonsignificant (p=0.399). Results of the Visia measurements showed a progressive reduction in the number of wrinkles, with a decrease of 0.8 percent (p=1.000) at T2 (Week 4) and 15 percent (p=0.010) at T3 (Week 12), compared to baseline. A more pronounced decrease in wrinkles was observed using the Visia instrument between T2 (Week 4) and T3 (Week 12), and the reduction from T1 (baseline) to T3 (Week 12) was statistically significant (Figure 1).
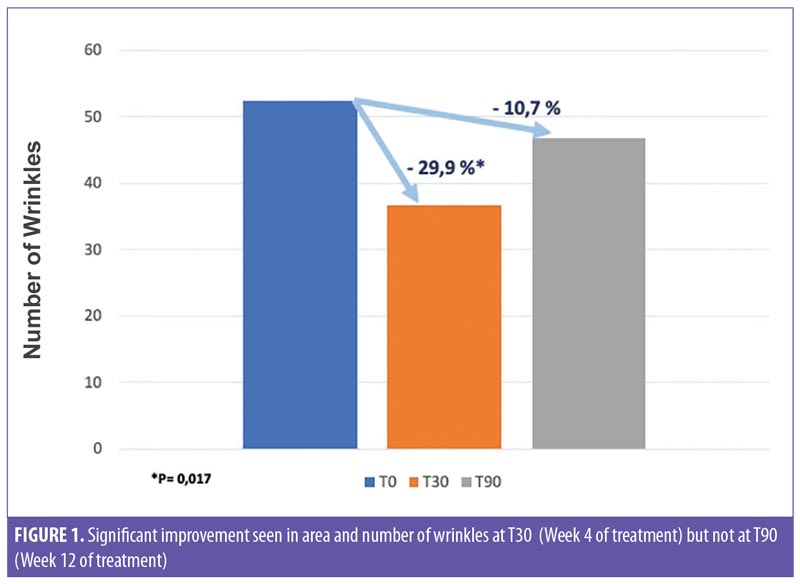

Firmness (R0) and elasticity (R6) parameters were evaluated by the Cutometer instrument. At T2 follow-up (Week 4), a 17.5-percent improvement in skin firmness was observed, compared to baseline, while the mean value of the viscoelasticity (R6 parameter) showed a statistically nonsignificant increase of 0.8 percent. At T3 follow-up (Week 12), a statistically significant improvement of 41.7 percent (p=0.002) was observed in firmness, compared to baseline, whereas viscoelasticity increased by 12.8 percent at T3 follow-up (Week 12) (Figure 3).
In regard to subjectively measured efficacy, an 8.8-percent improvement in the Rao-Goldman scale scores was observed at T2 (Week 4), and a trend of statistically significant improvement (17.5%, p=0.053) was reported at the end of the treatment period (T3, Week 12). Improvements in PGA and IGA scores were observed at T2 (Week 4) and T3 (Week 12). After 30 days of treatment (T2, Week 4), 15 percent of the participants indicated moderate improvement and five percent reported high improvement in overall appearance of aging using the PGA scale. After 90 days of treatment (T3, Week 12), 35 percent of the participants reported moderate improvement and 15 percent reported high improvement. Using the IGA scale, 15 percent of the participants were rated by the investigator as slightly improved and five percent were rated as moderately improved at T2 (Week 4) in overall appearance of aging, while 25 percent were rated as slightly improved and 10 percent were rated as moderately improved at T3 (Week 12).
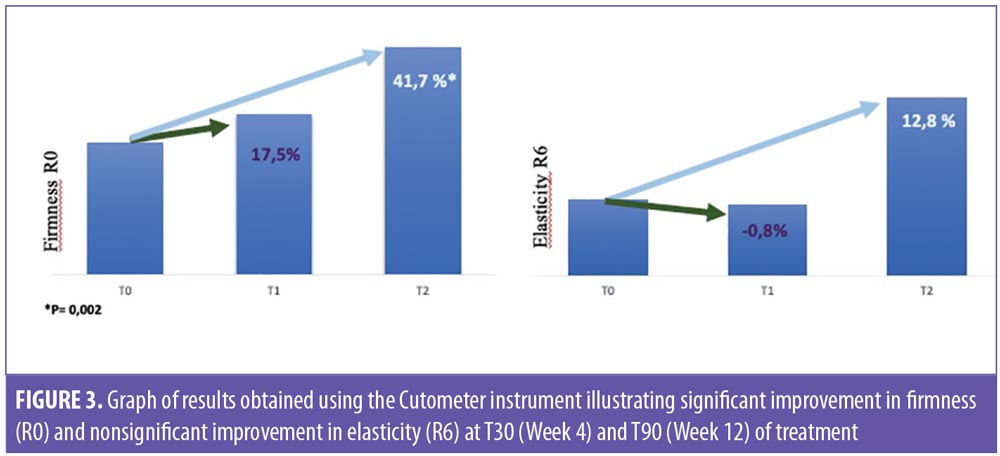
Tolerance for treatment was assessed as good or very good in 86.5 percent of the volunteers. One patient reported adverse events, specifically itching, dryness, and desquamation. Globally, the physical characteristics of the product were judged favorably by the participants in the study. All participants (100%) reported a statistically significant improvement in skin texture, with more brightness and more rejuvenated skin (p < 0.001). Likewise, 95 percent of the participants reported their facial skin felt more flexible and elastic (p=0.0001), and 65 percent indicated that the treatment provided moisturization to the skin (p=0.1316) (Figure 4).
Discussion
Numerous studies have confirmed the negative impact that certain environmental and lifestyle factors can have on skin barrier quality and appearance, including loss of firmness, wrinkle formation, hyperpigmentation, and skin cancer. Understanding how these factors interact with the skin may help scientists to develop effective and safe antiaging strategies for human skin.2
Several ingredients used in cosmetic products have demonstrated their ability to mitigate or correct signs of aging in the skin.2 In this study, we evaluated a topical antiaging treatment regimen that demonstrated improvements in several parameters associated with aging skin. Based on our results, we believe this topical regimen might serve as an effective treatment option for improving the appearance of aging skin, as well as potentially be used as preventative agent against certain environmental and lifestyle factors known to accelerate the aging skin process, such as air pollution, sun exposure, and cigarette smoke.
The first pillar of prevention using this treatment regimen is blocking the damage produced by environmental factors. The extract of EDA has demonstrated high antagonist activity against AhR, to modulate melanogenesis, epidermal barrier function, photoinduced biological responses, and innate immunity. EDA technology used in the study formulations has been shown to increase loricrin, which can promote an optimal cutaneous barrier in the skin against environmental pollution and tobacco smoke. The second pillar, improving the characteristics of aging skin, is attributed to the novel retinoid technology (RST), which combines retinol in an optimal delivery system and hydroxypinacolone retinoate.
In our study, the observed improvement in facial skin elasticity and firmness suggest the study regimen demonstrates action on dermal collagen and elastic fibers. This is in accordance with the well-known capability of retinoids to increase collagen content in the upper papillary dermis by inhibiting collagen degradation and increasing collagen synthesis.16 Topical administration of retinoids in the study regimen resulted in an improvement in several objective facial skin parameters, including a significant improvement in elasticity (41.7%, p=0.002) and a decrease in wrinkles (15%, p=0.010) after 90 days treatment, compared to baseline. Increased skin smoothness and reduced wrinkles seen with topical retinoid treatment is the result of epidermal hyperplasia, compacting of the stratum corneum, thickening of the granular layer, and increased epidermal and dermal glycosaminoglycan deposits.16
Based on our own clinical experience, we believe the Visioline instrument is more sensitive than the Visia instrument in the detection of wrinkles. After 30 days of treatment, the Visioline identified significant improvement in wrinkles, whereas Visia detected nonsignificant improvement. However, after 90 days of treatment, Visioline showed a nonsignificant increase in the number of wrinkles and wrinkle area, compared to that seen with Visia. We theorize that skin dryness might have contributed to the detection of an increased wrinkle recount with the Visioline instrument at T2 (Week 4). In consideration of these results, moisturization should be incorporated into the treatment regimen to prevent this side effect of retinoids.
The observed functional and structural changes in our study were correlated with subjective improvements in skin texture, brightness, and overall rejuvenation indicated by 100 percent of study participant (p<0.001).
Discontinuation of retinoid therapy has resulted in significant reversal of observed antiaging effects in some studies;17 therefore, it is important that products containing retinoids be well-tolerated to allow continuous treatment. Almost 90 percent of our study participants showed good or very good tolerance. Although, a high concentration of retinol is present in the study regimen, we believe the encapsulated delivery system, along with the combination of niacinamide as an anti-inflammatory agent, contributed to the level of product tolerance observed in our study.10
Limitations. The small number of patients, lack of heterogeneity among participants, absence of control group, single-center study design, and the survey format used as one of the assessment methods all limit the results we observed during our study. Larger and longer-term, double-blind, placebo-controlled trials in more diverse population samples are needed to confirm our findings.
Conclusion
An antiaging treatment that blocks daily environmental contaminants, stimulates repair mechanisms, and is well-tolerated for long-term use is essential to maintaining healthier skin. The antiaging treatment regimen used in this study included a progressive transition cream that allowed the facial skin of the participants to better tolerate the intensive retinoid formulation. This antiaging treatment regimen not only demonstrated objective and subjective effectiveness in improving firmness and viscoelasticity and reducing wrinkles, but was also well tolerated for up to 90 days when applied to the facial skin of adult Caucasian women with Fitzpatrick Skin Types III and IV. Our results suggest that this novel antiaging treatment regimen could potentially serve as an effective and long-term topical treatment option for improving signs of facial aging and protecting the skin from external factors associated with acceleration of the skin aging process, such exposure to UV radiation, air pollution, and cigarette smoke.
References
- Krutmann J, Liu W, Li L, et al. Pollution and skin: from epidemiological and mechanistic studies to clinical implications. J Dermatol Sci. 2014;76(3):163–168.
- Krutmann J, Bouloc A, Sore G, et al. The skin aging exposome. J Dermatol Sci. 2017;85(3):152–161.
- Abbas S, Alam S, Singh KP, et al. Aryl hydrocarbon receptor activation contributes to benzanthrone-induced hyperpigmentation via modulation of melanogenic signaling pathways. Chem Res Toxicol. 2017;30(2):625–634.
- Kim KE, Cho D, Park HJ. Air pollution and skin diseases: Adverse effects of airborne particulate matter on various skin diseases. Life Sci. 2016;152:126–134.
- Tigges J, Krutmann J, Fritsche E, et al. The hallmarks of fibroblast ageing. Mech Ageing Dev. 2014;138:26–44.
- World Health Organization. Ambient air pollution: a global assessment of exposure and burden of disease. Available at: http://www.who.int/mediacentre/news/releases/2016/air-pollution-estimates/en.
- Burke KE. Mechanisms of aging and development. A new understanding of environmental damage to the skin and prevention with topical antioxidants. Mech Ageing Dev. 2018;172:123–130.
- Schembari A, Nieuwenhuijsen MJ, Salvador J, et al. Traffic-related air pollution and congenital anomalies in Barcelona. Environ Health Perspect. 2014;122(3):317–323.
- Cameli N, Mariano M, Abril E, Berardesca E. Clinical evaluation of the efficacy of a new retinoic-complex (Retinsphere®) product for the treatment of skin photoaging. Esp Derm. 2012;14(1):29–34.
- Truchuelo MT, Jiménez N, Jaén P. Assessment of the efficacy and tolerance of a new combination of retinoids and depigmenting agents in the treatment of melasma. J Cosmet Dermatol. 2014;13(4):261–268.
- Ortiz-Espín A, Morel E, Juarranz A, et al. An extract from the plant Deschampsia antarctica protects fibroblasts from senescence induced by hydrogen peroxide. Oxid Med Cell Longev. 2017;2017:2694945.
- Lucena S, Pérez-Davó A, Delgado P, et al. An aqueous extract of Deschampsia antarctica (EDA) exerts clear protective effects on human skin cell against dioxins treatments. J Investig Dermatol. 2018;138(9):B11.
- Forbat E, Al-Niaimi F, Ali FR. Use of nicotinamide in dermatology. Clin Exp Dermatol. 2017;42(2):137–144.
- Wohlrab J, Kreft D. Niacinamide—mechanisms of action and its topical use in dermatology. Skin Pharmacol Physiol. 2014;27(6):311–315.
- Tsunoda T, Takabayashi K. Stability of all-trans-retinol in cream. J Soc Cosmet Chem. 1995;46:191–198.
- 16aHsu J, Skover G, Goldman MP. Evaluating the efficacy in improving facial photodamage with a mixture of topical antioxidants. J Drugs Dermatol. 2007;6:1141–1148.
- 16b Schlager JG, Rosumeck S, Werner RN.Topical treatments for scalp psoriasis.Cochrane
- Database Syst Rev. 2016 Feb 26;2:CD009687. doi: 10.1002/14651858.CD009687.pub2. Review.
- 16Hubbard BA, Unger JG, Rohrich RJ. Reversal of skin aging with topical retinoids. Plast Reconstr Surg. 2014;133(4):481e–490e.
- 17Olsen EA, Katz HI, Levine N, et al. Sustained improvement in photodamaged skin with reduced tretinoin emollient cream treatment regimen: effect of once-weekly and three-times-weekly applications. J Am Acad Dermatol. 1997;37(2 Pt 1):227–230.

