 J Clin Aesthet Dermatol. 2022;15(5):29–34.
J Clin Aesthet Dermatol. 2022;15(5):29–34.
by Pooja Bains, MBBS, MD; Simplepreet Kaur, MBBS, MD; and Komalpreet Kaur, MBBS, MD
Dr. Bains is an associate professor with Department of Dermatology Venereology & Leprosy at Sri Guru Ram Das Institute of Medical Sciences & Research in Amritsar, India. Dr. S. Kaur is a consultant deramtologist with the Department of Dermatology Venereology & Leprosy the Government Multispeciality Hospital Sector in Chandigarh, India. Dr K. Kaur is a junior resident at the Department of Dermatology Venereology & Leprosy at Sri Guru Ram Das Institute of Medical Sciences & Research in Amritsar, India.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. Female androgenetic alopecia (FAGA) is a patterned hair loss caused by progressive miniaturization of hair follicles. This leads to reduction in the number and thickness of hairs, especially in the central, frontal, and parietal scalp regions. Telogen effluvium (TE) is characterized by diffuse hair loss within months of a significant systemic stressor because of premature follicular transition from the anagen to the telogen.
Objective. This article aims to highlight the dermoscopic differences between TE and FAGA compared to healthy female controls.
Methods. A total of 124 female patients, which included 31 women with clinical diagnosis of FAGA, 33 with TE, and 60 controls, were enrolled. Two dermatologists independently assessed each patient clinically as well as with dermoscope, recorded the history and examination findings on a proforma, and made a diagnosis. These dermoscopic images were later revised in photographs on the computer.
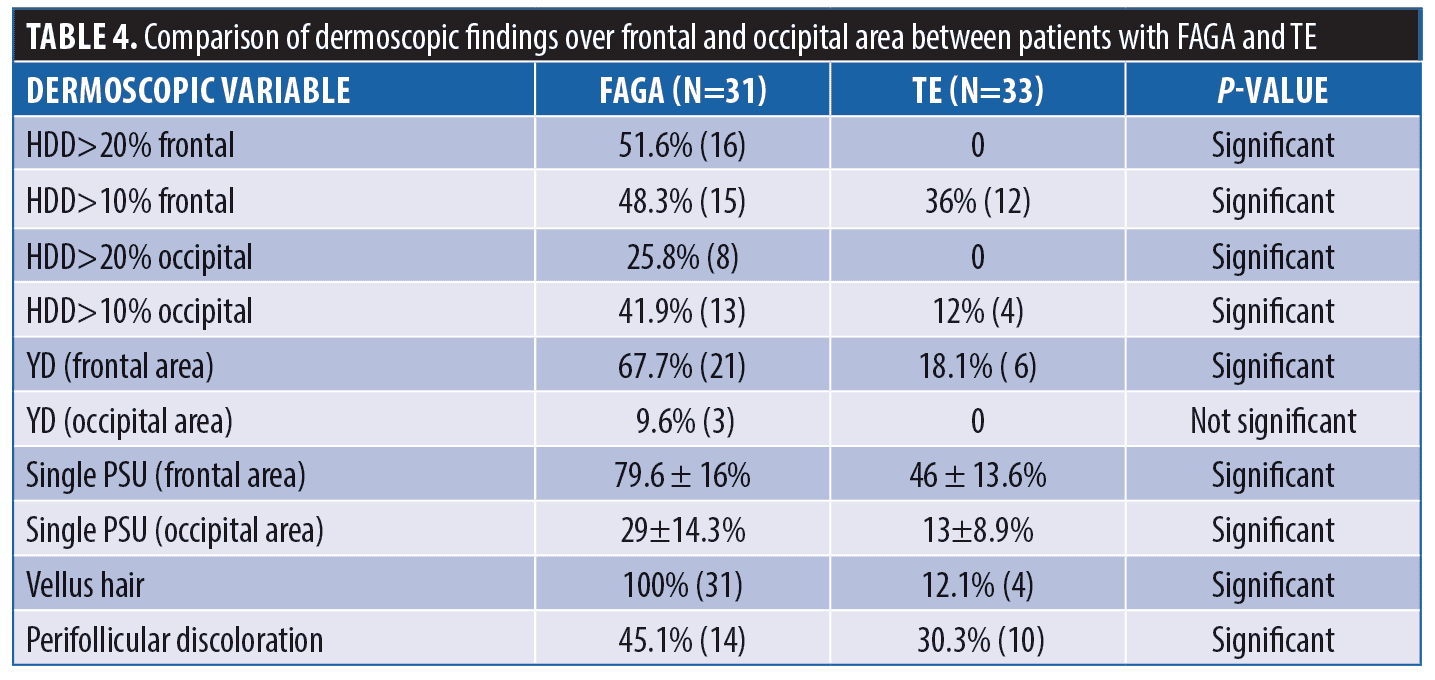
Results. There was a statistically significant difference in hair diameter diversity (HDD) between patients with FAGA versus TE and FAGA versus controls (p<0.0001). The difference in the mean percentage of single PSU in both frontal and occipital areas in FAGA versus controls and FAGA versus TE patients was statistically significant. The vellus hair were significantly higher in the FAGA patients than TE and control.
Conclusion. Dermoscopic features of FAGA and TE will help in early detection on the basis of increased proportion of thin and vellus hairs, HDD, perifollicular discoloration, and the presence of a variable number of yellow dots.
Keywords: Dermoscopy, female androgenetic alopecia, telogen effluvium, hair diameter diversity, vellus hair, perifollicular discoloration, yellow dots
Pattern hair loss in women is also referred to as female androgenetic alopecia (FAGA). It is caused by progressive miniaturization of hair follicles and subsequent reduction in the number of hairs, especially in the central, frontal, and parietal scalp regions.1 The diagnosis of FAGA is primarily clinical based on the patient‘s history and the pattern of hair loss.2 Telogen effluvium (TE) is another leading cause of female hair loss but it is a self-limiting process and almost never causes obvious baldness, whereas FAGA progresses in time, leading to a significant decrease in hair thickness.3 It is often difficult to distinguish FAGA from TE, especially in the early stages. Clinical data, laboratory findings, and biopsy may be needed for a definitive diagnosis.4 One of the most recent tools that helps differentiate FAGA from TE is dermoscopy of hair and scalp, a noninvasive tool that is increasingly used in dermatological practice. FAGA is characterized dermoscopically according to an increased proportion of thin and vellus hairs, hair diameter diversity, perifollicular discoloration, and the presence of a variable number of yellow dots.5 These signs are more conspicuous in the frontal area in comparison to the occipital area.2 The clinical diagnosis of FAGA augmented by the dermoscopic signs allows timely treatment before the occurrence of significant reduction in hair volume.2 This article aims to highlight the dermoscopic differences between TE, FAGA, and its comparison to healthy female controls, as there are very few studies on the comparative dermoscopic patterns of FAGA, TE, and healthy female controls.
Methods
The study was conducted after obtaining approval from the Institutional Ethics Committee. It was conducted on a total of 124 female patients attending the dermatology out-patient department of our institute between September 2019 to June 2020. This study included 31 females with clinical diagnosis of FAGA, 33 females with TE, and 60 controls. The exclusion criteria included pregnancy, hair loss treatment in the prior three months, and chronic debilitating diseases. Subjects with other scalp disorders like trichotillomania, alopecia areata, tinea capitis, seborrheic dermatitis or psoriasis were also excluded. Female patients coming for other dermatological conditions (excluding scalp), relatives accompanying the patients, hospital staff, and other healthy volunteers with normal hair cycle and in similar age group were included as controls.The controls were recruited during the same period of time and using same exclusion criteria. Screening hematologic investigations were performed to assess underlying thyroid disease, iron deficiency, or hormonal abnormality. Two dermatologists independently assessed each patient clinically as well as with dermoscope, recorded the history and examination findings on a proforma, and made a diagnosis.The dermoscopic assessment was made without knowledge of clinical severity and diagnosis. These dermoscopic images were later revised in photographs on the computer. A Dermlite DL3N dermoscope and a Cybershot Sony DSC-W210 12.1 megapixel camera were used with no conflict of interest. During this study, at least two dermoscopic images from the frontal and occipital scalp of females suffering from FAGA were analyzed along with corresponding areas in TE and healthy controls. Each case of FAGA was graded according to Sinclair scale into Sinclair 1, Sinclair 2, Sinclair 3, Sinclair 4 and Sinclair 5.6 All subjects were asked to shampoo their hair one day prior to the procedure and to avoid oiling or coloring their hair. The frontal and occipital scalp were examined in the midline (sagittal plane) approximately three centimeters above the hairline. Frontal scalp and occipital scalp of cases were compared with that of controls. Similarly, frontal area of cases was compared with the occipital area of their own.
Statistical analysis. The data were analyzed statistically using GraphPad Prism 7 statistical software (GraphPad Software Inc., La Jolla, California). Mean values with standard deviations (SD) were calculated for quantitative data, and nominal data were presented as percentages. Various clinical and dermoscopic parameters were analyzed statistically by using student t-test and McNemar’s test. A p-value of less than 0.05 was considered to be significant.
Results
Demographic data. The data obtained from all cases and controls were analyzed. The total number of FAGA cases were 31 with a mean age was 35 ± 11(20-64) years , 33 patients with TE with a mean age of 32 ±12 (18-60) years and 34± 12(18-65) years in 60 healthy controls.
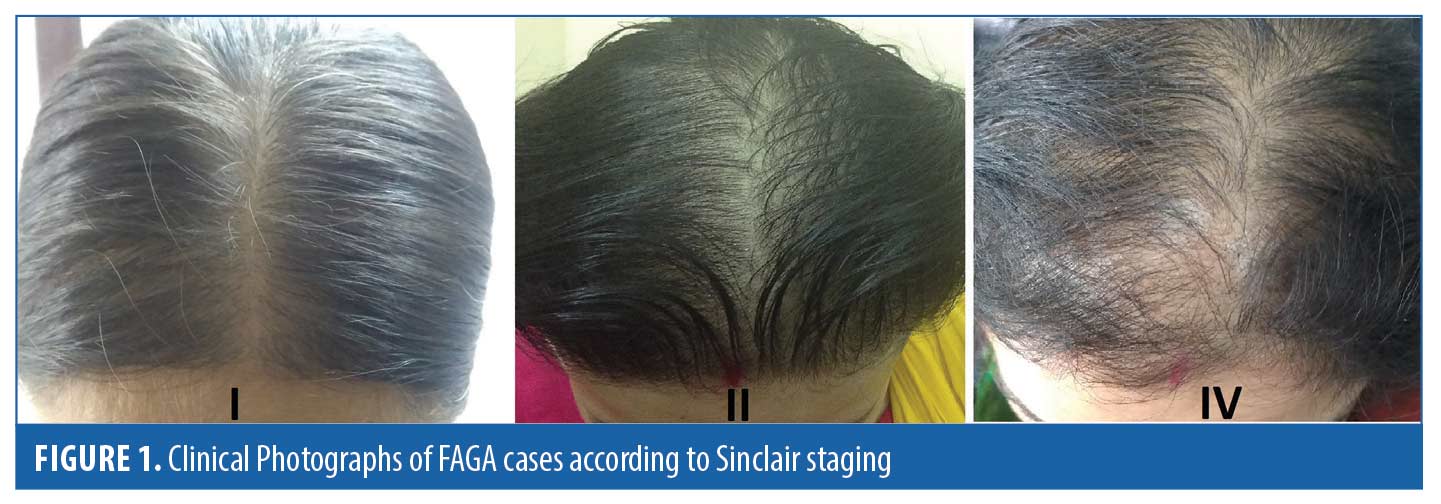
Demographic data for FAGA. The duration of disease ranged from minimum eight months to 15 years with a mean of 38.5 months ± 51.3. There was a positive paternal history of androgenetic alopecia in 9.6 percent of cases (n=3/31) while only two patients (2/31) had similar hair loss pattern in mother. The menstrual cycles were irregular in five out of 31 cases (16.1%), and 19.3% (6/31) were postmenopausal. Of 31 cases of FAGA, two cases had high Prolactin levels and five cases had hypothyroidism. There was no significant correlation between age of presentation and the duration of disease. Out of total 31 cases of FAGA,18 females (58%) were under 40 years.The authors classified each patient of FAGA according to Sinclair scale. Of the participants, 64.1 percent (n=20) were in Stage 1, 22.5 percent (n=9) were in Stage 2, and 12.9 percent (n=4) were in Stage 4 (Figure 1). There was a significant positive correlation between age of patient and the higher grade of Sinclair staging (R=0.63 and p=0.001). Also, the disease duration and Sinclair staging showed a significant positive correlation (R=0.42 p=0.01). No associations were found between dermoscopic signs and Sinclair staging of FAGA.
Demographic data for TE. The duration of disease ranged from minimum 15 days to eight months with a mean of 3.1± 2months. Twenty-six females (48.4%) presented with TE in early age (i.e., <40 years). The menstrual cycles were irregular in nine out of 33 cases (27.2%) and eight out of 33 cases(24.2%) were postmenopausal. Hypothyroidism was detected in 24.2 percent (8/33) and anemia in 57.5 percent (19/33) cases of TE.
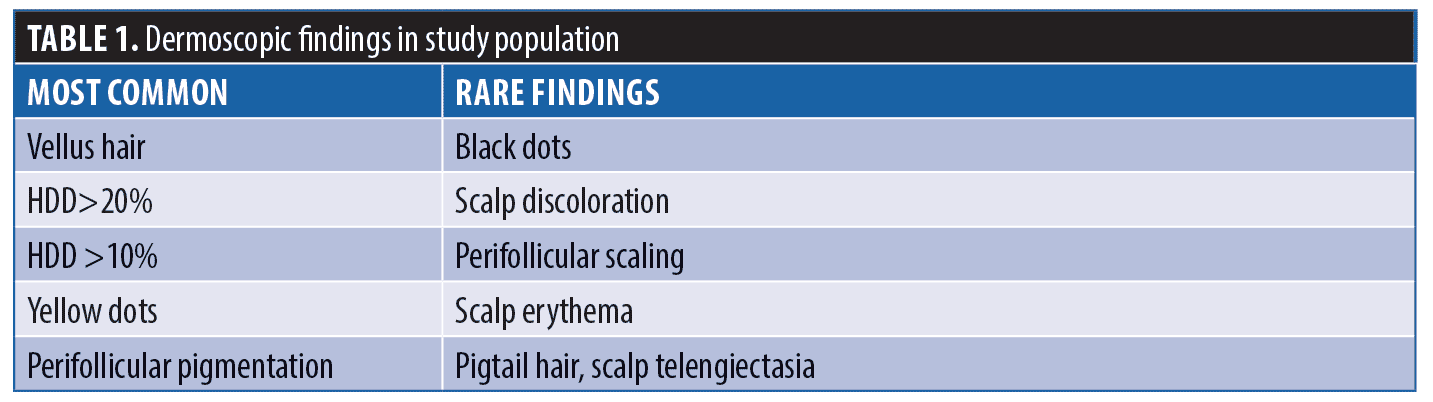
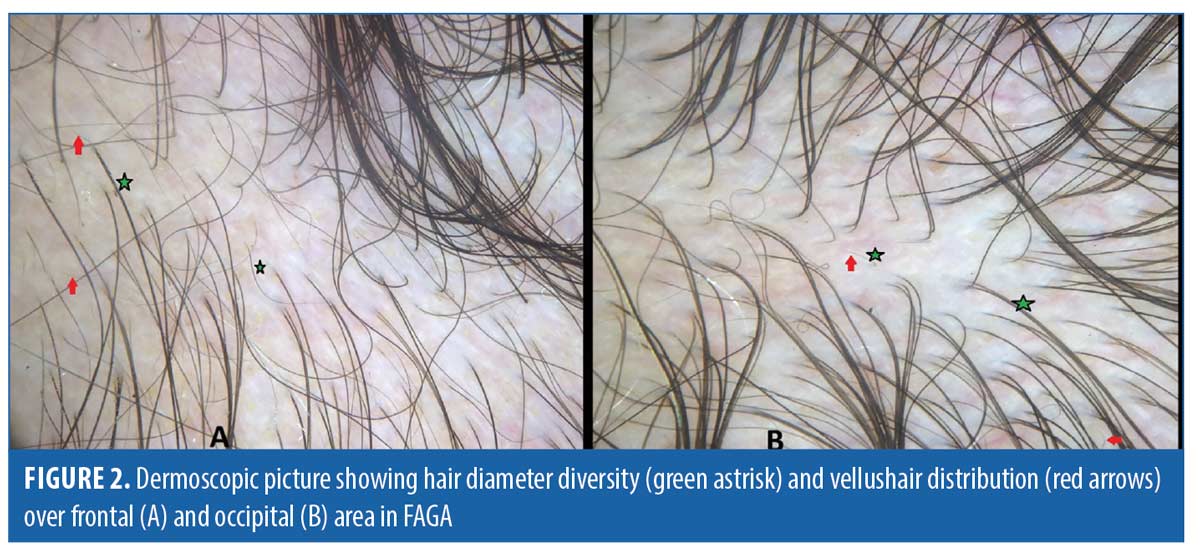
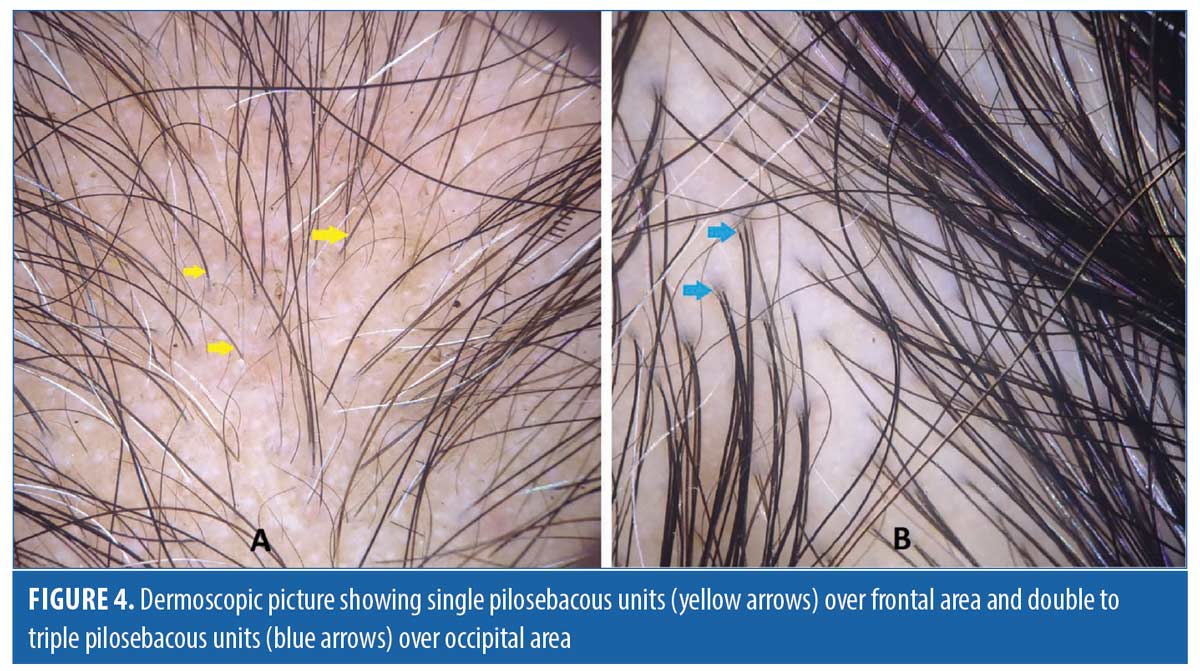
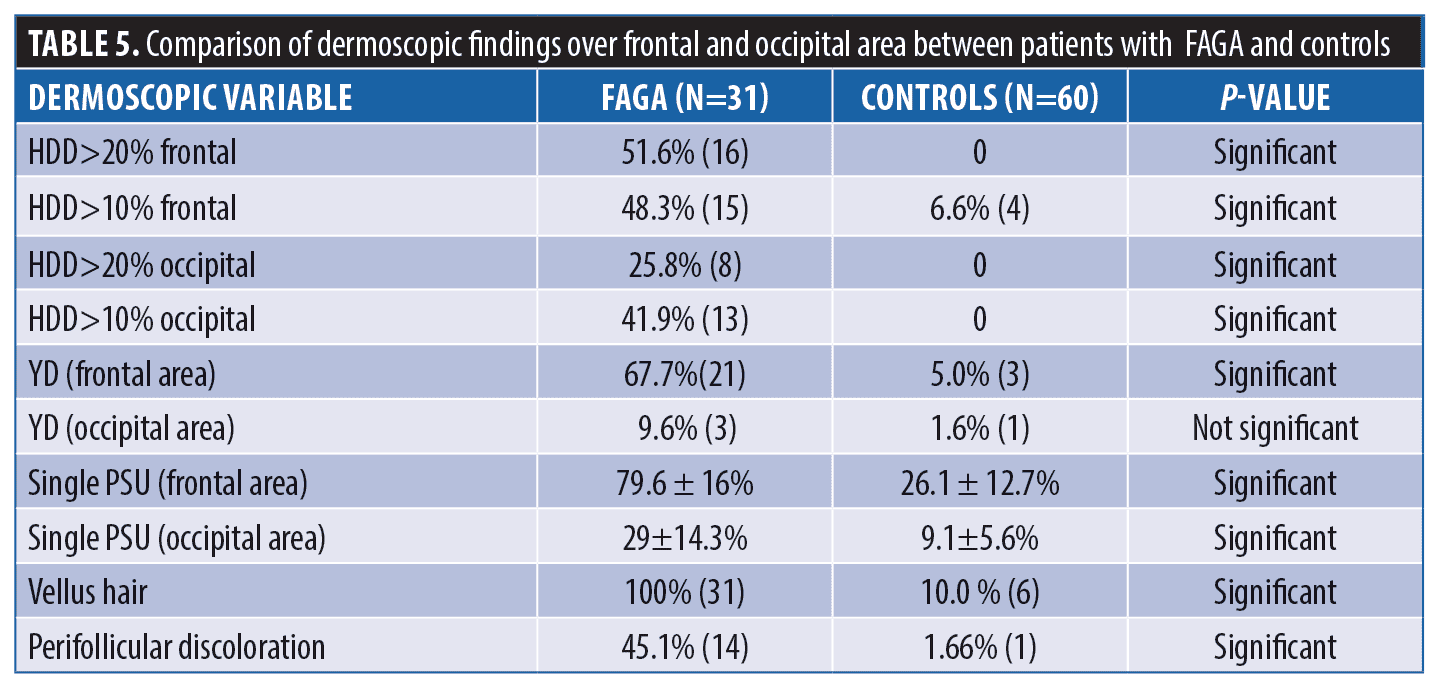
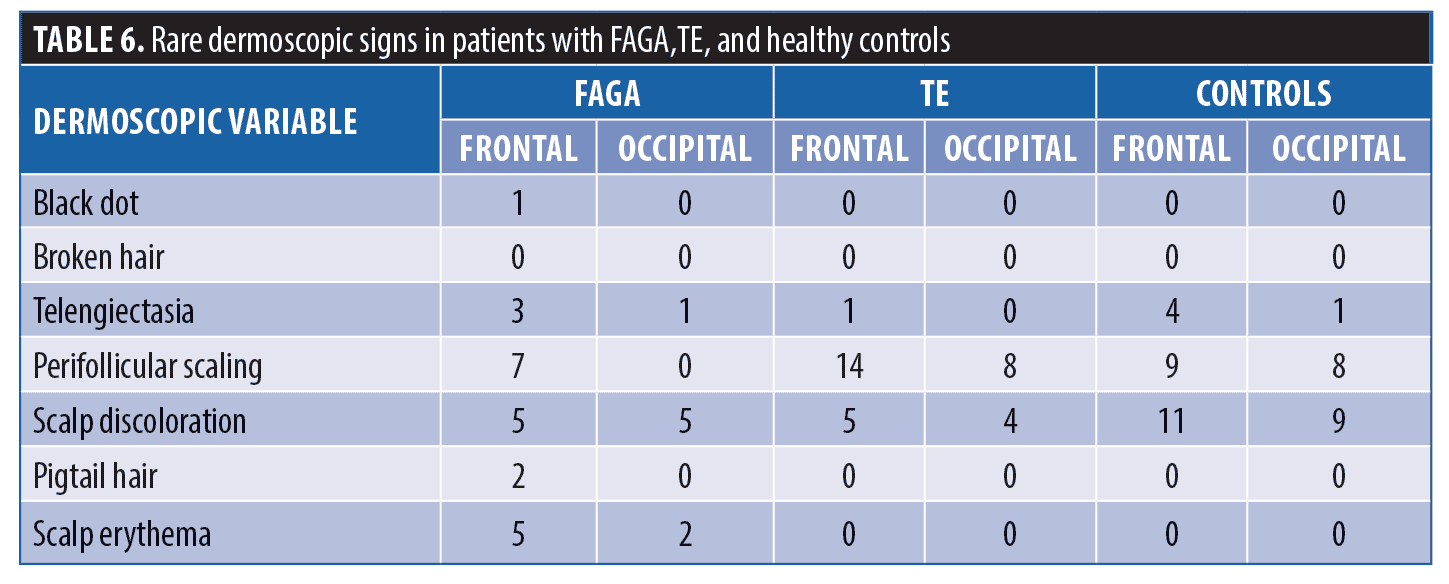
Dermoscopic findings in patients with FAGA. The common dermoscopic findings in FAGA were hair diameter diversity (HDD), single hair pilosebaceous unit (PSU), yellow dots (YD),vellus hair (VH),perifollicular pigmentation (PFP), and scalp pigmentation. Among the rare findings were perifollicular scaling, scalp erythema, telengiectasia, broken hair, black dots, and pigtail hair. These rare parameters were also evaluated as per the study design. Table 1 enumerates the various dermoscopic findings in our study population. All patients (31/31) in the FAGA group showed increased vellus hairs in the frontal area, 83 percent had vellus hair in the parietal area, and 74 percent in the occipital area. There was a significant difference between vellus hair in frontal and occipital area in FAGA (Figures 2A, 2B). On comparison of HDD > 20 percent between frontal and occipital area in FAGA patients, it was found to be statistically significant (Figure 2A, 2B). The difference in HDD >10 percent between frontal and occipital areas in FAGA patients was not significant. The difference in yellow dots in frontal and occipital hair in FAGA patients was statistically significant. About 67.7 percent of patients with FAGA had yellow dots in the frontal area, while 9.67 percent of patients had yellow dots in occipital area (Figure 3). The mean value of single PSU in the frontal area was 79.6±16 percent while in the occipital area it was 29± 14.3 percent, which is statistically significant. Perifollicular pigmentation was seen in frontal area in 11/31 (35.4%) patients, and none of the FAGA patients had perifollicular pigmentation in occipital area (Figure 4A, 4B). Table 2 shows the comparison of dermoscopic findings in frontal and occipital scalp in FAGA cases.
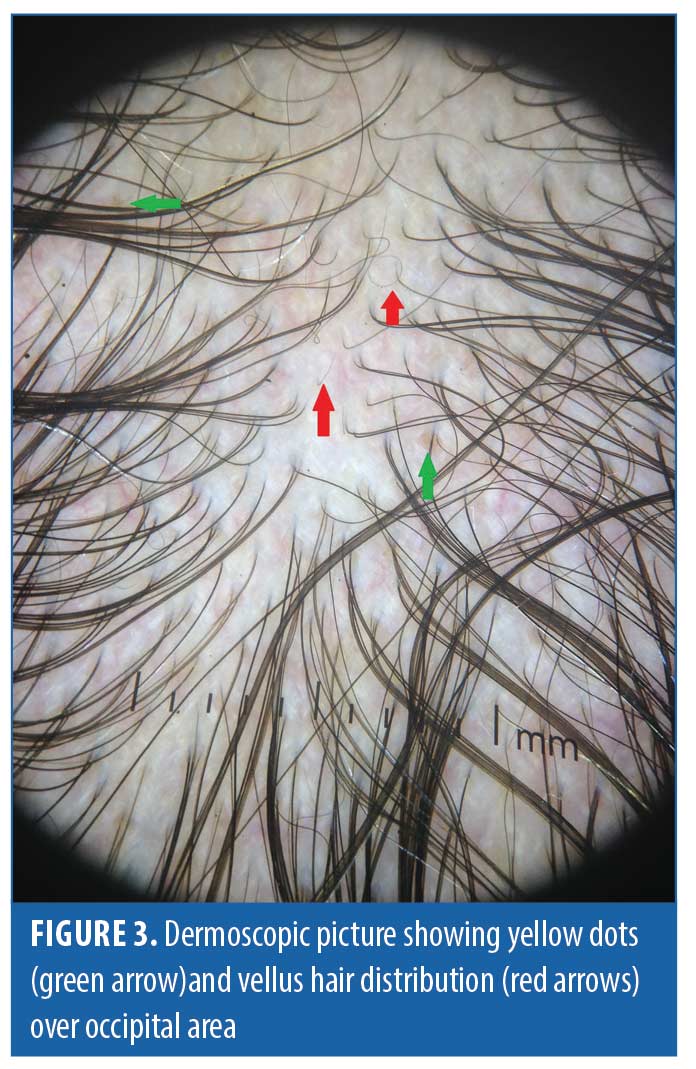
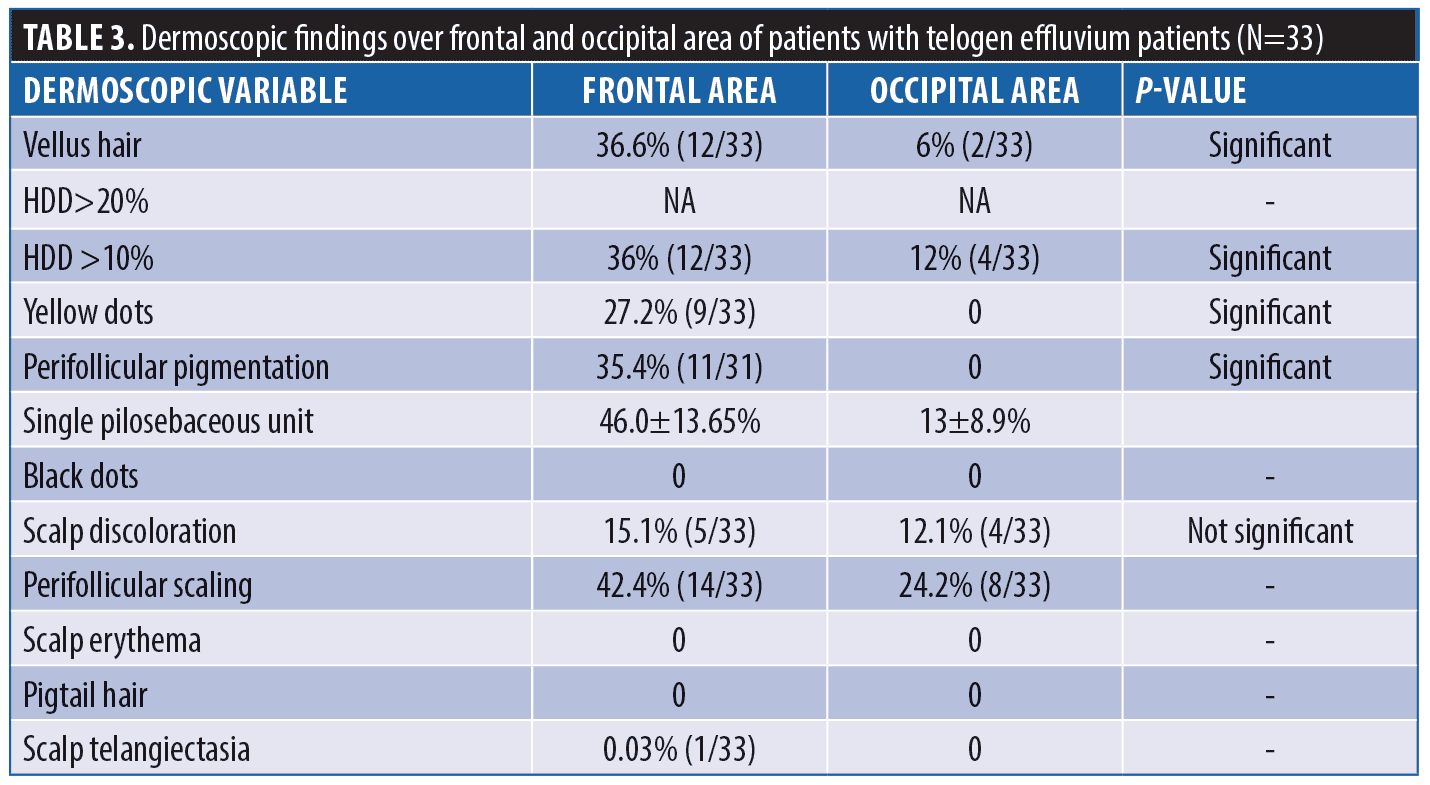
Dermoscopic findings in TE. In TE, HDD >10 percent was seen in 12 out of 33 patients (36.3%) in the frontal area and in four patients (12%) in the occipital area. No patient exhibited HDD of > 20 percent. Vellus hair was appreciated in the frontal area in 12/33 (36.3%) patients of TE. Table 3 shows the various dermoscopic findings in TE patients.
Dermoscopic findings in controls. In healthy female controls, HDD >10 percent was observed in none of them in both frontal and occipital areas. The most common sign seen in controls was scalp discolouration (11/60), which could be attributed to trend of hair color application. The occipital area of the controls did not differ significantly from their frontal area vis a vis various dermoscopic signs.
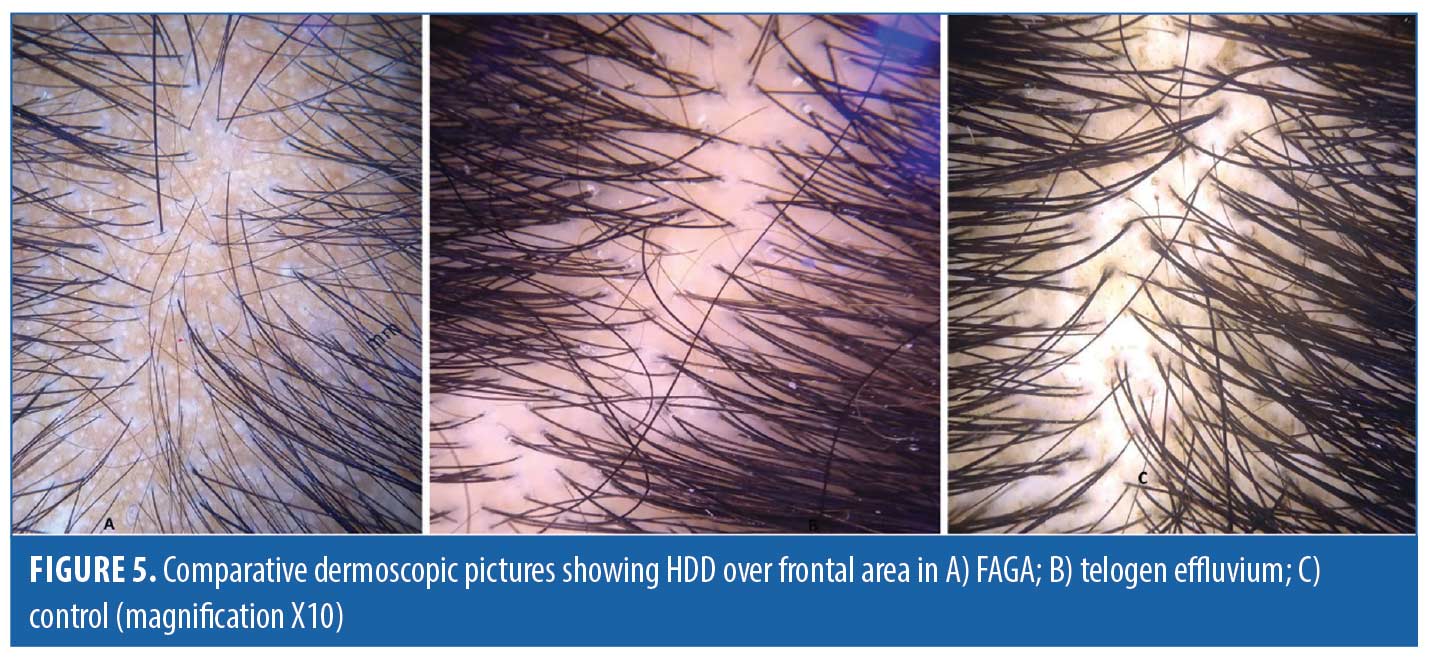
Comparison of FAGA, TE, and healthy controls. Dermoscopic findings over the frontal area and the occipital area of cases and control group are described in Tables 4 and 5, respectively. The rare dermoscopic findings have been tabulated in Table 6. There was a statistically significant difference in HDD in both areas between patients of FAGA versus TE and FAGA versus controls (p<0.0001) as depicted in Figures 5 and 6. The mean percentage of single PSU in the frontal area in FAGA, TE, and control groups were 79.6±16 percent, 46.0±13.65 percent, and 26.1±12.7 percent, respectively. The difference in the mean percentage of single PSU in both frontal and occipital areas in FAGA versus controls and FAGA versus TE patients was statistically significant. Our study found a significant difference in YD in the frontal area between FAGA and TE group and also between FAGA and control. This difference in YD percentage was not seen in the occipital area. The vellus hair were significantly higher in the FAGA patients than TE and control. There was a statistically significant difference in perifollicular pigmentation in FAGA versus TE and FAGA versus controls.

Discussion
Follicular miniaturization in the scalp is the hallmark of FPHL. FPHL tends to occur in genetically predisposed patients with altered hair follicle cycling and miniaturization of hair follicles leading to the transformation of terminal to shorter and finer vellus hair follicles. The pattern of hair loss sparing the occiput can be explained by the higher level of 5α reductase and androgen receptors in frontal hair follicles. Since aromatase content in frontal hair is six times higher in females, it protects the scalp from undergoing total baldness.7 The studies by De Lacharriere et al, Inui et al, Ramos et al, and Mubki et al considered hair diameter diversity as an important feature and an accurate, easily accessible, and reliable clinical sign in diagnosis of FAGA and that all dermoscopic abnormalities are more prominent in frontal area compared with the occipital area.8–11 Thus, we planned to evaluate and compare frontal and occipital scalps of study subjects and compare the dermoscopic findings of FAGA, TE, and healthy female controls.
According to our study, there was no statistically significant correlation between family history of similar patterened hair loss and early age of onset in FAGA patients. This is similar to the findings by Tawfik et al.2 Contrary to this, Zhang et al reported significant correlation between family history and early age of onset in FAGA patients.12 Our results show that there was no significant correlation between age of presentation and the duration of disease. However, there was a significant positive correlation between age of patient and the higher grade of Sinclair staging (R=0.63 and p=0.001). Also, disease duration and Sinclair staging showed a significant positive correlation (R=0.42 p=0.01). All these findings are similar to the findings by Tawfik et al and Zhang et al.2,12
Hair diameter diversity, or anisotrichosis, is observed in the affected scalp region of all FAGA patients.13 It is caused by progressive and unsynchronized miniaturization of hair follicles in the genetically predisposed scalp regions, thus, replacing the terminal hairs by vellus hairs. HDD of more than 20 percent has been regarded as a hallmark of AGA in various studies done in past. We found HDD of more than 20 percent in the frontal area in 16/31 (51.6 %) patients of FAGA. However, HDD of more than 10 percent could be seen in 48.3 percent (15/31) of FAGA patients in the frontal area. In study done by Inui et al, HDD > 20 percent was present in the frontal scalp of all the cases, and Hu et al reported HDD >10 percent in all cases of FAGA.9,12 In our study, there was a statistically significant difference in HDD in both areas between patients of FAGA versus TE and FAGA versus controls. Nagar et al also found the presence of anisotrichosis to be significantly associated with the frontal scalp of FAGA cases when compared with the frontal scalp of controls.15 When we compared the difference in HDD > 20 percent between frontal and occipital area in FAGA patients, it was statistically significant. Tawfik et al noticed increased percentage of HDD in frontal and temporal area as compared to occipital area of scalp.2 Nagar et al also reported a statistically significant difference in HDD in frontal and occipital area.15 On dermoscopy, increased vellus hair was observed in FAGA patients in both frontal and occipital area, and vellus hair were statistically significant more in frontal area as compared to occipital area. The proportion of vellus hair were significantly higher in the FAGA patients than the other two groups. Rakowska et al’s study observed a shift towards vellus hair in both frontal and occipital area in FAGA patients.3 The frontal versus occipital difference in HDD >10 percent was present in TE while healthy controls exhibited no difference. Nagar et al commented in their study that, on comparing the frontal and occipital scalp in healthy female controls, it showed statistically significant hair diameter diversity.15
The current study showed percentage of single PSU in the frontal area in FAGA,TE and control groups as 79.6±16 percent, 46.0±13.65 percent, and 26.1±12.7 percent, respectively. This difference in the mean percentage of single PSU in frontal and occipital areas in FAGA versus controls and FAGA versus TE is statistically significant. The study done by Rakowska et al also found a significant difference in single PSU between FAGA and controls as well as between FAGA and TE in both frontal and occipital area. Rakowska et al also reported a high percentage of single PSU on the frontal scalp, and Tawfik et al showed a high percentage of 97.4 percent (77/79 patients).2,3 In a healthy person, the number of hair in one pilosebaceous unit varies from one to three. A predominant prevelance of single PSU in the frontal area is one of the main features in distinguishing FAGA from other diseases of hair.16
The perifollicular pigmentation was observed in 35.4 percent of FAGA patients in the frontal area and nil in occipital area. It is also known as peripilar sign that signifies the presence of perifollicular lymphocytic infiltrate in initial stages of AGA.17 In studies of Asian population, perifollicular pigmentation is less appreciated as compared to white population, possibly due to the concealment by Asian subjects’ skin color.9,14 The detection rate of perifollicular pigmentation in white subjects very high, at 90 percent.18,19 In Nagar et al study on an Indian population, it was found in only 18.7 percent of cases in the frontal scalp.15 In our study, there was a statistically significant difference in perifollicular pigmentation in FAGA versus controls and FAGA versus TE. The Rakowska et al study found that perifollicular discoloration was significantly more frequent in FAGA as compared with healthy controls or patients with CTE.3 Our study found that, in FAGA, the percentage of perifollicular discoloration in the frontal area is significantly higher as compared to occiput. Rakowska et al observed that the ratio of frontal to occipital perifollicular dicoloration higher than 3:1 is highly indicative of FAGA.3
Yellow dots are empty follicular ostia (atrichia) due to sebaceous gland persistence after severe miniaturization of the follicles or sebaceous gland hyperplasia under the influence of androgens.9 Our study reported YD in 67.7 percent of FAGA cases in the frontal area and 0.09 percent in the occipital area. YD has a very variable presence in different studies, ranging from 1.67 percent to 66 percent. The ratio of YD in FAGA cases was 25:143 in Kibar et al’s study,13:50 in Deloche et al’s study, and 1:6 in study done by Inui et al.5,9,18 Zhang et at found YD as an insignificant finding in FAGA patients, whereas Rakowska et al reported higher YD in FAGA patients as compared to TE and controls.3,12 Tawfik et al showed that the yellow dots in androgenetic alopecia were positively correlated with the advanced Ludwig and Sinclair stages of hair loss though there was no correlation between staging and YD in the current study.2 However, the difference in yellow dots in frontal and occipital hair in FAGA patients was statistically significant, and the difference between yellow dots in FAGA, TE, and controls in the frontal area was also significant.
A few studies found an association between various dermoscopic signs with advanced staging in FAGA patients. Kibar et al observed no difference between trichoscopic findings in the different severity groups when they classified FAGA cases according to the Ebling and Olsen classifications.5 However, in the Ludwig classification, arborizing red lines were detected to be more common in Stage 1, and BD was detected to be more common in Stage 3. In study done by Zhang et al, white peripilar signs, scalp pigmentation ,and focal atrichia positively correlated with Ludwig’s Stage 3.12 No associations were found between dermoscopic signs and staging of FAGA in our study.
Conclusion
This article highlights the dermoscopic differences between TE, FAGA ,and its comparison to healthy female controls. These dermoscopic features will help in difficult to differentiate cases. Dermoscopy of scalp has proven its worth by reducing the need of biopsy. It helps in detecting FAGA on the basis of increased proportion of thin and vellus hairs, hair diameter diversity, perifollicular discoloration, and the presence of a variable number of yellow dots. The clinical diagnosis of FAGA augmented by the dermoscopic signs allows timely treatment before the occurrence of significant reduction in hair volume.
Limitations. Biopsies were not performed in every patient as the diagnosis was evident clinically in many patients and an invasive procedure seemed unwarranted.
References
- Paus R, Oslen EA, Messenger AG. Hair growth disorders. In: Wolff K, Goldsmith LA, Katz SI, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York: McGraw Hill; 2008.753–77.
- Tawfik SS, Sorour OA, Alariny AF, et al. White and yellow dots as new trichoscopic signs of severe female androgenetic alopecia in dark skin phototypes. Int J Dermatol. 2018;57(10):1221-8.
- Rakowska A, Slowinska M, Kowalska-Oledzka E, et al. Dermoscopy in female androgenic alopecia: method standardization and diagnostic criteria. Int J Trichology. 2009;1(2):123-30.
- Werner B, Mulinari-Brenner F. Clinical and histological challenge in the differential diagnosis of diffuse alopecia: female androgenetic alopecia, telogen effluvium and alopecia areata – part I. An Bras Dermatol. 2012;87(5):742-7.
- Kibar M, Aktan S, Bilgin M. Scalp dermatoscopic findings in androgenetic alopecia and their relations with disease severity. Ann Dermatol. 2014;26(4):478-84.
- Gupta M, Mysore V. Classification of Patterened hair loss: A review. J Cutan Aesthet Surg. 2016;9(1):3-12.
- Singal A, Sonthalia S, Verma P. Female pattern hair loss. Indian J Dermatol Venereol Leprol. 2013;79:626-40
- de Lacharrière O, Deloche C, Misciali C, et al. Hair diameter diversity: a clinical sign reflecting the follicle miniaturization. Arch Dermatol. 2001;137(5):641-6.
- Inui S, Nakajima T, Itami S. Scalp dermoscopy of androgenetic alopecia in Asian people. J Dermatol. 2009;36(2):82-5.
- Ramos LD, Santili MC, Bezerra FC, et al. Dermoscopic findings in female androgenetic alopecia. An Bras Dermatol. 2012;87(5):691-4.
- Mubki T, Rudnicka L, Olszewska M, et al. Evaluation and diagnosis of the hair loss patient: part II. Trichoscopic and laboratory evaluations. J Am Acad Dermatol. 2014;71(431):e1– e11.
- Zhang X, Caulloo S, Zhao Y, et al. Female pattern hair loss: clinico-laboratory findings and trichoscopy depending on disease severity. Int J Trichology. 2012;4(1):23-8.
- Sewell L, Elston D, Dorion R. “Anisotrichosis”: A novel term to describe pattern alopecia. J Am Acad Dermatol. 2007;56:856.
- Hu R, Xu F, Han Y, et al. Trichoscopic findings of androgenetic alopecia and their association with disease severity. J Dermatol. 2015;42(6):602-7.
- Nagar R, Dhudshia R. Utility of trichoscopy to diagnose early female pattern hair loss in resourcepoor setting: A cross-sectional study. Indian J Dermatol Venereol Leprol. 2019;85(6):681.
- Rakowska A. Trichoscopy (hair and scalp videodermoscopy) in the healthy female. Method standardization and norms for measurable parameters. J Dermatol Case Rep. 2009;3(1):14-19.
- Tosti A. Hair shaft disorders. In: Tosti A, ed. Dermoscopy of Hair and Scalp: Pathological and Clinical Correlation. Illustrated ed. USA: CRC Press; 2007.51–53.
- Deloche C, de Lacharriere O, Misciali C et al. Histological features of peripilar signs associated with androgenetic alopecia. Arch Dermatol Res. 2004;295:422–8.
- Petit L, Claudine, Pierard F et al. Subclinical speckled perifollicular melanosis of the scalp. Eur J Dermatol. 2002;12:565–8.

