 J Clin Aesthet Dermatol. 2022;15(6):65-67.
J Clin Aesthet Dermatol. 2022;15(6):65-67.
by Rohan Singh, BS; Ayman Grada, MD, MS; Alan Fleischer Jr., MD; and Steven R. Feldman, MD, PhD
Mr. Singh is with the Center for Dermatology Research, Department of Dermatology at Wake Forest School of Medicine in Winston-Salem, North Carolina. Dr. Grada is with Grada Dermatology Research in Chesterbrook, Pennsylvania. Dr. Fleischer is with the Department of Dermatology at University of Cincinnati College of Medicine in Cincinnati, Ohio. Dr. Feldman is with the Center for Dermatology Research, Department of Dermatology at Wake Forest School of Medicine in Winston-Salem, North Carolina; the Department of Pathology at Wake Forest School of Medicine in Winston-Salem, North Carolina; the Department of Social Sciences and Health Policy at Wake Forest School of Medicine in Winston-Salem, North Carolina; and the Department of Dermatology at University of Southern Denmark in Odense, Denmark.
FUNDING: No funding was provided for this study.
DISCLOSURES: Dr. Feldman has received research, speaking and/or consulting support from a variety of companies, including Galderma, GSK/Stiefel, Almirall, Leo Pharma, Baxter, Boeringer Ingelheim, Mylan, Celgene, Pfizer, Valeant, Taro, Abbvie, Cosmederm, Anacor, Astellas, Janssen, Lilly, Merck, Merz, Novartis, Regeneron, Sanofi, Novan, Parion, Qurient, National Biological Corporation, Caremark, Advance Medical, Sun Pharma, Suncare Research, Informa, UpToDate and National Psoriasis Foundation. He is founder and majority owner of www.DrScore.com and founder and part owner of Causa Research. Dr. Grada is the former Head of R&D and Medical Affairs at Almirall (US). Dr. Fleischer has received consulting support from Almirall, Incyte Qurient, and SCM Lifescience. Dr. Fleischer is an investigator for Galderma and Trevi (research support). Mr. Singh has no conflicts of interest to disclose.
ABSTRACT: Objective. A nationally representative database was used to assess how soon patients were informed to return to clinic after cryosurgical treatment or prescription of topical 5-fluoruracil (5-FU) for actinic keratosis (AK).
Methods. The National Ambulatory Medical Care Survey was used to capture diagnoses and medications associated with visits to U.S. outpatient physicians for the treatment of AKs between 2011-2016.
Results. Patients treated with topical 5-FU were commonly told to return in two months or more (53%) or were not given a specific time to return at all (23%). Patients treated with cryosurgery were commonly told to return in two months or more (60%) or were not given a specific time to return at all (23%).
Limitations. This study was limited by the accuracy of AK diagnosis and treatment recording.
Conclusion. Adequate follow-up after cryosurgical or topical 5-FU treatment of AK allows physicians to assess response to treatment. When treatment for AKs fail due to lack of efficacy or intolerance, it is anticipated that patients may not return to clinic for long periods as return visits are scheduled months after cryosurgery or topical 5-FU is prescribed. Additionally, premature follow-up may not be adequate to ensure treatment-related inflammation has subsided. According to recently defined core outcome sets for AKs, if treatment fails due to intolerability, evaluation at 2 to 4 months could allow physicians to switch AK treatments. Follow-up 6 to 12 months post-treatment might better assess efficacy and potential reoccurrence of AKs.
Keywords: Actinic keratosis, cryosurgery, field cancerization, field-directed therapy, follow-up visits, topical 5-fluorouracil
Actinic or solar keratoses (AKs) are premalignant cutaneous lesions with a 0.025- to 16-percent chance of malignant transformation per lesion per year.1 Approximately 60 percent of squamous cell carcinomas arise from pre-existing AKs.2,3 Moreover, AKs are one of the most common skin conditions treated by dermatologists.4 AKs are frequently treated with either destructive treatments or topical medications.5,6 Topical 5-fluorouracil cream (5-FU) is the most common topical treatment for AKs, and cryosurgery is the most common treatment for AKs overall.7
Topical 5-FU is more effective than cryosurgery for treatment of AKs.8-10 However, topical 5-FU is associated with considerable local skin toxicity and can cause prolonged patient discomfort.8 Patients often fail treatment with topical 5-FU due to poor tolerability, and several weeks of treatment can lead to severe local skin reactions in the treated areas, particularly on the face.4,8 While participants in clinical trials have high rates of adherence, patients who experience treatment-related adverse effects might have lower adherence, masking real-world effectiveness.11,12
Moreover, when patients are treated with either destructive treatments or topical 5-FU, they may be told to return as needed or to come back after many months. In studies, patients might return in a year for assessment of treatment effect or at six-month intervals.10,13 According to recently defined core outcome sets for clinical trials assessing efficacy of AKs treatment, follow-up to assess treatment efficacy should be measured 2 to 4 months after treatment, allowing sufficient time to resolve any treatment-related inflammation while minimizing the chance for new AKs to emerge.14 Long-term remission in the field of treatment should also be assessed at least 6 to 12 months after treatment in order to detect reoccurrence.14
Adequate follow up for the treatment of AKs can allow physicians to assess response to treatment. However, the timing of topical 5-FU and cryosurgery return visits in clinical practice is not well characterized. We examined a nationally representative database to assess how often and when healthcare providers inform patients to return to clinic after cryosurgical treatment and prescription of topical 5-FU.
Methods
The National Ambulatory Medical Care Survey (NAMCS) is an annual national survey, ongoing since 1989, of U.S. office-based, non-federally employed physicians conducted by the Division of Health Care Statistics, the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC). Physicians provide a log of patient symptoms, diagnoses, treatment, and patient demographic data. The survey uses a multistage probability sample design and is weighted to reflect national estimates of outpatient medical care services in the United States. The NAMCS can provide reliable national estimates on physician office visits for the management of AK.
The NAMCS captures diagnoses and medications associated with visits to U.S. outpatient physicians. We used data from the most recent available six-year period (2011–2016) to identify office visits at which AK (International Disease Classification-9 (ICD-9) or ICD-10 code L57.0) was the primary and only diagnosis. We used procedure codes to identify visits at which cryosurgery was performed and analyzed drug mention data to determine when topical 5-FU was prescribed. The NAMCS can also be used to determine, within the limitations of the coding, the scheduled time to next return visit.15 The NAMCS captures information on follow up visits and encodes the information as follows:
- [RETAPPT1] Return in less than one week
- [RETAPPT2] Return in one week to less than two months
- [RETAPPT3] Return in two months or greater
- [RETUNSP] Return unspecified
- [RETNEED] Return as needed (p.r.n.)
We used this time to return visit variable to assess when patients treated with 5-fluorouracil are told to return for follow up. Data were analyzed using the SAS Software 9.4 and 95 percent confidence intervals were calculated for the frequency of return visits.
Results
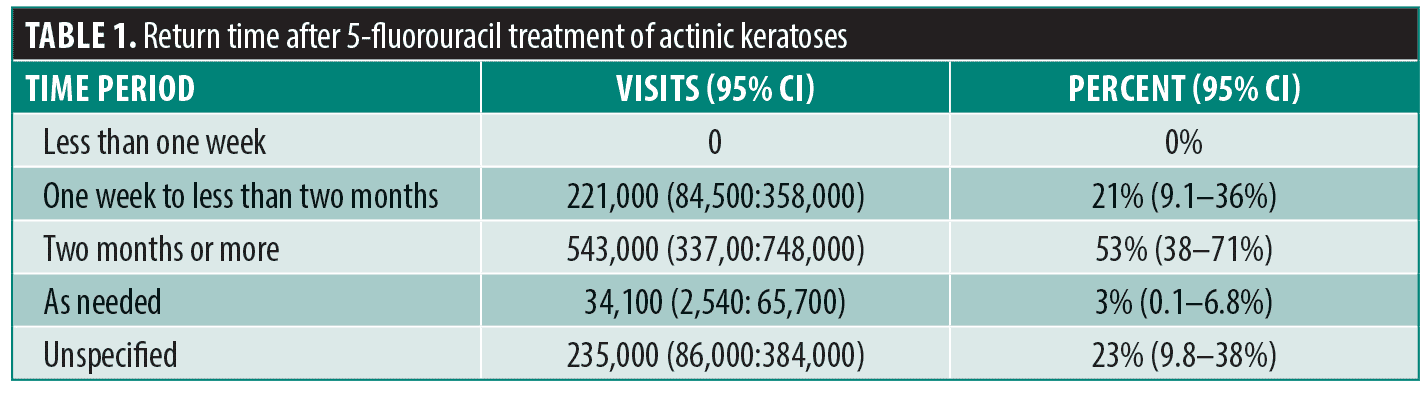
After applying visit weights, there were approximately 29 million actinic keratosis visits identified at which cryosurgery was performed or topical 5-FU was prescribed over the 2011-2016 study interval. There were approximately one million AK visits at which topical 5-FU was prescribed over the 2011-2016 study interval.No patient was told to return in less than one week. Only a minority of patients (21%; 95% CI: 9.1–36%), were told to return in less than two months. Patients were commonly told to return in two months or more (53%; 95% CI: 38–71%) or were not given a specific time to return at all (as needed or unspecified, 23%; 95% CI: 9.8–38%) (Table 1).
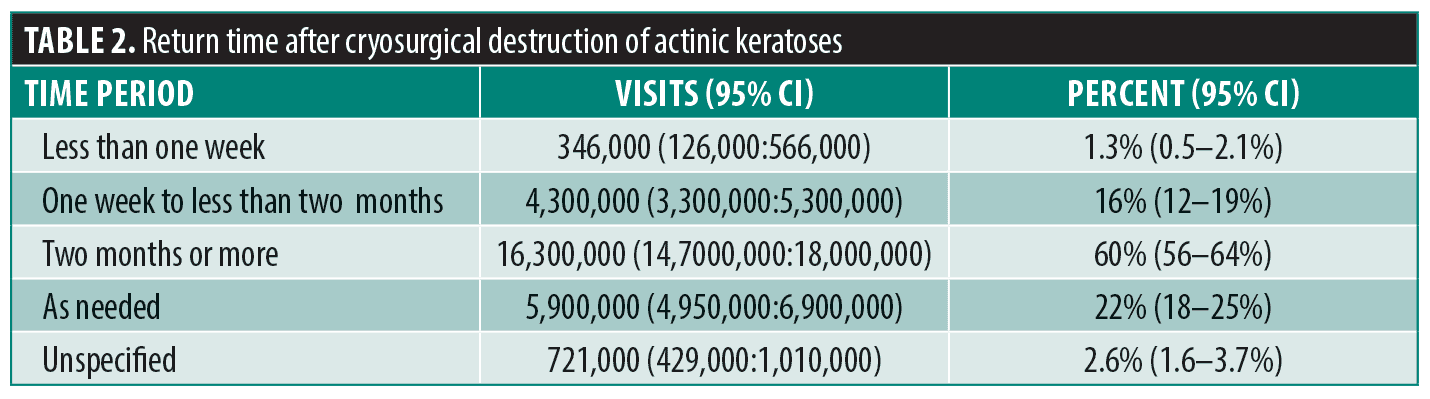
Destruction of actinic keratoses were far more common than was use of topical 5-FU. There were approximately 28 million actinic keratosis visits at which cryosurgery was performed over the 2011–2016 study interval. The pattern of return visits was similar, with only a minority of patients (16%; 95% CI: 12–19%) told to return in less than two months. Patients were commonly told to return in two months or more (60%; 95% CI: 56–64%) or were not given a specific time to return at all (as needed or unspecified, 22%; 95% CI: 18–25%) (Table 2).
Discussion
Cryosurgical destructive treatments are the most common treatment for AKs, even though this destructive approach focuses on lesions and not the entire treatment field, contrary to topical agents. Despite the superior efficacy of topical 5-FU, cryosurgery can be more time-efficient and is not affected by patient adherence.8-10,16 Topical treatment with 5-FU is associated with considerable local toxicity, limiting its use. While topical 5-FU is a poorly tolerated treatment for actinic keratoses, it has been the most widely used topical treatment for actinic keratoses.7 Particularly, topical 5-FU can be used alone or in combination with destructive treatment modalities and the treatment course can range from 2 to 6 weeks of once or twice daily application.17,18 Patients frequently do not tolerate the treatment and often are unwilling to consider repeat treatment.8 Treatment-related adverse effects may also limit patient adherence, thus the efficacy of topical 5-FU in a real-world setting may not be consistent with those reported in clinical trials.11,12
When patients fail the usual treatments (cryosurgery or topical 5-FU treatment) for AKs, it can be anticipated that patients might not return to the clinic for long periods of time, as patients are typically given return visits many months after cryosurgery is performed or after topical 5-FU treatment is prescribed, if they are given a scheduled return visit at all. A long delay between prescription and first follow up visit might compromise adherence to treatment (follow-up visits encourage better adherence); however, follow-up within two months of treatment prescription might not be an adequate time to ensure that treatment-related inflammation has subsided to assess efficacy.14 According to our results, approximately 21 percent of patients treated with topical 5-FU are scheduled for follow-up within two months of treatment, which might not be adequate time to assess treatment efficacy, and approximately 26 percent of patients are not given a specified follow-up visit. Moreover, approximately 17 percent of patients treated with cryosurgery are scheduled for follow-up within two months of treatment and approximately 25 percent are not given a specified follow-up visit. Repeat treatment with topical 5-FU would rarely, if ever, be indicated for patients who fail treatment, irrespective of how long it has been since topical 5-FU treatment failure. Premature follow-up might not adequately assess treatment efficacy.
Because destructive or topical treatment for AKs may fail because of lack of efficacy, the efficacy could be assessed at 6 to 12 months based on the primary outcomes used in published trials and the recently defined core outcome sets for clinical trials.9,10,14 Moreover, if treatment fails because of intolerability to severe local skin reactions associated with topical 5-FU treatment, evaluation 2 to 4 months after treatment could allow physicians to switch to a different topical AK treatment after such reaction has subsided as appropriate.14
Limitations. This study was limited by the accuracy of AK diagnosis, treatment recording, and potential out of office patient follow-up.
References
- Ratushny V, Gober MD, Hick R, et al. From keratinocyte to cancer: the pathogenesis and modeling of cutaneous squamous cell carcinoma. J Clin Invest. 2012 Feb;122(2):464–472.
- Criscione VD, Weinstock MA, Naylor MF, et al; Department of Veteran Affairs Topical Tretinoin Chemoprevention Trial Group. Actinic keratoses: natural history and risk of malignant transformation in the veterans affairs topical tretinoin chemoprevention trial. Cancer. 2009 Jun 1;115(11):2523–2530.
- Marks R, Rennie G, Selwood TS. Malignant transformation of solar keratoses to squamous cell carcinoma. Lancet. 1988 Apr 9;1(8589):795–797.
- Dianzani C, Conforti C, Giuffrida R, et al. Current therapies for actinic keratosis. Int J Dermatol. 2020 Jun;59(6):677–684. Epub 2020 Feb 3.
- Feldman SR, Fleischer AB Jr, Williford PM, Jorizzo JL. Destructive procedures are the standard of care for treatment of actinic keratoses. J Am Acad Dermatol. 1999 Jan;40(1):43–47.
- Gupta AK, Cooper EA, Feldman SR, et al. A survey of office visits for actinic keratosis as reported by NAMCS, 1990-1999. National Ambulatory Medical Care Survey. Cutis. 2002 Aug;70(2 Suppl):8–13.
- Ranpariya VK, Muddasani S, Mahon AB, et al. Frequency of procedural and medical treatments of actinic keratosis. J Am Acad Dermatol. 2021 Mar 20:S0190-9622(21)00598-3.
- Emmerich VK, Cull D, Kelly KA, et al. Patient assessment of 5-fluorouracil and imiquimod for the treatment of actinic keratoses: a retrospective study of real-world effectiveness. J Dermatolog Treat. 2021 May 5:1-4.
- Krawtchenko N, Roewert-Huber J, Ulrich M, et al. A randomised study of topical 5% imiquimod vs. topical 5-fluorouracil vs. cryosurgery in immunocompetent patients with actinic keratoses: a comparison of clinical and histological outcomes including 1-year follow-up. Br J Dermatol. 2007 Dec;157 Suppl 2:34–40.
- Jansen MHE, Kessels JPHM, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019 Mar 7;380(10):935–946.
- van Onzenoort HA, Menger FE, Neef C, et al. Participation in a clinical trial enhances adherence and persistence to treatment: a retrospective cohort study. Hypertension. 2011 Oct;58(4):573–578.
- Snyder S, Crandell I, Davis SA, et al. Medical adherence to acne therapy: a systematic review. Am J Clin Dermatol. 2014 Apr;15(2): 87–94.
- Pomerantz H, Hogan D, Eilers D, et al. Veterans affairs keratinocyte carcinoma chemoprevention (VAKCC) trial group. long-term efficacy of topical fluorouracil cream, 5%, for treating actinic keratosis: a randomized clinical trial. JAMA Dermatol. 2015 Sep;151(9):952–960.
- Reynolds KA, Schlessinger DI, Vasic J, et al. Core outcome set for actinic keratosis clinical trials. JAMA Dermatol. 2020 Mar 1;156(3):326–333.
- Muddasani S, Heron CE, Fleischer AB Jr, et al. Time intervals until the first return office visit after new medications. J Drugs Dermatol. 2020 Dec 1;19(12):1226–1230.
- Navarrete-Dechent C, Marghoob AA, Marchetti MA. Contemporary management of actinic keratosis. J Dermatolog Treat. 2021 Aug;32(5):572–574.
- Hansen JB, Larsson T, Dunkelly-Allen N, et al. Real-world effectiveness and safety of field- and lesion-directed treatments for actinic keratosis. J Drugs Dermatol. 2020 Aug 1;19(8):756–762.
- Management of actinic keratosis. Drug Ther Bull. 2013 Jul;51(7):81–84.

