J Clin Aesthet Dermatol. 2025;18(10):48–54.
by Steven H. Dayan, MD; Corey L. Hartman, MD, FAAD; Carlo Di Gregorio, MD, PhD; Luiz Avelar, MD; Alessandra Haddad, MD, PhD; Bill Andriopoulos, PhD; and Torun Bromée, PhD
Dr. Dayan is with DeNova Research in Chicago, Illinois. Dr. Hartman is with Skin Wellness Dermatology in Homewood, Alabama. Dr. Di Gregorio is with a plastic surgery private practice in Palermo, Italy. Dr. Avelar is with Luiz Eduardo Avelar Cirurgia Plástica in Belo Horizonte, Brazil. Dr. Haddad is with Hospital Israelita Albert Einstein in São Paulo, Brazil. Dr. Andriopoulos is with Galderma Laboratories in Dallas, Texas. Dr. Bromée is with Galderma in Uppsala, Sweden.
FUNDING: Galderma funded the study, provided the study product, and funded medical writing support.
DISCLOSURES: Dr. Dayan is a consultant and investigator for Galderma. Drs. Hartman, Avelar, and Haddad are investigators for Galderma. Dr. Di Gregorio is an investigator for Galderma and the Chief of the Italian Galderma Medical Faculty. Drs. Andriopoulos and Bromée are employees of Galderma.
Abstract: Objective: The authors sought to evaluate a flexible, hyaluronic acid (HA) filler, Restylane® Defyne™ (HADEF) (Galderma), for combined treatment of chin, nasolabial folds (NLFs), and marionette lines (MLs), in a predefined stepwise order, comparing Down-up (ie, chin first) versus Top-down (NLFs and MLs first) treatment approaches. This postmarketing study complements prior pivotal investigations that demonstrated the safety and effectiveness of HADEF treatments of the lower face, by providing a standardized treatment algorithm for combining several treatment areas in the lower face. Methods: HADEF was injected at Day 1 in the first treatment area and at Week 3 in the second area (randomized to either Down-up or Top-down order), with optional touch-up (any area) at Week 6. Assessments included Global Aesthetic Improvement Scale (GAIS), skin firmness, facial harmony, patient satisfaction, and safety until Week 9. Results: Both approaches achieved similar, favorable results at Week 9, with 100% of patients in both groups (Down-up, n=31; Top-down, n=29) demonstrating aesthetic improvement on the GAIS, improved skin firmness and facial harmony, and natural-looking results. Of patients seeking aesthetic improvement of the submental area, 95% in the Top-down group and 100% in the Down-up achieved improvement. Patient-reported endpoints supported these results, with high satisfaction throughout the study. HADEF was well tolerated throughout the study. Limitations: The results should be considered indicative rather than definitive given the post-marketing design of the study. Conclusion: Both stepwise approaches may be used for administering HADEF when treating the combined areas of chin, NLFs, and MLs. Keywords: Injection approach, hyaluronic acid filler, chin, nasolabial folds, marionette lines, patient satisfaction
Introduction
Restylane® Defyne™ (HADEF) is a hyaluronic acid (HA) filler for aesthetic use, providing flexible support and contour enhancement.2,3 HADEF is approved for the correction of moderate-to-severe, deep facial wrinkles and folds, such as NLFs, in the United States (US) since 20161 and Conformité Européenne (CE) marked in the European Union (EU) since 2010. In 2021, the label was extended in the US to also include chin augmentation in the mid-to-deep dermis (subcutaneous and/or supraperiosteal) to improve the chin profile in adults with mild-to-moderate chin retrusion, yielding high aesthetic improvement and patient satisfaction.4 In addition, it is approved for nasolabial folds (NLFs) in China5 and recently received marketing approval for chin augmentation in China, following demonstration of safety and effectiveness also in an Asian population.6 The product has also been shown to be effective in correcting lower facial wrinkles while maintaining natural movement in various facial expressions.7,8
The present post-marketing study investigated HADEF for combined treatment of several lower facial areas, including the chin, NLFs, and marionette lines (MLs), administered at different sessions. For the first time, two different stepwise injection approaches were compared: “Down-up” from the chin up to the NLFs and MLs, versus “Top-down”, treating the same areas in reverse order. This study aimed to evaluate these treatment approaches to see if they impact the treatment outcome, including aesthetic improvement, facial balance, and patient satisfaction. In real-world practice, it is of interest to understand if different injection approaches impact the overall treatment outcome. Results from the study can support future treatment guidelines used by aesthetic health care practitioners when injecting HADEF in the chin, NLFs, and MLs.
Methods
Study design. This randomized, multicenter study (NCT04520997) was conducted from December 2020 to September 2021 at two clinics in Brazil, two clinics in the US, and one clinic in Italy. The chin, NLFs, and MLs were treated in a predefined stepwise order with HADEF to compare the Down-up versus Top-down treatment approaches. The study protocol was approved by Independent Ethics Committees/Institutional Review Boards, and conformed to Good Clinical Practice and the Declaration of Helsinki. Patients signed informed consent and photoconsent for participation in this study.
Enrolled patients were adults over 21 years who could benefit from HA filler treatment of the lower face, including chin, NLFs, and MLs. Main exclusion criteria included scars or deformities, active skin disease, inflammation or related conditions such as infection, perioral dermatitis, and herpes infection near or in the area to be treated, or previous hypersensitivity to any injectable HA gel or anesthesia. For the area below the level of the lower orbital rim, patients were also excluded if they had undergone previous surgery or non-permanent filler treatment (HA-based/collagen-based) within the past 12 months, or had received semi-permanent filler treatment within the past 24 months, or had ever received any permanent filler treatment.
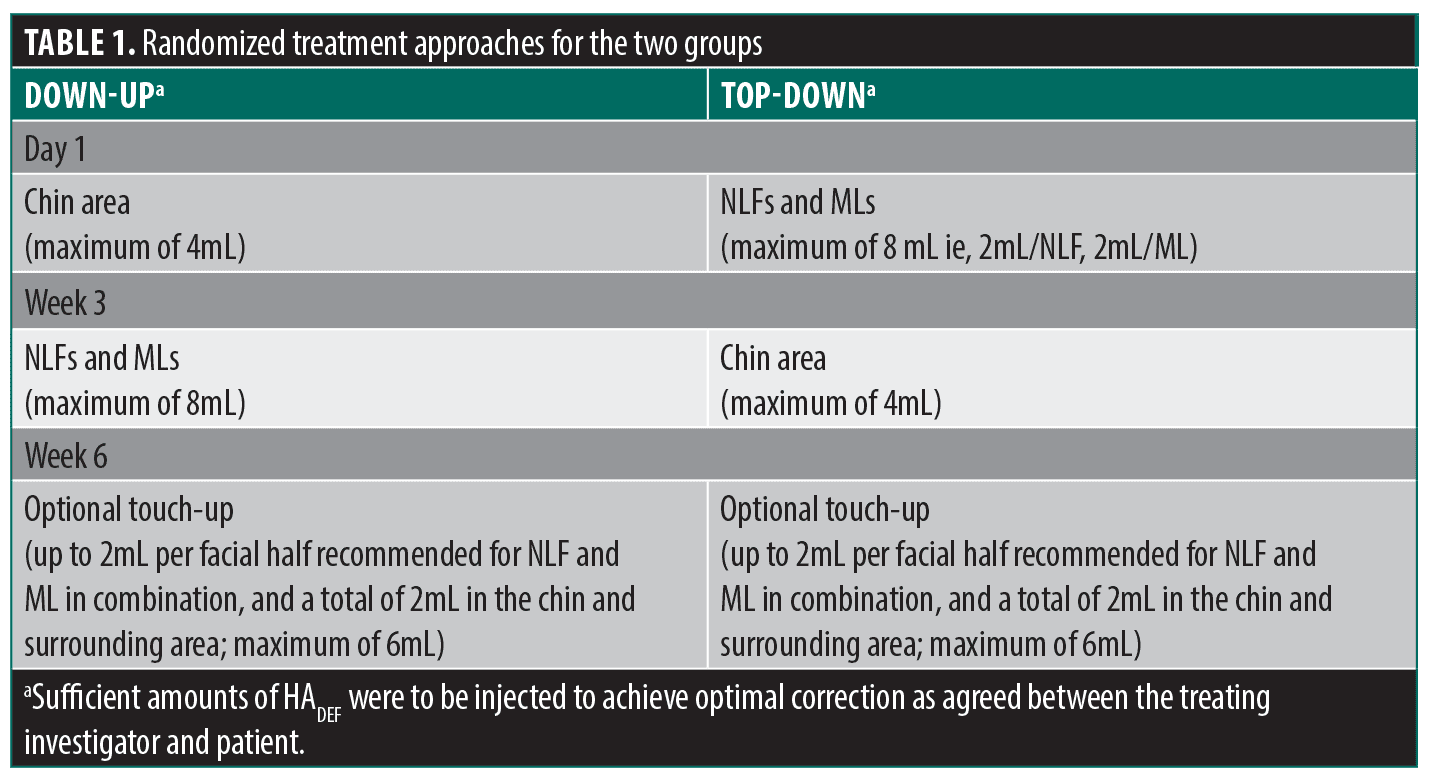
Treatment. Patients were randomized in a 1:1 ratio to one of two groups (Down-up or Top-down), both receiving treatment with HADEF (containing 20mg HA/mL and lidocaine hydrochloride 3mg/mL). In the Down-up group, injections were administered at Day 1 in the chin and at Week 3 in the NLFs and MLs, and in the Top-down group, injections were administered at Day 1 in the NLF and MLs, and at Week 3 in the chin. An optional touch-up in any of the treated areas that had not obtained optimal results was offered to both groups at Week 6 (Table 1). The injection technique used was at the discretion of the treating investigator, but the same predominant injection technique was to be used for all patients per site to limit variability. Injection depth could vary based on the patient’s treatment needs.
Assessments. Effectiveness objectives evaluated the impact of the treatment approach on aesthetic outcome, including aesthetic improvement of the lower face, facial harmony, and patient satisfaction. The following effectiveness endpoints were measured: aesthetic improvement of the lower face on a 5-grade Global Aesthetic Improvement Scale (GAIS) from “worse” to “very much improved,” as assessed by investigators and patients; naturalness of results and improvement in skin firmness around the chin, assessed by investigators and patients; improvement in facial harmony (facial symmetry, facial proportions, and chin width), assessed by investigators; improvement in the submental area, assessed by investigators; patient satisfaction questionnaire and two patient-reported FACE-Q scales (Satisfaction with Overall Facial Appearance9 and Satisfaction with Outcome10) comprising a total of 16 questions. Finally, an independent photographic reviewer (IPR) assessed facial harmony, attractiveness and masculinity/femininity by evaluating blinded pairs of photographs, and a layman board evaluated attractiveness (both comparing photos from baseline and Week 3, 6, and 9). Safety was evaluated by standard collection of adverse events throughout the study.
Statistical methods. For both effectiveness and safety endpoints, data were summarized using descriptive statistics, and there was no statistical hypothesis defined for this post-marketing study. The modified intention-to-treat (MITT) population consisted of all treatment-compliant patients (treated at both baseline and Week 3) and was the population used for all effectiveness analyses. Safety evaluations were based on the safety population, defined as all patients who were injected at least once with the study product. For each FACE-Q questionnaire, results were summarized into a Rasch-transformed total score (0–100) at each visit, with higher total scores reflecting a better outcome. For the FACE-Q Satisfaction with Facial Appearance Overall questionnaire, the total score change from baseline was also calculated for each follow-up visit and analyzed with the Wilcoxon signed-rank test. A p-value of less than 0.05 was considered statistically significant. No other statistical tests were used. The sample size of 60 patients (30 in each treatment group) was not based on a statistical calculation but was judged as sufficient for evaluation of the study’s objectives.
Results
Patients and treatment. Of 62 patients randomized (one randomized in error), 61 received the first treatment at baseline, 60 also received treatment at Week 3, and 58 completed the study. One participant withdrew consent after the first treatment. Two patients completed treatment but did not complete the study (one could not attend the last visit, and one was lost to follow-up after Week 6). One patient was randomized to the Top-down group but received Down-up treatment by mistake and was analyzed according to the treatment received.
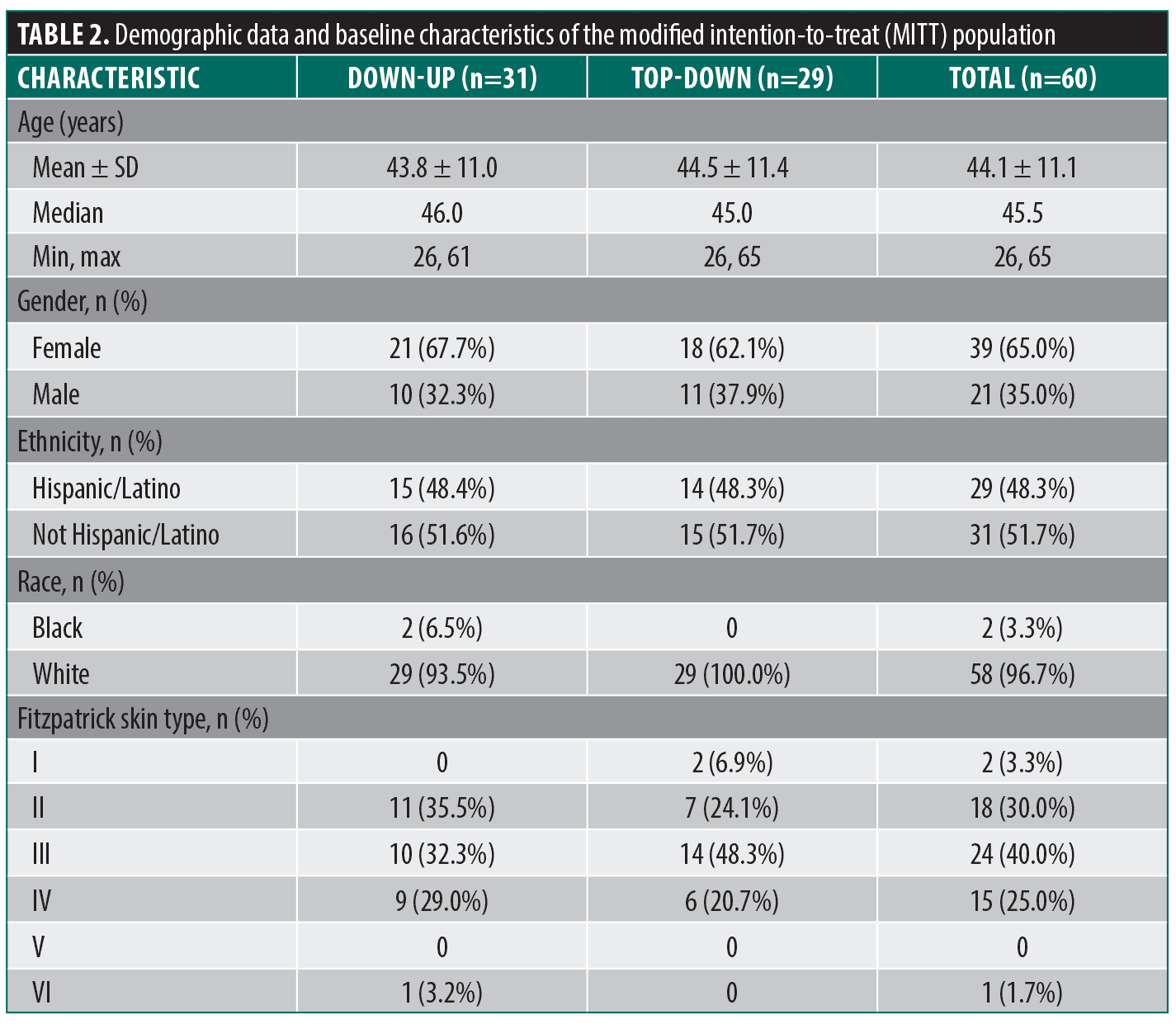
Demographic data for the MITT population are presented in Table 2. Overall, patients had an average age of 44 years, 65% were female, 95% had Fitzpatrick skin type II-IV, and approximately 48% were of Hispanic/Latino ethnicity.
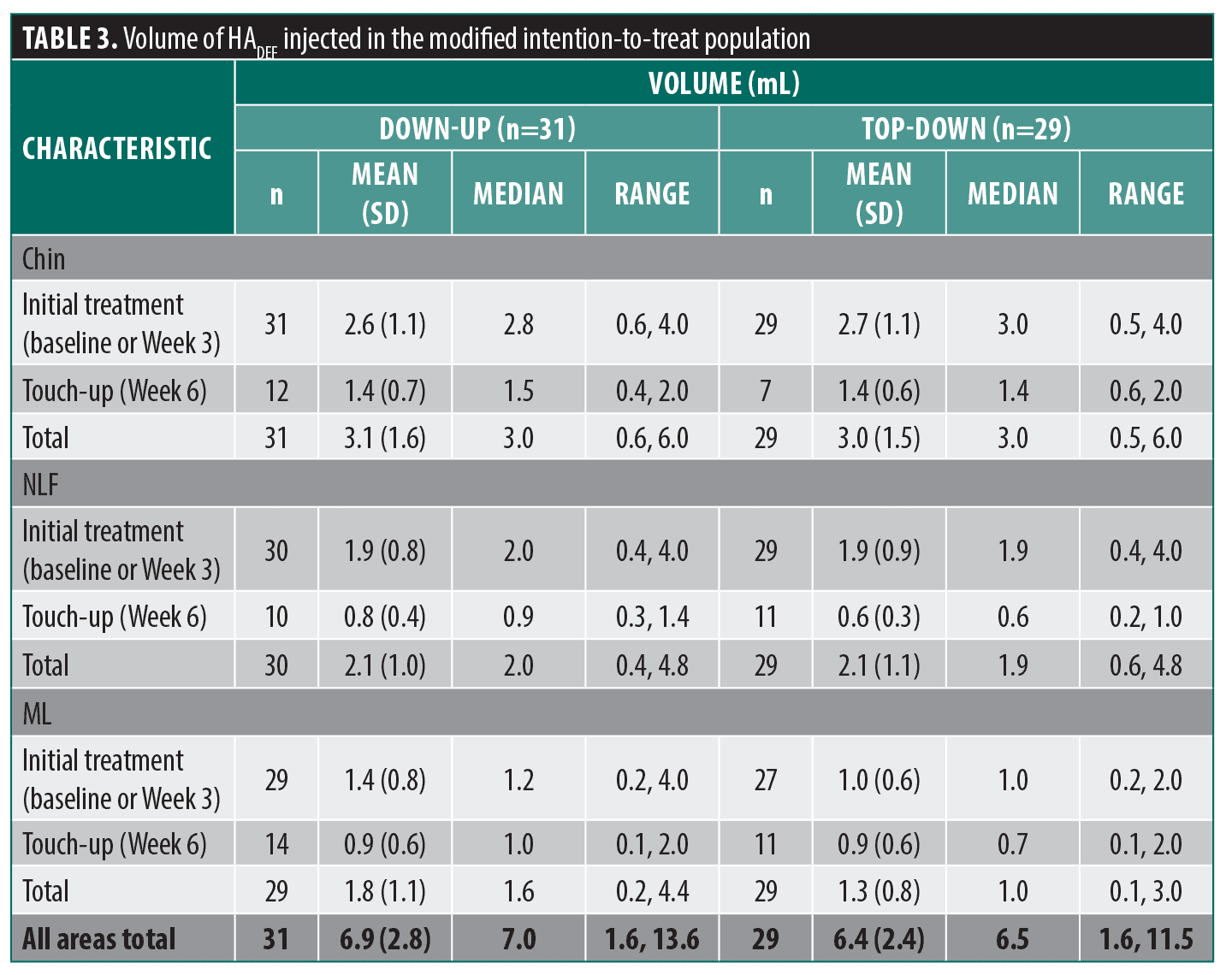
A slightly larger mean total (initial and touch-up) volume of HADEF (6.9mL) was used in the Down-up group, compared to the Top-down (6.4 mL). Similar volumes were used in the Top-down and Down-up groups for each of the NLF and chin. However, in the MLs a slightly larger mean volume was used in the Down-up group (1.8mL) versus the Top-down (1.3mL) (Table 3).
The most common depths of injection were subcutaneous and supraperiosteal for the chin and subcutaneous for NLFs and MLs. The main injection methods for chin were retrograde linear threading, microbolus, fanning and serial puncture. For NLFs, the main methods were retrograde linear threading, micro-bolus and fanning, and for MLs, retrograde linear threading and fanning.
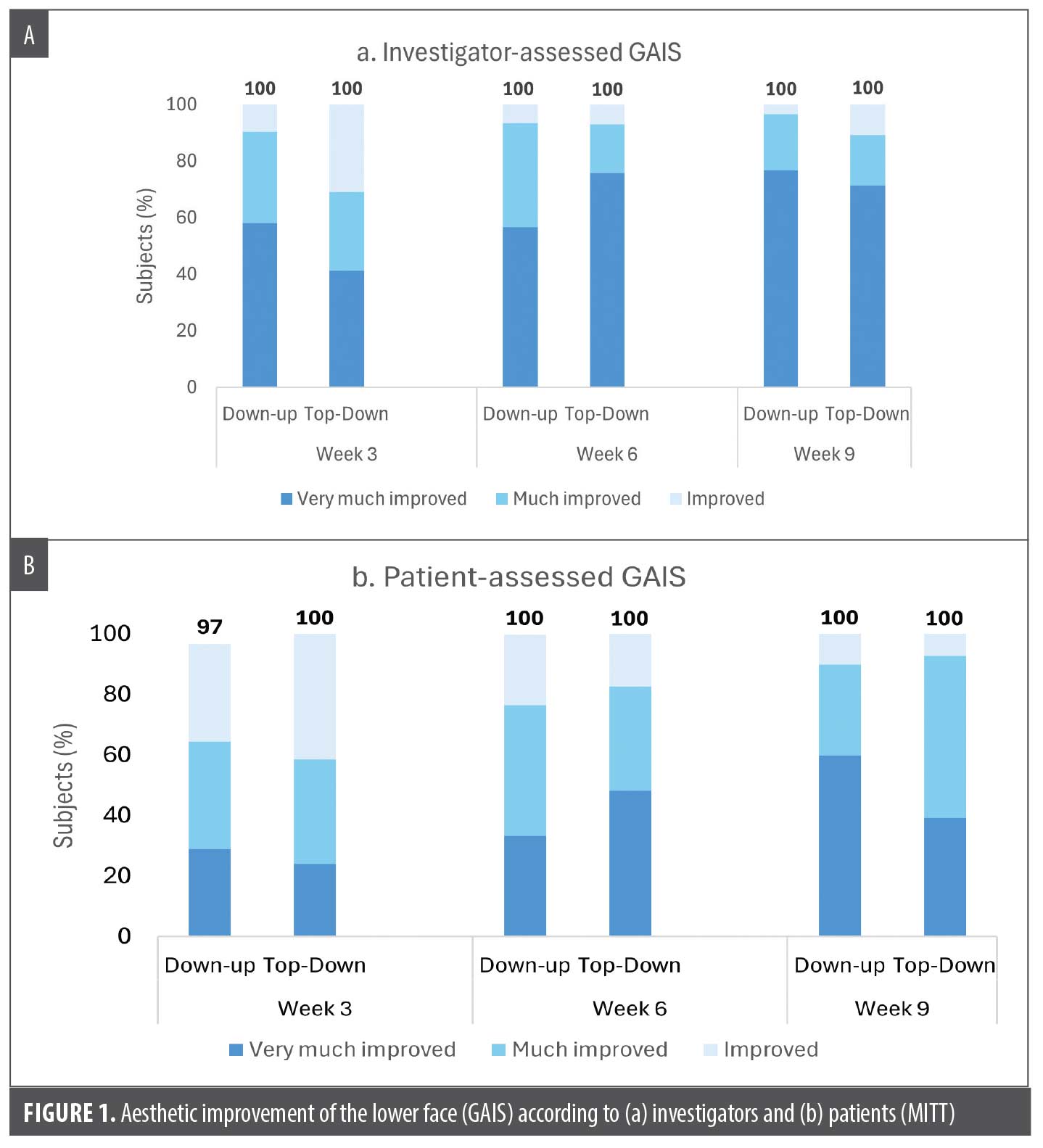
Effectiveness. Aesthetic improvement compared to pretreatment based on GAIS was high at all visits for both groups as assessed by the patient or the investigator. All patients (100%) were assessed by the investigator to be improved (“improved,” “much improved,” or “very much improved”) at 3, 6, and 9 weeks following the initial injection (both groups) (Figure 1A). All patients except one (97%) assessed themselves as improved at Week 3, and all were improved at Weeks 6 and 9 (both groups) (Figure 1B).
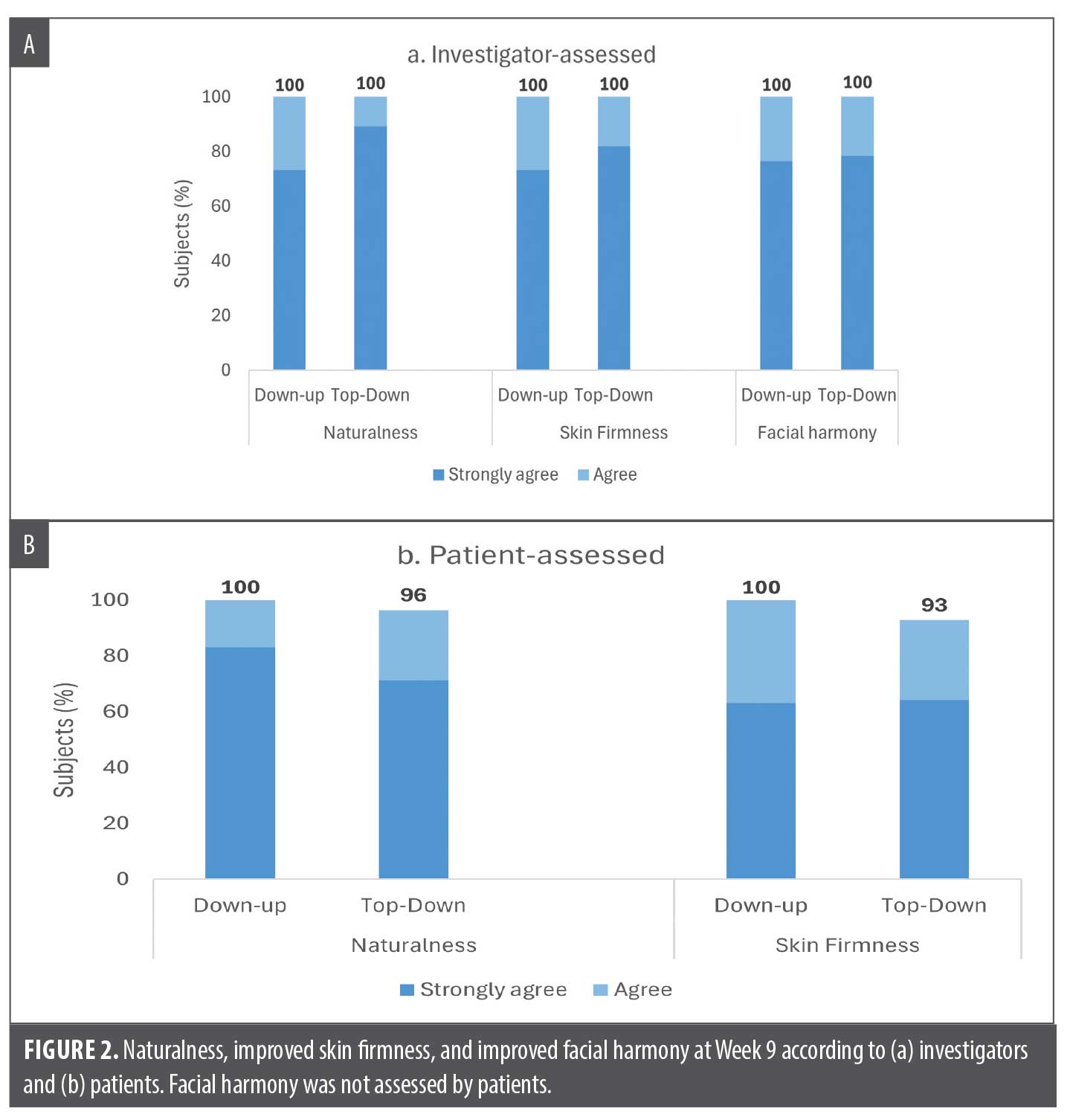
The naturalness of results and improvement in skin firmness around the chin were also rated as high throughout the study by both investigators and patients. At all timepoints for both groups, the majority of patients (at least 97% by investigators and at least 83% by the patients themselves) were assessed to have natural-looking results and firmer-looking skin around the chin after treatment. Facial harmony, assessed by investigators, was improved in 90% (Top-down) versus 100% (Down-up) at Week 3, in 100% of patients in both groups at Week 6, and also maintained in 100% of patients at Week 9. In summary, at Week 9 all patients (100%) in both treatment groups were assessed by the investigators to have natural-looking results, improved skin firmness, and improved facial harmony (Figure 2A). Likewise, at least 93% of patients from both groups reported themselves as having natural-looking results and improved skin firmness at Week 9 (Figure 2B).
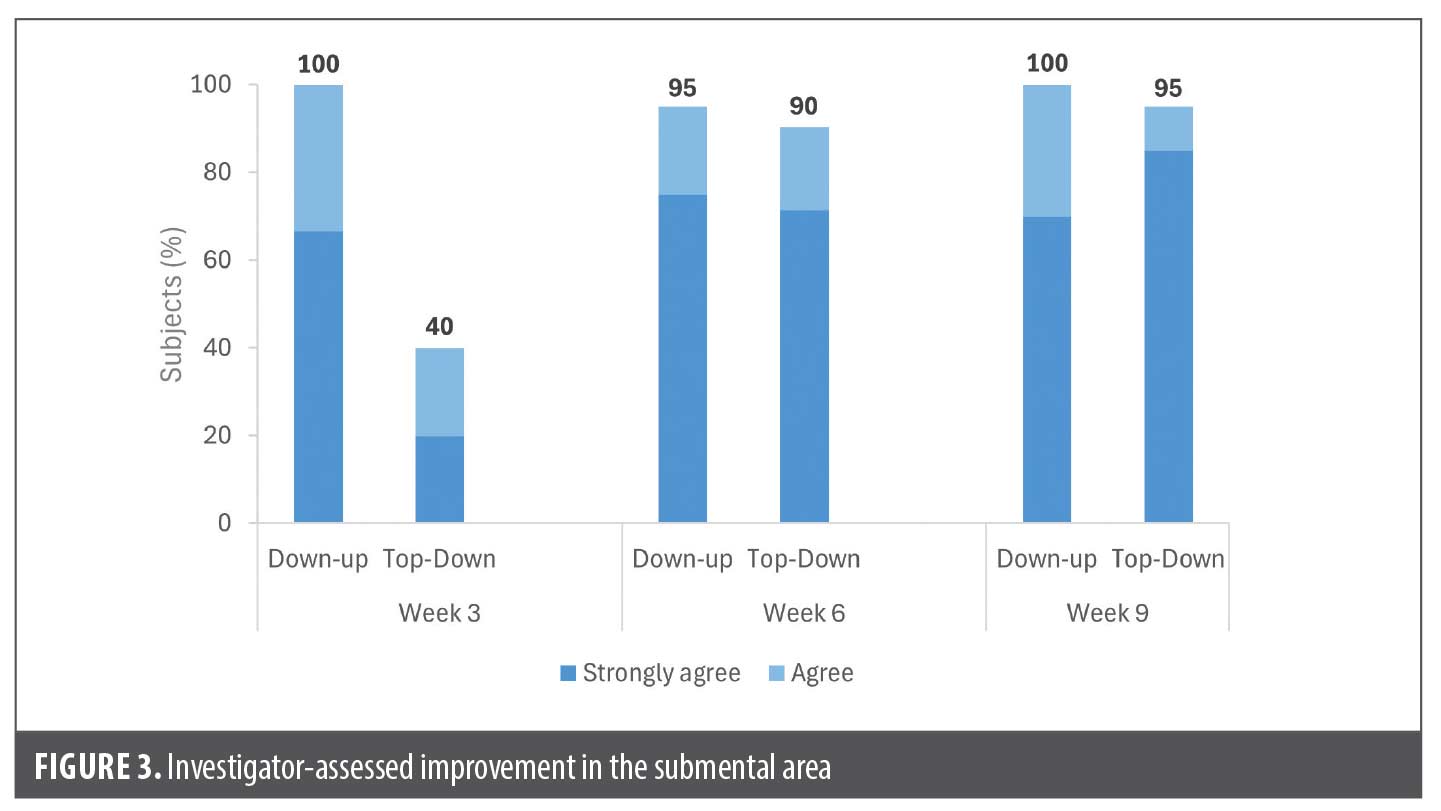
The submental area was assessed by investigators to need improvement at baseline in over two-thirds of patients in both groups (68%, 21 of 31 patients in Down-up group and 72%, 21 of 29 patients in Top-down group). Among these patients, the majority (at least 95%) in both groups achieved improvement at Week 9, and most of the improvement was reported at the visit immediately following chin treatment, ie, Week 3 for the Down-up group and Week 6 for the Top-down group (Figure 3).
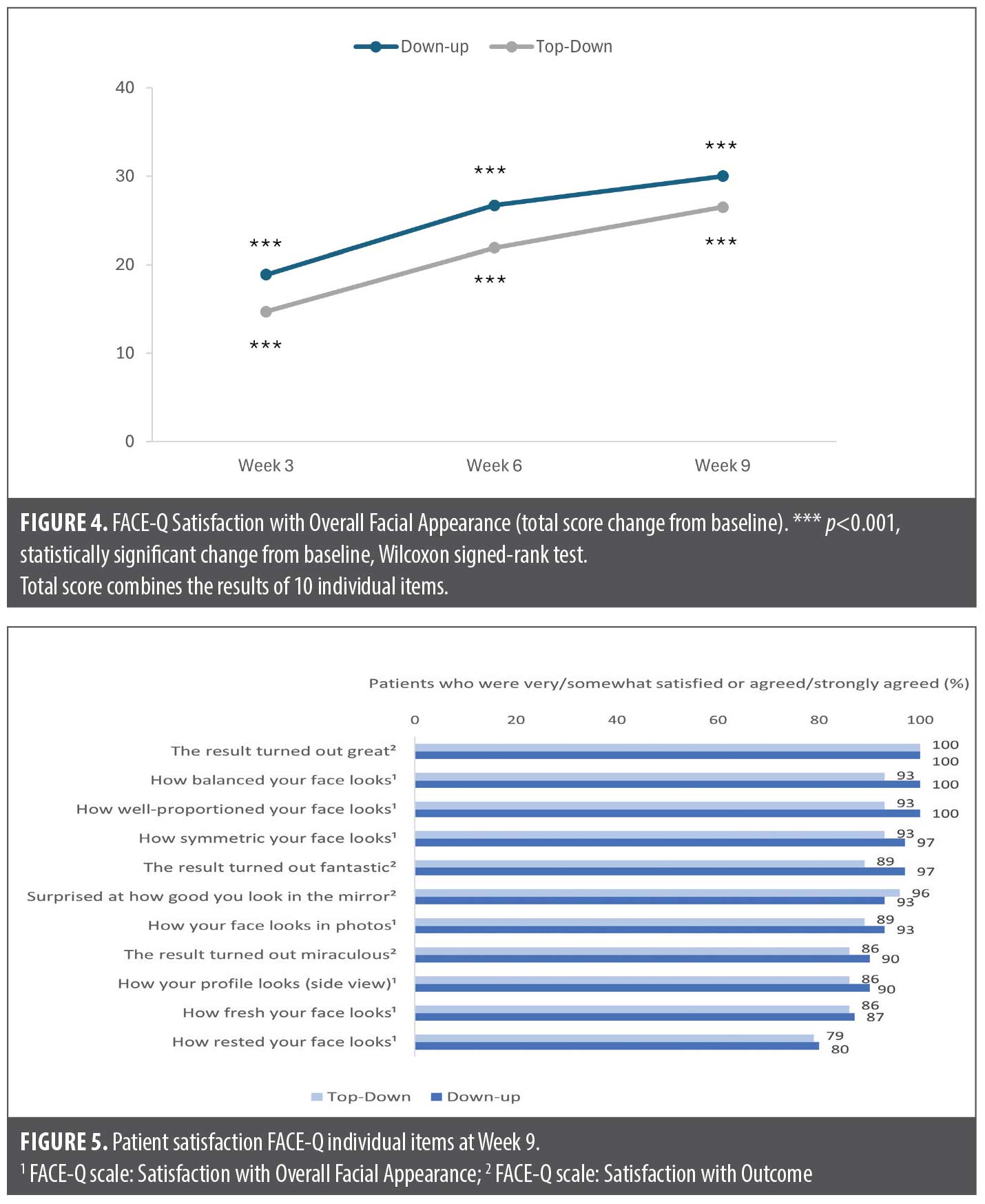
Both FACE-Q scales showed high patient satisfaction after treatment with similar results across both treatment groups (Figure 4 and Figure 5). FACE-Q Satisfaction with Overall Facial Appearance was statistically significantly improved from Baseline at each visit (p<0.001), with the highest total score improvement at Week 9, +30.0 in the Down-up group and +26.5 in the Top-down group (Figure 4). Based on the individual items of the Satisfaction with Overall Facial Appearance questionnaire, the majority of patients in both groups were very satisfied or somewhat satisfied at Week 9 with how balanced (100% [Down-up]; 93% [Top-down]), well-proportioned (100% [Down-up]; 93% [Top-down]) and rested their face looked (80% [Down-up]; 79% [Top-down]) (Figure 5). FACE-Q Satisfaction with Outcome mean total scores were high in both groups at Week 3 after one treatment (76.6 for Down-up and 67.4 for Top-down), and this increased further to Week 9 (80.7 for Down-up and 77.9 for Top-down). In addition at Week 9, all patients (100%) in both groups definitely agreed or somewhat agreed that the results turned out great, with ≥89% in both groups reporting fantastic results and ≥86% reporting miraculous results (Figure 5).
Results of the patient satisfaction questionnaire showed overall improved satisfaction in both groups. Before treatment, 42% of patients (Down-up) and 24% (Top-down) were satisfied with how well-defined the chin looked, and this increased to 97% and 89% at Week 9. For chin shape, there was a similar improvement in satisfaction from 42% and 31% in each group at baseline to 100% and 93% at Week 9. Patients were also highly satisfied with lower face contour in both groups at Week 9 (100% [Down-up]; 96% [Top-down]). Furthermore, at Week 3, 100% in the Down-up group (chin only) and 93% in the Top-down group (NLFs/MLs only) reported their appearance to be improved after their first treatment. After completion of all treatments in both groups, patients reported similar rates of improved appearance at Week 9 (100% [Down-up]; 93% [Top-down]).
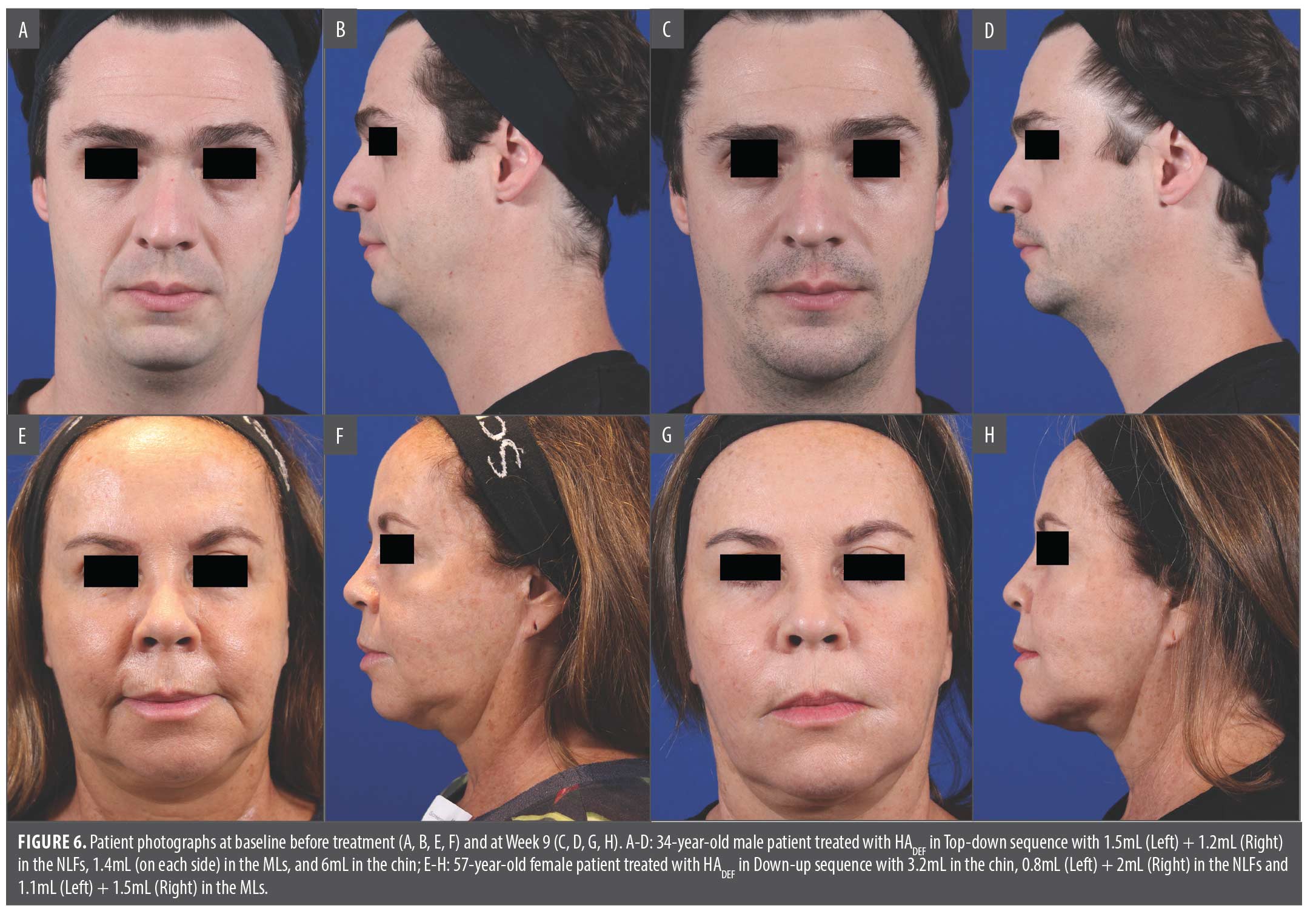
Photographic outcomes of Down-up and Top-down treatment are provided in Figure 6.
IPR-blinded assessment of photographs at Week 3, after the first treatment, showed a slight tendency in favor of the Down-up treatment for all variables assessed. The IPR reviewer reported a higher proportion of patients with improved facial harmony at Week 3 compared to baseline in the Down-up (74%) vs. Top-down (59%) group, as well as greater attractiveness (74% Down-up vs. 62% Top-down) and more masculinity/femininity (81% Down-up vs. 62% Top-down). At Weeks 6 and 9, there was no longer any tendency of a higher improvement in the Down-up group, as both groups showed similar, high rates of improvement from baseline of approximately 73-80% for all variables. The layman board blinded photo evaluations of attractiveness did not favor either group at any timepoint (data not shown).
Safety. Treatment-related AEs were reported in 19% (6 of 32 patients; 8 events) in the Down-up and 14% (4 of 29 patients; 6 events) in the Top-down groups. All of these AEs were non-serious, mild, and resolved within one week. In addition, they were all anticipated events related to the injection procedure, ie, implant site pain (19% of patients in the Down-up group and 10% in the Top-down group), implant site bruising (3% and 7%, respectively), and implant site swelling (both groups 3%).
Discussion
This randomized multicenter study assessed the clinical outcomes following treatment of the chin, NLFs, and MLs with HADEF, using two different stepwise approaches, Top-down and Down-up. Both approaches achieved similar, favorable results at nine weeks after treatment, including overall aesthetic improvement, improved skin firmness and facial harmony, aesthetic improvement of the submental area for those who needed it, natural-looking results, and high patient satisfaction. Furthermore, HADEF was well tolerated throughout the study period. The results support that lower face treatment using HADEF can achieve the desired aesthetic outcome and a high level of patient satisfaction regardless of the treatment order.
Investigator-reported submental improvement at Week 3 (Figure 3) and the IPR results following treatment of only one of the areas (chin or MLs/NLFs) tended to favor the Down-up approach, reflecting the importance of the chin area for facial harmony and that treating the chin first may provide a faster and more noticeable improvement of facial appearance. This would suggest that the Down-up approach may be the preferable treatment order. However, since a between-group difference at Week 3 was not a consistent observation in all effectiveness assessments, and since the results at Week 9 overall showed little difference between the groups, any difference resulting from the order of treatment seems to be temporary and of minor importance for the final clinical outcome.
A slightly smaller mean volume (0.5mL less) was used in the MLs in the Top-down group versus the Down-up group. One can speculate that this reflected individual patient needs (the group sizes were rather small), or maybe injectors were more cautious to not overinject MLs before treatment of the chin.
The safety results of the study did not indicate any new types of adverse events beyond those already described for HADEF in the product’s “Instructions for Use.”1
Limitations. Limitations of this study include a small sample size, largely nonblinded design (blinded IPR and layman board assessors), and short duration of the study, which makes the results indicative rather than definitive and limits the conclusions we can make about long-term results. However, the safe and effective use of HADEF in the lower face, including NLFs and chin for up to 12 months after treatment, has already been demonstrated in several pivotal investigations,11,4–6 and the sample size of 60 patients was regarded as sufficient for the purpose of this comparison. With this in mind, the findings that either the Top-down or Down-up treatment approach for administering lower face HA filler treatments can lead to satisfactory outcomes is relevant information for clinical practitioners. In addition, the study results may inspire future studies of longer duration, among larger populations, with more diverse treatment approaches.
In conclusion, this study supports that both stepwise approaches may be used for administering HADEF when treating the chin, NLFs, and MLs, and achieve similar, favorable results at nine weeks after aesthetic treatment. Furthermore, results support the use of HADEF in combined treatment areas with high aesthetic improvement and patient satisfaction.
Ethical Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards and Good Clinical Practice. The study protocol was approved by Independent Ethics Committees/Institutional Review Boards.
References
- Restylane Defyne [directions for use]. 2023. Accessed 3/20/23 from https://www.accessdata.fda.gov/cdrh_docs/pdf14/P140029S027C.pdf.
- Lundgren B, Sandkvist U, Bordier N, Gauthier B. Using a new photo scale to compare product integration of different hyaluronan-based fillers after injection in human ex vivo skin. J Drugs Dermatol. 2018;17(9):982-986
- Öhrlund Å. Evaluation of rheometry amplitude sweep cross-over point as an index of flexibility for HA fillers. J Cosmetics, Dermatological Sciences and Applications. 2018;8:47-54
- Marcus K, Moradi A, Kaufman-Janette J, et al. A randomized trial to assess effectiveness and safety of a hyaluronic acid filler for chin augmentation and correction of chin retrusion. Plast Reconstr Surg. 2022;150(6):1240e-1248e.
- Xie Y, Wu W, Xu J, et al. A randomized, multicenter study on a flexible hyaluronic acid filler in treatment of moderate to severe nasolabial folds in a Chinese population. J Cosmet Dermatol. 2022;21(10):4288-4293
- Xie Y, Zhao H, Wu W, et al. Chin augmentation and treatment of chin retrusion with a flexible hyaluronic acid filler in Asian subjects: A randomized, controlled, evaluator-blinded study. Aesthetic Plast Surg. 2024;48(5):1030-1036
- Philipp-Dormston WG, Wong C, Schuster B, et al. Evaluating perceived naturalness of facial expression after fillers to the nasolabial folds and lower face with standardized video and photography. Dermatol Surg. 2018;44:826-832
- Solish N, Bertucci V, Percec I, et al. Dynamics of hyaluronic acid fillers formulated to maintain natural facial expression. J Cosmet Dermatol. 2019;18:738-746
- Pusic AL, Klassen AF, Scott AM, Cano SJ. Development and psychometric evaluation of the FACE-Q satisfaction with appearance scale: a new patient-reported outcome instrument for facial aesthetics patients. Clin Plast Surg. 2013;40:249-260
- Klassen AF, Cano SJ, Schwitzer JA, et al. FACE-Q scales for health-related quality of life, early life impact, satisfaction with outcomes, and decision to have treatment: development and validation. Plast Reconstr Surg. 2015;135:375-386
- Baumann L, Weiss RA, Grekin S, et al. Comparison of hyaluronic acid gel with (HARDL) and without lidocaine (HAJUP) in the treatment of moderate-to-severe nasolabial folds: A Randomized, Evaluator-Blinded Study. Dermatol Surg. 2018;44(6):833-840.