 by Philip Milam, MD; Michael Berger, PharmD; Bhuvaneswari Ramaswamy, MD; Raquel Reinbolt, MD; Robert Wesolowski, MD; and Benjamin H. Kaffenberger, MD
by Philip Milam, MD; Michael Berger, PharmD; Bhuvaneswari Ramaswamy, MD; Raquel Reinbolt, MD; Robert Wesolowski, MD; and Benjamin H. Kaffenberger, MD
Drs. Milam and Kaffenberger are with the Division of Dermatology at The Ohio State University Wexner Medical Center in Columbus, Ohio. Drs. Berger, Ramaswamy, Reinbolt, and Weslowski are with the Division of Medical Oncology at The Ohio State University Comprehensive Cancer Center in Columbus, Ohio.
FUNDING: No funding was provided for this study.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Objective.We describe three patients treated with ado-trastuzumab emtansine who developed either palmar flushing or spider angiomas. We then correlate these findings with radiologic imaging results of the liver.
Design. Three consecutive referrals to dermatology for patient skin complaints while taking ado-trastuzumab emtansine were evaluated and found to have either telangiectases or palmar flushing. Two patients who did not have prominent nodularity on computed tomography of the liver underwent transient elastography.
Results. All three patients with stigmata of liver disease that developed during the course of ado-trastuzumab emtansine demonstrated parenchymal liver abnormalities.
Conclusion. Patients presenting with spider angiomas and/or palmar flushing on ado-trastuzumab emtansine should be considered for elastography studies to ensure early structural damage is not occurring. Further investigation is needed to define the precise etiology for these telangiectases and the role of elastography imaging in monitoring patients on long-term ado-trastuzumab emtansine.
KEYWORDS: Ado-trastuzumab emtansine, cirrhosis, metastatic breast cancer, nodular regenerative hyperplasia, palmar flushing, pseudocirrhosis, T-DM1, telangiectasia
J Clin Aesthet Dermatol. 2019;12(7):23–26
Ado-trastuzumab emtansine (T-DM1) is an antibody-drug conjugate containing the antihuman epidermal growth receptor 2 (HER2) antibody trastuzumab linked with the tubulin inhibitor emtansine. It is approved by the United States Food and Drug Administration (FDA) as an intravenous infusion given every three weeks in patients with advanced breast cancer who have failed trastuzumab and a taxane. We describe three women who presented with cutaneous stigmata of liver disease without displaying high-grade transaminase elevations during their treatment.
Case Presentations
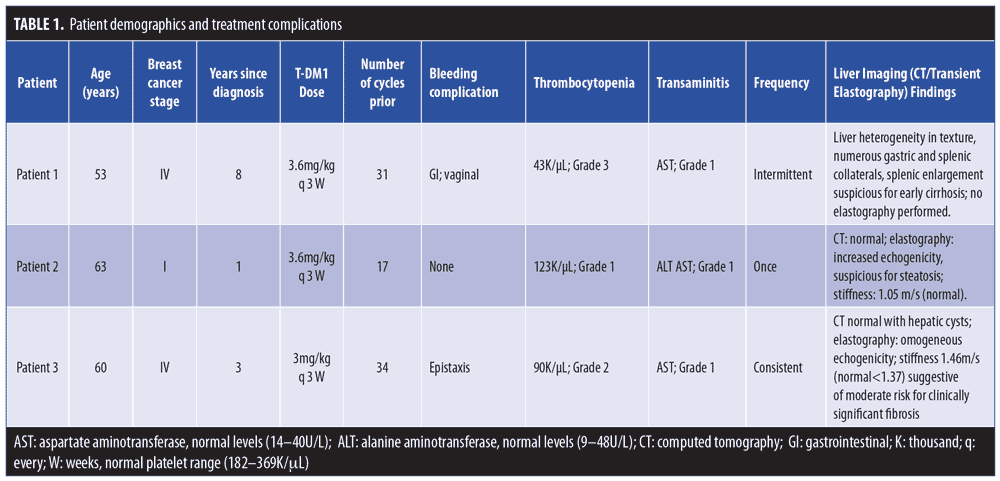
Case 1. A woman in her 50s was diagnosed with Stage IIIC breast cancer in 2009. Despite multiple therapies, she had developed progressive brain and pulmonary metastases. She started single-agent T-DM1, which stabilized her metastatic disease. After 31 cycles, she was found to have innumerable spider angiomas (Figure 1). Abdominal computed tomography (CT) and magnetic resonance imaging (MRI) demonstrated subtle liver nodularity, splenic enlargement, and diffuse heterogeneity consistent with early cirrhosis.
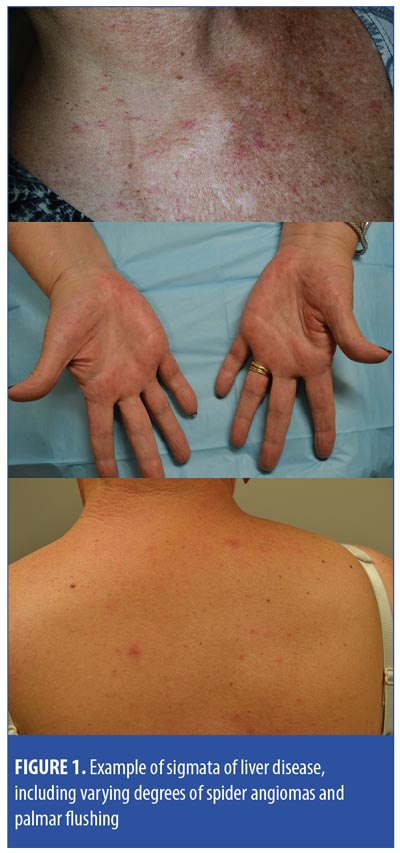
Case 2. A woman in her 60s with Stage I breast cancer was enrolled in a clinical trial for adjuvant therapy with T-DM1 for one year. Following completion, she presented with palmar erythema and a solitary spider angioma contralateral to her radiation (Figure 1). Her aspartate transaminase/alanine aminotransferase (AST/ALT) values were normal save for in one instance that was attributed to concomitant acetaminophen. Transient elastography identified hepatic steatosis.
Case 3. A woman in her 60s presented with Stage IV breast cancer. Despite multiple therapies, she developed disease progression in her liver, at which point, single-agent T-DM1 was started. She received 18 cycles before requiring dose reduction for treatment-related peripheral neuropathy; eventually, usage was stopped altogether. T-DM1 dosing was resumed one year later, and the patient received an additional 25 cycles. After her 17th cycle, she was found to have spider angiomas on the upper chest, back, and face. Transient elastography demonstrated increased liver stiffness.
Table 1 summarizes patient information and treatment complications.
Discussion
T-DM1 is composed of DM1 (a maytansine derivative that binds to ?-tubulin and inhibits microtubule assembly) that is covalently conjugated to trastuzumab by a thioether bond.1 T-DM1 has demonstrated the ability to extend progression-free and overall survival compared to standard-of-care therapy in patients with metastatic breast cancer who had progressed on prior trastuzumab-containing chemotherapy regimens.2,3 Despite the additional cytotoxicity, T-DM1 is generally well-tolerated, but laboratory risks of thrombocytopenia and transaminase elevation were noted to occur in 30 to 40 percent of patients.2,3 While not seen during clinical trials, several investigators have published reports of spider angiomas, telangiectases, and mucosal telangiectases in this population.4,5 In one case series of five such patients, the authors did not detect HER2 expression in endothelial cells; however, they did observe mild mucosal bleeding in three of the five patients, despite insignificant or mild thrombocytopenia and proposed mucosal telangiectases as the etiology of these events.4 In a follow-up series, the authors demonstrated mucosal bleeding events in an additional five patients, also without significant thrombocytopenia.5 The authors note that all 10 patients were reported to have Grade 1 transaminitis throughout their treatment course of T-DM1. With the concern for hereditary hemorrhagic telangiectasia, another report demonstrated widespread mucosal involvement together with skin involvement, gastrointestinal bleeding, and pulmonary hypertension—all findings associated with hereditary hemorrhagic telangiectasia.6 While these authors suspected an etiology similar to hereditary hemorrhagic telangiectasia syndrome, the cases did not demonstrate a linking mechanism without HER2 expression in endothelial cells, and nearly all patients had at least Grade 1 liver toxicity. Elsewhere, translational research has demonstrated that hepatocytes express HER2 receptors and, while the trastuzumab molecule itself is not toxic, hepatocyte apoptosis occurs in a dose-dependent manner when the microtubule inhibitor DM1 is linked to the trastuzumab molecule.7 Although mild elevations in transaminases were seen in these patients, no additional clinical liver studies have been correlated as we show through elastography.
Interestingly, in our patients, the density of cutaneous stigmata was associated with the degree of liver fibrosis. For two patients, however, structural damage in the liver was confirmed only with transient elastography and was not noted on routine computed tomography imaging. This is remarkable because, while liver toxicity has been well-described with T-DM1, structural damage (primarily reported as nodular regenerative hyperplasia) is rare and has been observed only in seven patients during clinical trials.8 Transient elastography, along with magnetic resonance elastography, are techniques that employ a vibrating transducer to detect the density of the liver and are particularly useful in patients with skin and autoimmune diseases treated with long-term methotrexate.9 Magnetic resonance elastography analyzes the propogation of acoustic waves through the target tissue to assess its elasticity, and then a contrast magnetic resonance protocol translates the fibrotic characteristics into an image.9 Transient elastography is similar, but uses an ultrasound transducer to examine certain acoustic windows of the liver as determined by the technician.9 Compared to the gold-standard liver biopsy, both techniques show extremely high agreement in the staging of hepatic fibrosis in a noninvasive manner.10,11 Interestingly, methotrexate can also result in a similar phenomenon wherein patients have low-grade or no transaminitis, which might ultimately present as liver fibrosis.12 Whether these changes are progressive and to what degree these changes occur in a larger population remain to be seen.
The development of spider angiomas and palmar erythema occurs in the minority of patients with liver cirrhosis and traditionally has been associated with hyperestrogenemia due to decreased liver metabolism or alcohol-mediated vasodilation.13 Newer studies demonstrate that, while levels of estradiol are elevated in patients with liver cirrhosis, spider angiomas are not necessarily associated with alcoholism, but rather are most strongly associated with younger age and angiogenic factors such as vascular endothelial growth factor, basic fibroblastic growth factor, and substance P.14,15 These angiogenic factors have not been analyzed in patients with metastatic breast cancer and T-DM1, but tumor response in these cases might result in the release of angiogenic factors.
Two diagnoses that need to be differentiated include nodular regenerative hyperplasia and pseudocirrhosis. Nodular regenerative hyperplasia is another structural liver change that is now associated with T-DM1 use, albeit rarely.8 This condition is a chronic fibrosing condition of the liver comprising scattered pockets of hepatocyte hyperplasia surrounded by areas of fibrosis, and most frequently results in noncirrhotic portal hypertension. This condition has been associated with death during clinical trials.8 While clinical trial patients who developed this change were heavily pretreated, this phenomenon has now been reported in a patient who received T-DM1 as first-line for her metastatic breast cancer, suggesting nodular regenerative hyperplasia might be most strongly linked with T-DM1.16 This condition is still one that is diagnosed by liver biopsy, although it stands to benefit that future publications report on whether it can be defined elastographically. Pseudocirrhosis, another closely linked phenomenon, can also include histologic features of nodular regenerative hyperplasia. It is usually seen in metastatic breast cancer patients when metastatic tumors respond to chemotherapy, resulting in fibrosis and an irregular contraction of the liver capsule, desmoplasia of infiltrating cells, and portal hypertension.17 This is ultimately differentiated from cirrhosis by liver biopsy, but pathophysiological function similarly causes portal venous hypertension.18,19 None of our patients were biopsied to rule out pseudocirrhosis or nodular regenerative hyperplasia, and it is possible that these conditions might all intersect pathophysiologically. Further, at this time neither pseudocirrhosis nor nodular regenerative hyperplasia of metastatic breast cancer have been linked with spider angiomas, palmar flushing, or other nonportal, vein-associated cutaneous features of liver disease. Interestingly, nodular regenerative hyperplasia has been associated with ataxia telangiectasia syndrome, hereditary hemorrhagic telangiectases, and generalized essential telangiectasia syndromes and thus, perhaps, represents a shared reactionary pathway.20–22
Our report identifies that T-DM1 might be associated with liver structural damage, despite a lack of high-grade transaminitis. At this time, there exists mechanistic evidence and population-level evidence to link T-DM1 directly to hepatotoxicity, but further research is needed to differentiate this condition from pseudocirrhosis and nodular regenerative hyperplasia. Based on this study, we believe patients demonstrating telangiectases or palmar flushing should be evaluated and potentially monitored using a liver elastography technique. These changes are demanding of further study, particularly to discern whether they relate to a hyperestrogenemic state or elevated levels of vascular peptides, and to ascertain the time course after starting T-DM1.
References
- LoRusso PM, Weiss D, Guardino E, et al. Trastuzumab emtansine: a unique antibody-drug conjugate in development for human epidermal growth factor receptor 2-positive cancer. Clin Cancer Res. 2011;17(20):6437–6447.
- Krop IE, Kim SB2, González-Martín A, et al. Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(7):689–699.
- Verma S, Miles D, Gianni L, et al. Trastuzumab Emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367(19):1783–1791.
- Sibaud V, Niec RE, Schindler K, et al. Ado-trastuzumab emtansine-associated telangiectasias in metastatic breast cancer: a case series. Breast Cancer Res Treat. 2014;146(2): 451–456.
- Sibaud V, Vigarios E, Combemale P, et al. T-DM1-related telangiectasias: a potential role in secondary bleeding events. Ann Oncol. 2015;26(2):436–437.
- Kwon Y, Gomberg-Maitland M, Pritzker M, Thenappan T. Telangiectasia and pulmonary arterial hypertension following treatment with trastuzumab emtansine: a case report. Chest. 2016;149(4):e103–e105.
- Yan H, Endo Y, Shen Y, et al. Ado-trastuzumab emtansine targets hepatocytes via human epidermal growth factor receptor 2 to induce hepatotoxicity. Mol Cancer Ther. 2016;15(3): 480–490.
- Diéras V, Harbeck N, Budd GT, et al. Trastuzumab emtansine in human epidermal growth factor receptor 2-positive metastatic breast cancer: an integrated safety analysis. J Clin Oncol. 2014;32(25):2750–2757.
- Kaffenberger BH, Kaffenberger JA, Wong H, et al. Magnetic resonance elastography and transient elastography as non-invasive analyses for liver fibrosis: can they obviate the need for liver biopsy in psoriasis patients treated with methotrexate?. Int J Dermatol. 2015;54(7):752–756.
- Friedrich-Rust M, Ong MF, Martens S, et al. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134(4):960–974.
- Wang QB, Zhu H, Liu HL, Zhang B. Performance of magnetic resonance elastography and diffusion-weighted imaging for the staging of hepatic fibrosis: a meta-analysis. Hepatology. 2012;56(1):239–247.
- Lindsay K, Fraser AD, Layton A, et al. Liver fibrosis in patients with psoriasis and psoriatic arthritis on long-term, high cumulative dose methotrexate therapy. Rheumatology (Oxford). 2009;48(5): 569–572.
- Liu SW, Lien MH, Fenske NA. The effects of alcohol and drug abuse on the skin. Clin Dermatol. 2010;28(4):391–399.
- Li CP, Lee FY, Hwang SJ, et al. Spider angiomas in patients with liver cirrhosis: role of vascular endothelial growth factor and basic fibroblast growth factor. World J Gastroenterol. 2003;9(12):2832–2835.
- Li CP, Lee FY, Hwang SJ, et al. Role of substance P in the pathogenesis of spider angiomas in patients with nonalcoholic liver cirrhosis. Am J Gastroenterol. 1999;94(2):502–507.
- Prochaska LH, Damjanov I, Ash RM, et al. Trastuzumab emtansine associated nodular regenerative hyperplasia: a case report and review of literature. Cancer Treat Commun. 2016;5:26–30.
- Adike A, Karlin N, Menias C, Carey EJ. Pseudocirrhosis: a case series and literature review. Case Rep Gastroenterol. 2016;10(2): 381–391.
- Jüngst C, Krämer J, Schneider G, et al. Subacute liver failure by pseudocirrhotic metastatic breast cancer infiltration. Ann Hepatol. 2013;12(5): 834–836.
- Diamond JR, Finlayson CA, Borges VF. Hepatic complications of breast cancer. Lancet Oncol. 2009;10(6):615–621.
- Milligan KL, Schirm K, Leonard S, et al. Ataxia Telangiectasia associated with nodular regenerative hyperplasia. J Clin Immunol. 2016;36(8):739–742.
- Rothweiler S, Heim MH, Semela D, et al. Nodular regenerative hyperplasia in a patient with generalized essential telangiectasia: endotheliopathy as a causal factor. Hepatology. 2014;59(6):2419–2421.
- Scardapane A, Ficco M, Sabbà C, et al. Hepatic nodular regenerative lesions in patients with hereditary haemorrhagic telangiectasia: computed tomography and magnetic resonance findings. Radiol Med. 2013;118(1):1–13.

