 J Clin Aesthet Dermatol. 2021;14(9 Suppl 1):S29–S35
J Clin Aesthet Dermatol. 2021;14(9 Suppl 1):S29–S35
by Emma Davies, RN, INP; Devan Vaghela, MBBS, MRCP; Cormac Convery, MB ChB, MSc; Lee Walker, BDS, MFDS, RCS; and Gillian Murray, MPharm, PG Clin Pharm INP
Ms. Davies is the clinical director of Save Face UK. Dr. Vaghela is with the Department of Microbiology at the Norfolk and Norwich University Hospitals United Kingdom and with V&A Aesthetics Clinic in London United Kingdom. Dr. Convery is with the Ever Clinic Glasgow in Glasgow, Scotland. Ms. Murray is with the Clinical Academic Kings College London in London, England. Dr. Walker is with B City Clinic in Liverpool, England. All authors are founding board members of the Complications in Medical Aesthetics Collaborative (CMAC).
FUNDING: No funding was provided for the preparation of this article.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: The Complications in Medical Aesthetics Collaborative (CMAC) is a nonprofit organization established to promote best patient outcomes through educating clinicians in the prevention, diagnosis, and management of complications that can arise following nonsurgical cosmetic procedures. The organization is a global community sharing information, learning, experience, and data to promote best practices. There is no reliable data on the risk or incidence of acute infection following a nonsurgical cosmetic procedure, but it is considered to be a rare complication. This article explores the evidence base for precautions and best practice standards, including an examination of the rational for standard aftercare advice and guidance on prevention, diagnosis, and management protocols.
KEYWORDS: Acute infection dermal fillers, acute infection cosmetic treatment, skin infection, antibiotic stewardship, acute infection treatment, acute skin infection diagnosis, dermal fillers risks, complications dermal fillers
Acute bacterial skin and soft tissue infections (SSTIs) can occur following any procedure in which the normal integrity of the skin is breached. There are no reliable data on the incidence of acute infections following nonsurgical cosmetic procedures; this could be because patients seek treatment from their primary care physicians or other healthcare providers, for a second opinion, rather than the clinician who performed the procedure, which prevents reliable audit in practice. There is no agency for central reporting. Acute infection following a nonsurgical cosmetic procedure is likely to be categorized as injector- or patient-related, so these adverse events may not be included in post-marketing surveillance reports.
Acute bacterial SSTIs following nonsurgical cosmetic procedures occur rarely if good infection prevention and control measures are taken, the patient’s history is screened for risk factors, and the patient is adherent to aftercare instructions. Acute bacterial SSTIs may be rarely seen or reported, but bacterial contamination at the time of injection can lead to delayed complications that are difficult to treat.1 This guideline presents the etiology, pathogenesis, prevention, diagnosis, and management of acute bacterial SSTIs in nonsurgical medical facial cosmetic practice.
Bodily Defenses and Pathogenesis
The skin serves as one of the body’s first lines of defense against harmful microbes. The antimicrobial barrier function of the skin is primarily localized to the stratum corneum, which limits the growth of bacteria with its low water content, acidic pH, resident microflora, and surface lipids.2 The diverse communities of beneficial bacteria that colonize healthy skin, collectively known as the skin microbiota, are referred to as commensals. Commensals inhibit the colonization by pathogenic species through site competition and the production of antimicrobial substances.3 If the stratum corneum is breached, immune cells, such as Langerhans, T-lymphocytes, and macrophages, act as second lines of defense.4 Superior to the groin, the most common species are Staphylococcus aureus (S. aureus), Streptococcus pyogenes (S. pyogenes), and Corynebacterium sp.
Commensal organisms can be pathogenic when the stratum corneum is breached by way of trauma, causing invasion into underlying tissues and interaction with host defenses. In immunocompetent individuals with healthy skin, these invasions are typically dealt with effectively by the body’s natural defense mechanisms.5 In nonsurgical cosmetic procedures, the risks for infection are increased due to potential innoculation by way of insanitary injectable products or by challenging the normal barrier functions of the skin.
Risk Factors of Infection
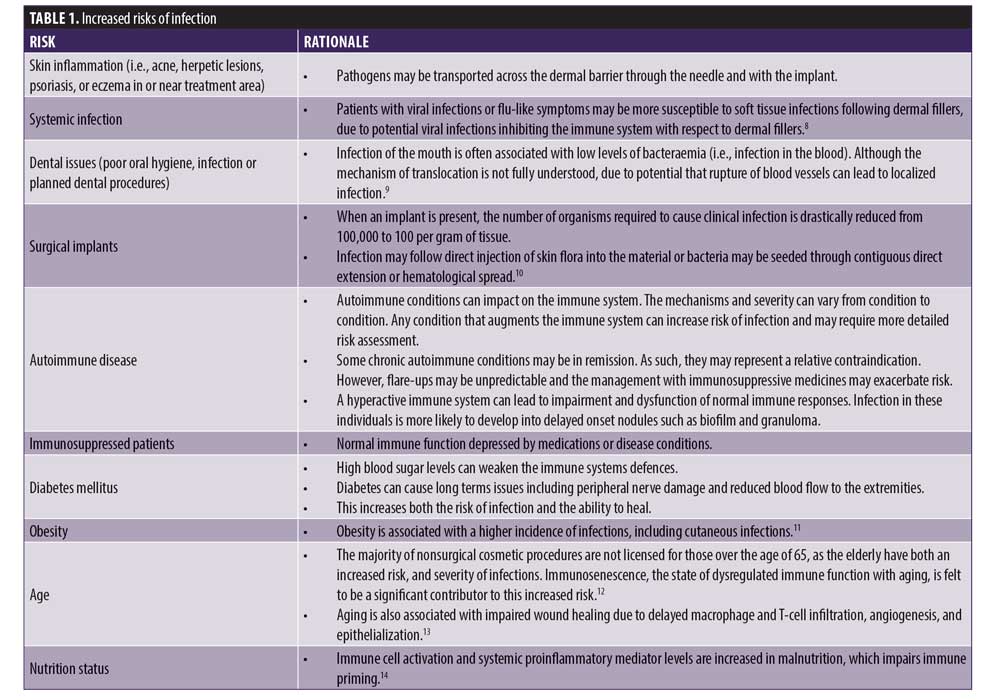
Patient-related risks. Patient-related risk factors correlate to a poorer prognosis, more rapid progression of disease, slower healing, and more resistant pathogens.6 Prior to performing a cosmetic procedure, a full history should be taken and a thorough examination of the patient performed to identify any skin conditions that might increase risk of infection. Table 1 describes patient-related risk factors.
Procedural risks. There is naturally a high bacterial population on “clean skin”— around one million organisms per square centimeter.14 When foreign material is injected into the body, such as a dermal filler, the host immunity can be compromised, making it possible for as few as 100 Staphylococcus aureus bacteria to initiate infection.16 The risks associated with injection of vaccine or drugs that rapidly diffuse into subcutaneous tissue cannot be transposed to those associated with dermal implants/fillers because injectables create a volume of material that is more difficult for the immune system of the body to access, and these materials remain in situ for several months.16
Ultimately, it is the clinician’s responsibility to understand and manage all the identified risks for infection in each patient before, during, and after performing any cosmetic procedures. When infection does occur, it is most likely due to contamination during the procedure.9 Strict adherence to infection prevention and control measures should be maintained.
An online survey of over 5,000 United States (US)-based clinicians who prepare or administer parenteral medicines revealed the following alarming statistics.
Six percent of respondents said they sometimes or always use single dose vials for more than one patient.
Nearly one percent of respondents said they sometimes or always reuse a syringe but change the needle to use on a second patient.
Over 15 percent of respondents said they reuse a syringe to enter a multidose vial.
Six and a half percent of respondents said they saved that vial for use on another patient.
Patients might not raise concerns during a cosmetic procedure, but if they develop a postprocedural infection, they might assign blame to the clinician who performed the procedure. Should a complication arise, documenting and adhering to a treatment protocol that reflects the highest standards of technique can provide important support to the practitioner presenting evidence as part of any claim.18
Infection Control Protocol
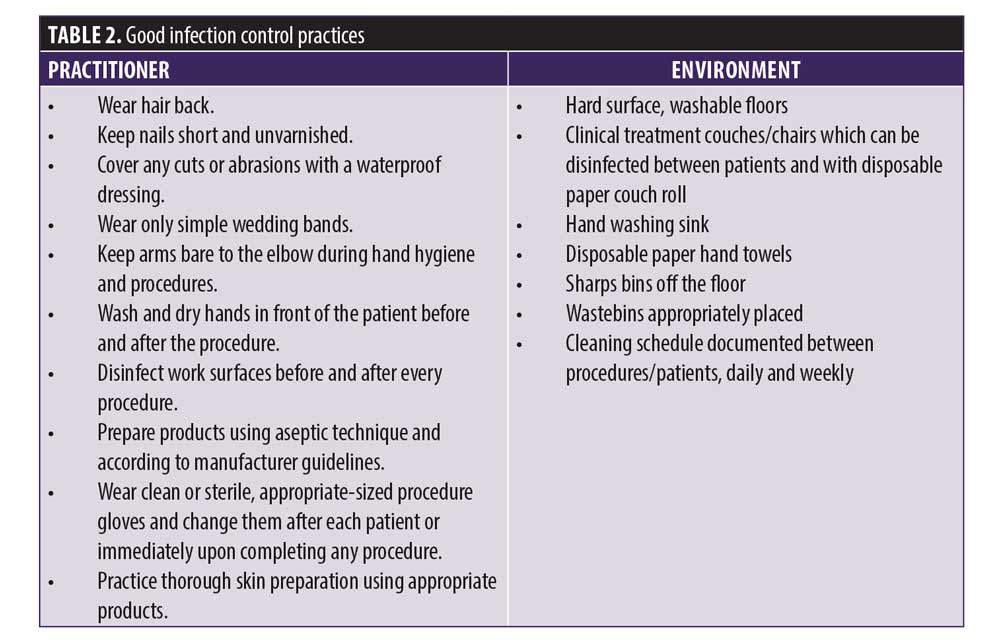
Practitioners have a duty of care to patients to ensure treatment is delivered safely according to best practice standards. Table 2 outlines good infection control practice. These steps should be routinely and consistently followed to minimize infection risks to the patient. Both the patient and the practitioner should feel confident that all precautions have been followed.
Skin preparation. Many patients will attend their appointments wearing cosmetic makeup. All makeup, not just that applied to the area that will be treated, and other potential topical contaminants should be removed with a facial wash prior to the procedure. Facial wipes that contain preservatives can cause irritation and leave behind residual chemicals, and they might not effectively remove all topical products on the skin. An antiseptic skin preparation should be used on clean skin. The optimal antiseptic skin preparation should offer the following:
- Significantly reduce microorganisms on intact skin
- Be broad spectrum
- Be fast-acting
- Have persistent effect
- Be nonirritating.
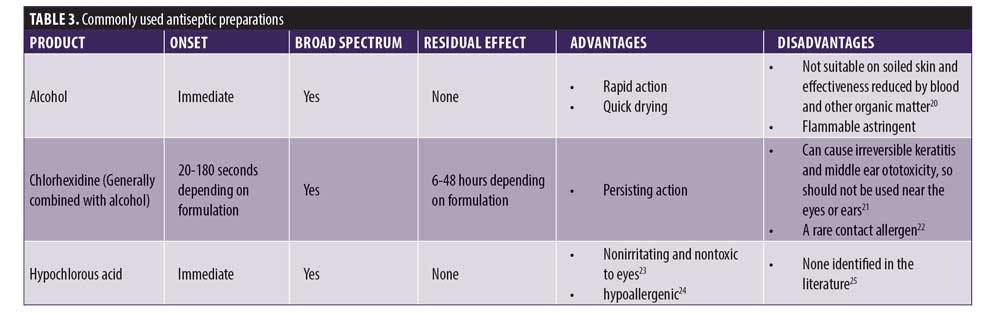
Skin cleansing has the aim of decreasing the bacterial counts of resident flora. It is not possible to sterilize skin because approximately 20 percent of skin bacteria are located in deep layers where antiseptics cannot penetrate.19 There are no clear, evidence-based guidelines regarding which antiseptic skin preparation is best to use in aesthetic practice. While the various available preparations all have advantages and disadvantages, any of the commonly used solutions will adequately do what they are supposed to do if used appropriately; however, CMAC does not recommend the use of alcohol alone as an antiseptic due to its lack of residual effect. See Table 3 for details of commonly used antiseptic preparations. After taking into consideration any sensitivity issues a patient may have, the skin should be cleansed with an antiseptic preparation after application of ice (if used) and removal of all makeup or other topical products from the skin. Keep in mind that ice is a potential source of contamination.26 The antiseptic preparation should be allowed to completely air dry on the skin.
Antiseptic skin preparations are available as solutions, swabs, pads saturated with a solution, and applicators containing a solution. Topical antiseptic products are not necessarily sterile and can themselves be a source of bacterial infection. In 2012, the US Food and Drug Administration (FDA) responded to concerns and reports of infections resulting from use of certain antiseptic products.28 Over-the-counter topical antiseptics for pre-injection skin preparation are not required to be sterile unless labeled as such; thus, it is possible that an antiseptic product can become contaminated during the manufacturing process. use. Consequently, the FDA advises that single-use products should be used when possible. The FDA also recommends that antiseptic solutions should not be diluted after opening and that sterile, single-use packaging/containers should be discarded properly after one use.29
Device and pharmaceutical preparation. Medical devices should be compliant with standards of safety and efficacy according to national regulations. Nondisposable parts should be sterilized before and after each use, and any single-use products associated with the device should only be used once and discarded according to manufacturer guidelines. Prescription medications should be sourced only from licensed and regulated pharmacies. Drugs should be used within their expiration date and be discarded appropriately in accordance with local regulations and policy.
Aesthetic products should be reconstituted using aseptic techniques, and any unused portions should be discarded as per manufacturer guidelines. If multidose vials are used, both the needle and syringe used to access the multidose vial should be sterile and the cap disinfected prior to penetration. Multidose vials should be stored according to manufacturer guidelines and discarded within 28 days unless the manufacturer advises otherwise.36
Administration of procedure. An aseptic, “no-touch’”technique should be adopted. Ensure the needle or cannula is not contaminated during injection procedures (i.e., do not let the needle or cannula touch the skin except at the injection/insertion point). If any nonsterile surface is inadvertently touched, the needle/cannula should be changed. It is important to accurately document your treatment protocol.
Aftercare instructions for patients. Risks of infection. All patients should be informed of the risk of infection and the importance of adherence to the aftercare instructions as part of the consent process. They should be provided with written aftercare instructions regarding signs and symptoms of complications. Generally, aftercare instructions commonly require patience adherence for 4 to 12 hours, but specific evidence supporting this advice is lacking. This time frame might correlate to the time taken for needle channels to close.
For treatments that disrupt the barrier functions of the skin, such as resurfacing procedures and chemical peels, aftercare instructions should follow manufacturer guidelines to optimize recovery of healthy skin barrier functions. Following injectable procedures, the goal should be to prevent bacterial contamination via the needle channels until they have closed.
While the needles used to administer dermal fillers are longer and of a wider gauge, studies evaluating microneedle channels for transdermal delivery of drugs report that needle channels close within 10 to 15 minutes.30 However, more recently, a study on hairless rats found that microchannels didn’t close until 15 to 72 hours had passed if occluded.31 Needle size and length do affect closure time, as would be expected.
While it is clear that needle channels breach the stratum corneum barrier function of the skin and allow the passage of drugs, studies evaluating microneedle channels are not looking at these channels as a lasting route for invasion of pathogenic microbes, where the additional defenses deployed in the skin should be expected to engage to prevent effective invasion. Thus, the evidence base for a timeframe of 4 to 12 hours for adherence to aftercare instructions is cautionary rather than supported by an evidence base. In nonsurgical injectable cosmetic procedures, the risks appear to be more significant during the procedure rather than postprocedure.
Using topical products (e.g., makeup, other over-the-counter [OTC] topicals). Cosmetics not only contain particles that can enter the injection points as irritants or foreign body particles but have also been shown to host a number of pathogenic microbes. P. aeruginosa, E. coli, S. epidermidis, and S. aureus have all been cultured from makeup samples in a department store, as part of an American TV program that aimed to illustrate the importance of taking care of cosmetics.31 Ultimately, there is a lack of directly applicable evidence, but it should not be assumed that makeup is sterile or clean; thus, avoiding the use of makeup or other OTC topical products for at least 12 hours is prudent advice to give to your patients.
Face touching. Human beings are habitual and unconscious face touchers. In a study published in 2008, Berkley students under video surveillance were found to touch their faces an average of 16 times per hour. Over a three hour period, the number of eye, nose, and lip touches varied from 3 to 104 times. Even in public, researchers saw 29 episodes of nose picking.33 There are many surfaces people commonly touch throughout the day, with varying levels of contamination. Consider the following:34
- Toilet seat: 1,201 bacteria per in2
- Kitchen counter: 1,736 bacteria per in2
- Pet food dish: 2,110 bacteria per in2
- Checkout screen: 4,500 bacteria per in2
- Doorknob: 8,643 bacteria per in2
- Mobile phones: 25,127 per in2
Thus, when we consider what we do with our hands and how many objects and surfaces we touch every hour of the day without washing our hands and then consider how many times we touch our faces, it’s surprising that frequent handwashing prior to the COVID-19 pandemic was not generally part of postprocedure advice given to patients. It would seem highly unlikely that, even with the best intentions, patients can avoid touching their faces; thus, advising patients to frequently wash their hands throughout the day would be appropriate.
Diagnosis of Infection
Signs and symptoms. Acute infection has a rapid onset, usually presenting within 3 to 4 days of exposure. Typical presenting features include erythema, edema, pain, and heat. More severe infections can present with systemic symptoms, such as a fever. Infection may initially be limited to the treatment area, but it can spread beyond the treatment area if left untreated. In severe infections, it may be possible to palpate crepitus and fluctuance due to fluid or gas collections under the skin. Skin numbness may be a late finding in severe infections.35
Differential diagnoses. Erythematous skin lesions do not always represent bacterial infections, and skin infection can occur coincidentally near the site of minimally invasive cosmetic procedures. However, in these cases, the infection may not necessarily be related to the dermal filler, unless the injection point was directly through an acne lesion. Allergic reactions are extremely rare, but it is important to distinguish a rash postprocedure to that of an infection. Herpetic lesions must be considered, especially if the dermal filler has been administered to the lips.
Types of Acute Infection
Cellulitis. Cellulitis is defined as non-necrotizing infection of the skin and the subcutaneous tissue that does not involve fascia or muscle. It is characterized by erythema, and the affected area is warm to the touch. Often the skin is tender on palpation. There is no mass-like lesion, and this type of infection is often self-limiting in immunocompetent people, though a small proportion of people may require antibiotic treatment.
Abscess. Abscesses are cavities with purulent exudate comprising white cells and bacteria (often dead). This type of infection may be caused by of number pathogenic organisms (e.g., S. aureus and ?-hemolytic streptococci) resulting in necrosis of local tissue. There is aggregation of white cells and chemokines, and the subsequent inflammatory response walls off the purulent exudate that is accumulating. Ongoing infection leads to further liquefaction of tissue and purulent exudate formation. This continues until the abscess wall is ruptured. If left untreated, an abscess can leave permanent skin and tissue atrophy.
Abscesses can be classified according to their anatomical site. In facial aesthetics, the two main sites are subcutaneous, which is most common, and intramuscular abscesses. The features of an abscess include a fluctuant mass that is tender on palpation and warm to the touch. There may be localized skin changes and the presence of a sinus at the point of injection. Often, the surrounding tissue will be unaffected and not painful. Subcutaneous abscesses are less likely to cause systemic signs, such as tachycardia and persistent pyrexia. Intramuscular abscesses tend to have more severe symptoms
Abscess formation can result from translocation of bacteria from the skin into the subacute tissue or due to the presence of periodontal infection at time of treatment. Formation of an abscess is often subacute, and symptoms can present at any time, though typically at least 14 days can pass before they manifest.
Osteomyelitis. Osteomyelitis is an infection of the bone. In aesthetic medicine, this type of infection can occur when the needle goes into the bone. It is exceedingly rare in medical aesthetics, though there have been case reports of such incidences.36 Bone is resistant to infection but, under certain circumstances (e.g., heavy bacterial inoculum, poor bone stock, patient comorbidities, the presence of prosthesis) infection is possible.
The characteristic feature of osteomyelitis is severe pain over the infected bone. The presence of a sinus implies severe infection. Symptoms are often delayed, with features presenting as much as a month after any procedure, but can occur at any point during the presence of dermal filler in the area. The presence of an implant increases the risk of infection.
Managing Infection
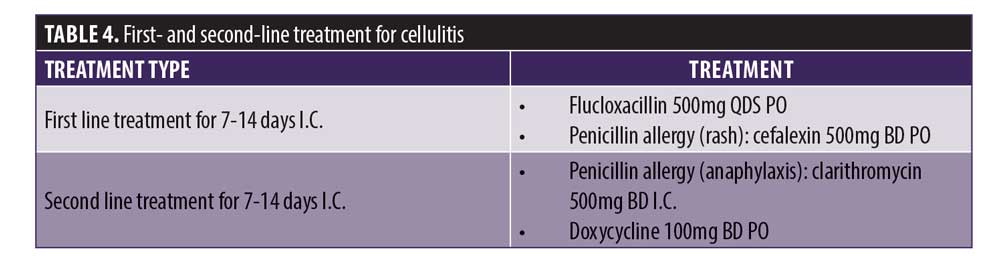
As part of the consent process, patients should be informed of the risks and symptoms of infection and and the importance of notifying the clinician for immediate assessment if symptoms of infection develop. This is particularly important in the presence of erythema, heat, tenderness, and/or swelling that is does not abate or worsens within the first 48 hours postprocedure. Nonprescribing clinicians should refer patients immediately to their prescribing practitioner for diagnosis and treatment. The general practitioner should be notified in accordance with professional standards and good medical practice with the consent of the patient. Medical history should be reviewed with particular attention to allergy/sensitivity or interactions with concomitant prescribed medicines. Consult your local formulary for first- and second-line treatments for acute skin infections, as local variations are common. If there is no response following four days of antibiotic treatment, a change in antibiotic regimen and swab for microbiology, culture, and sensitivity if a discharge is present should be considered.
Cellulitis. Cellulitis is a clinical diagnosis that often resolves without therapy if clinically mild or can be managed with a short course of antimicrobial therapy if required. Often there is no need to send any samples to the laboratory. The most common organisms are Gram-positive cocci (S. aureus and ?-Hemolytic streptococci), and these respond well to oral antibiotics if the infection is not severe. Table 4 lists the first- and second-line treatment for cellulitis. Treatment of cellulitis can take up to 14 days to resolve, but there should be clinical response within four days. If there is no response initially, this could be due to the following:
- Resistant organism including methicillin-resistant S. aureus (MRSA). Risk factors include being healthcare workers, living with healthcare worker or frequent hospital attender, or being an subcutaneous or intravenous drug abuser.
- Polymicrobial infection- particularly if a contaminated needle was used.
- Gram negative infection- particularly if a contaminated needle was used.
Periorbital cellulitis should be considered an emergency due to risk of orbital spread and subsequent blindness. This warrants immediate specialist referral to the on-call ophthalmologist.
In the UK, most ?-Hemolytic Streptococci are sensitive to ?-lactams such as flucloxacillin or amoxicillin. There is variation on resistant patterns to macrolides (clarithromycin, erythromycin), lincosamides (clindamycin) and fluoroquinolones (ciprofloxacin, levofloxacin).
It is important to be aware of a patient’s known allergy status so that the most appropriate antimicrobial therapy can be used empirically. Anaphylaxis, angioedema, and rash are classified as allergic reactions to penicillin (for full extensive list, see the British National Formulary). If a patient develops diarrhea on any antibiotic, consider Clostridium difficile infection. Notably, indigestion and nausea are not allergies and are classified as side effects of antibiotic therapy.
Paracetamol and/or ibuprofen may be required for pain management and control of fever. Wound management may also be needed, depending on the skin trauma and infection. Careful debridement and dressing care may be required if there has been associated necrosis.
Abscess. Definitive treatment of abscesses requires removal of the purulent exudate, and antimicrobial therapy may be required in some cases. Antimicrobial therapy alone often will not be sufficient to treat an abscess due to the lack of penetration through the abscess wall. In cases of antimicrobial therapy alone, prolonged courses of either intravenous or oral antibiotics are required (often greater than 30 days).
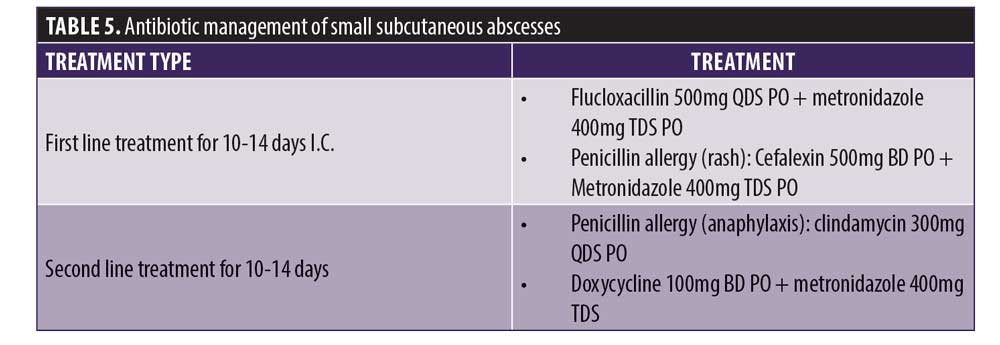
Small subcutaneous abscess. If there is an abscess that is superficial and small (<1cm in size), it can be managed within the clinic with aspiration of the purulent exudate with a 23g needle using aseptic technique. It may be necessary to liaise with the patient’s general practitioner to have the patient’s exudate samples sent to the laboratory for a microbiological diagnosis. If abscess formation occurs and the infection is fluctuant with systemic symptoms, incision and drainage are necessary. The patient may need to be referred to a specialist if the clinician is unable to do this. The most likely organism in this case is S. aureus, particularly if the infection is near the injection point. Antimicrobial therapy should be initiated after exudate has been aspirated. Table 5 states the antibiotic management of small subcutaneous abscesses.
Large subcutaneous abscess. This should be discussed with the local plastics or maxillofacial team for consideration of incision and drainage. Due to the risk of damaging of deeper structures and minimize scarring, a specialist should perform the closure (immediate or delayed). Do not start any antimicrobial treatment prior to speaking to the team as this will reduce the probability of obtaining a successful microbiological diagnosis. Treatment should only be started if the decision has been made to treat conservatively.
Deep subcutaneous abscesses/Intramuscular abscess. Patients with deep abscesses should be discussed with the local plastics/Max fax team, as they would warrant incision and drainage and possibly surgery. The referral should be made promptly and treated as urgent. Do not start any antimicrobial treatment prior to speaking to them, as this will reduce the probability of obtaining a successful microbiological diagnosis.
The most common organism involved in abscesses is S. aureus, particularly if the infection was caused by translocation of skin commensals. If the infection is thought to originate from the mouth due to history of acute or chronic periodontal disease, the organisms may be polymicrobial in nature.
Osteomyelitis. This is very rare in facial aesthetics but should be considered in patients who have had treatment involving dermal fillers who develop a fever and pain to the treated area of the face in the absence of any soft tissue mass. Particular risk factors include the presence of implants (e.g., chin and cheek). Referral to the local orthopedic or maxillofacial surgeans should be made promptly, particularly if there is a discharging sinus tract formed at the site of infection.
Antibiotic resistance. The selection of antimicrobial therapy is based on knowledge of the potential pathogens, the method of bacterial entry, disease severity, and clinical complications. For uncomplicated mild-to-moderate infections, the oral treatment suffices. For complicated severe infections, intravenous administration of antibiotics is warranted. Recognition of the potential for resistant pathogens causing SSTIs can assist in guiding appropriate selection of antibiotic therapy.
Antibiotics are a vital tool for modern medicine and are critical for treating infections, such as pneumonia, meningitis and tuberculosis. Antibiotics are also vital for avoiding infections during chemotherapy, caesarean sections and other surgeries. Antibiotics, unlike many other drugs used in medicine, become less effective the more they are used against their target organisms. Their overuse or inappropriate use allows bacteria to develop resistance.37 A failure to address the problem of antibiotic resistance could result in an estimated 10 million deaths every year globally by 2050, with a cost of $90.8 trillion in lost productivity to the global economy.37
Global concern regarding antibiotic resistance is compounded by the recent lack of discovery of new classes of antibiotics. It has been 30 years since a new class of antibiotics was introduced, and the pipeline of new antibiotics is nearly empty in the short term; only three of the 41 antibiotics in development have the potential to act against the majority of the most resistant bacteria. According to the US Center for Disease Control and Prevention (CDC), antibiotic-resistant infections affect two million Americans annually and result in more than 23,000 deaths. A new drug could be a decade away from discovery and approval by the FDA.38
Key responsibilities when prescribing antibiotics are correct diagnosis, using microbiology when available, and prescribing the right antibiotic in the right dose and only as long as necessary.39 Recently, antibiotic courses have tended to become shorter. Antibiotic resistance is driven by low doses rather than short courses of high doses and once a patient is feeling better it is unlikely their infection will relapse. Antibiotic courses are likely to continue to get shorter and be more tailored to the individual response, rather than specific durations.39 Therefore, it is important to monitor the patient. If they do not respond to the medication with four days, clinicians should review and consider the following potential reasons for non-esponse.
- Incorrect diagnosis
- Incorrect antibiotic
- Incorrect dosage
- Interaction of antibiotic with other medications taken by the patient
- New complication
- Nonadherence to prescribed treatment
Patient Follow-up
Having prescribed oral antibiotics, the patient should be receive follow-up care according to clinical need until the infection has resolved. It is good practice to have the initial follow-up within 48 hours after starting antibiotic therapy. Photographic documentation should be maintained, and the practitioner should keep a log of infection rates for audit purposes. Good follow-up, patient support, and full explanations to the patient is the best approach to stop a complication turning into a claim.
Conclusion
In nonsurgical cosmetic medicine, acute infection postprocedure is rare. The greatest risk of infection occurs during the procedure. Clinicians bear the responsibility to properly screen patients for potential risk factors by obtaining a complete medical history, performing a thorough physical exam, and counseling their patients of the risk of infection as part of the consent process. Aftercare advice should include information on the signs and symptoms of infection and the importance of notifying the clinician right away if symptoms of infection develop should be stressed so that the clinician can provide timely and appropriate management.
References
- Wagner RD, Fakhro A, Cox JA, Izaddoost SA. Etiology, prevention, and management of infectious complications of Dermal Fillers. Semin Plast Surg. 2016;30(2):83-86.
- Brodell LA, Rosenthal KS. Skin structure and function. Infect Dis Clin Pract. 2008;16(2):113-117.
- Hannigan GD, Grice EA. Microbial ecology of the skin in the era of metagenomics and molecular microbiology. Cold Spring Harb Perspect Med. 2013;3(12):a015362.
- Brodell L, LindsayA, Rosenthal K. Skin structure and function: the body’s primary defence against infection. Infect Dis Clin Pract. 2008;1(2);113-117.
- Ki V, Rotstein C. Bacterial skin and soft tissue infections in adults: a review of their epidemiology, pathogenesis, diagnosis, treatment and site of care. Can J Infect Dis Med Microbiol. 2008;19(2):173-184
- Swartz MN. Clinical practice. Cellulitis. N Engl J Med. 2004;350:904–12.
- Kikkert M. Innate Immune Evasion by Human Respiratory RNA Viruses. J Innate Immun. 2020;12(1):4-20.
- Cassuto D, Sundaram H. A problem-oriented approach to nodular complications from hyaluronic acid and calcium hydroxylapatite fillers: classification and recommendations for treatment. Plast Reconstr Surg. 2013;132(4 Suppl 2):48S-58S.
- Ibrahim O, Overman J, Arndt KA, Dover JS. Filler nodules: inflammatory or infectious? A review of biofilms and their implications on clinical practice. Dermatol Surg. 2018;44(1):53-60.
- Pugliese G, Gosnell C, Bartley JM, Robinson S. Injection practices among clinicians in United States health care settings . Am J Infect Control. 2010;38:789-98.
- Ghilotti F, Bellocco R, Ye W, et al. Obesity and risk of infections: results from men and women in the Swedish National March Cohort. Int J Epidemiol. 2019; 48(6):1783–1794.
- Castle SC. Impact of age-related immune dysfunction on risk of infections. Z Gerontol Geriatr. 2000;33(5):341-9.
- Gould L, Abadir P, Brem H, et al. Chronic wound repair and healing in older adults: current status and future research. Wound Repair Regen. 2015;23(1):1-13.
- Bourke CD, Berkley JA, Prendergast AJ. Immune dysfunction as a cause and consequence of malnutrition. Trends Immunol. 2016;37(6):386-398.
- Noble WC. Chapter 6. The human skin microflora and disease. In: Medical Importance of the Normal Microflora. Boston (Massachusetts): Springer;1999.
- Elek SD, Conen PE. The virulence of Staphylococcus pyogenes for man; a study of the problems of wound infection. Br J Exp Pathol. 1957;38(6):573-86.
- Johl SS, Burgett RA. Dermal filler agents: a practical review. Curr Opin Ophthalmol. 2006;17(5):471-479.
- Lawrence JC. The use of alcoholic wipes for disinfection of injection sites. J of Wound Care. 1994;3(1):1-14.
- Calfee DP, Farr BM. Comparison of four antiseptic preparations for skin in the prevention of contamination of percutaneously drawn blood cultures: a randomized trial. J Clin Microbiol. 2002;40(5):1660-1665.
- Engender Health. Updated 2011. Infection prevention; a reference booklet for healthcare professionals. Engender Health website. https://www.engenderhealth.org/files/pubs/qi/ip/ip-ref-eng.pdf. Accessed Apr 2021.
- Steinsapir KD, Woodward JA. Chlorhexidine keratitis: safety of chlorhexidine as a facial antiseptic. Dermatol Surg. 2017;43(1):1-6.
- FDA. Updated 2 Feb 2017. FDA drug safety communication: FDA warns about rare but serious allergic reactions with the skin antiseptic chlorhexidine gluconate. U.S. Food and Drug Administration website. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-warns-about-rare-serious-allergic-reactions-skin-antiseptic. Accessed Apr 2021.
- Wang L, Bassiri M, Najafi R, et al. Hypochlorous acid as a potential wound care agent: part I. Stabilized hypochlorous acid: a component of the inorganic armamentarium of innate immunity. J Burns Wounds. 2007;6:e5.
- Del Rosso JQ, Bhatia N. Status report on topical hypochlorous acid: clinical relevance of specific formulations, potential modes of action, and study outcomes. J Clin Aesthet Dermatol. 2018;11(11):36-39.
- McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance [published correction appears in Clin Microbiol Rev. 2001;14(1):227]. Clin Microbiol Rev. 1999;12(1):147-179.
- Rodriguez JM, Xie YL, Winthrop KL, et al. Mycobacterium chelonae facial infections following injection of dermal filler. Aesthet Surg J. 2013;33(2):265-269.
- AHRQ. Injection Safety/Safe Medication. Agency for Healthcare Research and Quality website. https://www.ahrq.gov/sites/default/files/wysiwyg/professionals/quality-patient-safety/patient-safety-resources/resources/esrd/injectionsafety.pdf. Accessed Apr 2021.
- Chang CY, Furlong LA. Microbial stowaways in topical antiseptic products. N Engl J Med. 2012;367:2170-2173.
- FDA. Updated 16 Oct 2015. Questions and answers: FDA requests label changes and single-use packaging for some over-the-counter topical antiseptic products to decrease risk of infection. U.S. Food and Drug Administration website. http://www.fda.gov/Drugs/DrugSafety/ucm374838.htm Accessed Apr 2021.
- Bal S, Kruithof AC, Liebl H, et al. In vivo visualization of microneedle conduits in human skin using laser scanning microscopy. Laser Phys Lett. 2010;7(3):242.
- Kalluri H, Banga A. Microneedles and transdermal drug delivery. J Drug Deliv Sci Technol. 2009;19(5):303–310.
- Good Morning America. 9 Jun 2010. CleanliNEST™ | GMA: How Dirty Is Your Makeup? Youtube website. http://youtu.be/nR8XgCccc88. Accessed Feb 2021.
- Nicas M, Best D. A study quantifying the hand-to-face contact rate and its potential application to predicting respiratory tract infection. J Occup Environ Hyg. 2008;5(6):347-352.
- Pontious A. StateFoodSafety Resources. StateFoodSafety website. https://www.statefoodsafety.com/Resources/Resources/the-dirty-cell-phone-25-127-bacteria-per-square-inch. Accessed Apr 2021.
- Ellis Simonsen SM, van Orman ER, Hatch BE, et al. Cellulitis incidence in a defined population. Epidemiol Infect. 2006;134(2):293-299.
- Wang HC, Wang,Y, Long X, Wang X. Mandibular osteomyelitis after hyaluronic acid injection. J Cosmet Dermatol. 2021;20: 457– 459.
- UK Government. 10 Dec 2015. Health matters: antimicrobial resistance. UK Government website. https://www.gov.uk/government/publications/health-matters-antimicrobial-resistance/health-matters-antimicrobial-resistance. Accessed Apr 2021.
- CDC. 23 Apr 2013. Antibiotic resistance threats in the United States. Centers for Disease Control and Prevention website. http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf. Accessed Mar 2021.
- Garner D. Microbiology nuts and bolts: key concepts of microbiology & infection, Third Edition. 2019.

