 by Hazel H. Oon, MD, MRCP, FAMS; Su-Ni Wong, MBBS, MMed, FRCP, FAMS; Derrick Chen Wee Aw, MBBS, MRCP, FAMS; Wai Kwong Cheong, MBBS, MRCP, FRCP; Chee Leok Goh, MD, MMed, FRCP, FAMS; and Hiok Hee Tan, MBBS, MRCP, FRCP, FAMS
by Hazel H. Oon, MD, MRCP, FAMS; Su-Ni Wong, MBBS, MMed, FRCP, FAMS; Derrick Chen Wee Aw, MBBS, MRCP, FAMS; Wai Kwong Cheong, MBBS, MRCP, FRCP; Chee Leok Goh, MD, MMed, FRCP, FAMS; and Hiok Hee Tan, MBBS, MRCP, FRCP, FAMS
Drs. Oon and Goh are with the National Skin Centre in Singapore. Dr. Wong is with Dr. SN Wong Skin, Hair, Nails & Laser Specialist Clinic in Singapore. Dr. Aw is with Sengkang General Hospital in Singapore. Dr. Cheong is with Specialist Skin Clinic and Associates in Singapore. Dr. Tan is with Thomson Specialist Skin Centre in Singapore.
FUNDING: The Dermatological Society of Singapore Acne Advisory Board received logistical support and funding from Menarini through an unrestricted educational grant, but has maintained editorial independence in the preparation of these guidelines.
DISCLOSURES: Dr. Oon has served as a researcher and advisory board member and has received honoraria from Galderma. The other authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Due to the multiethnic patient population with varying skin types in Singapore, clinicians often find the management of acne in their patients to be challenging. The authors developed these guidelines to provide comprehensive advice on individualized acne treatment and to provide a reference guide for all doctors who treat patients of Asian descent. Unique features of acne in Singapore are highlighted. We address concerns such as diet, special population needs, and the benefits, side effects, risks, and cost-effectiveness of currently available acne treatments. These treatment guidelines outline recommendations for the diagnosis, grading, and treatment of children, adolescents, and adults with acne of varying severity, and include advice pertaining to the use of cosmeceuticals and management of scars.
KEYWORDS: acne vulgaris, topical therapy, systemic therapy, benzoyl peroxide, retinoid, antibiotics, fixed combination, diet, hormonal therapy, laser therapy, light therapy, contraceptives, Propionibacterium acnes, acne scar, irritation, adjunctive therapy, cosmeceuticals, Singapore, treatment guidelines
J Clin Aesthet Dermatol. 2019;12(7):34–50
Acne is a chronic inflammatory disease of the pilosebaceous units, characterized by the formation of comedones, erythematous papules, pustules, and/or nodules (i.e., pseudocysts) that can be accompanied by scarring.1 Acne affects the face more than the trunk and is most common in individuals aged 15 to 24 years, with a typical onset in adolescence or early adulthood.2,3
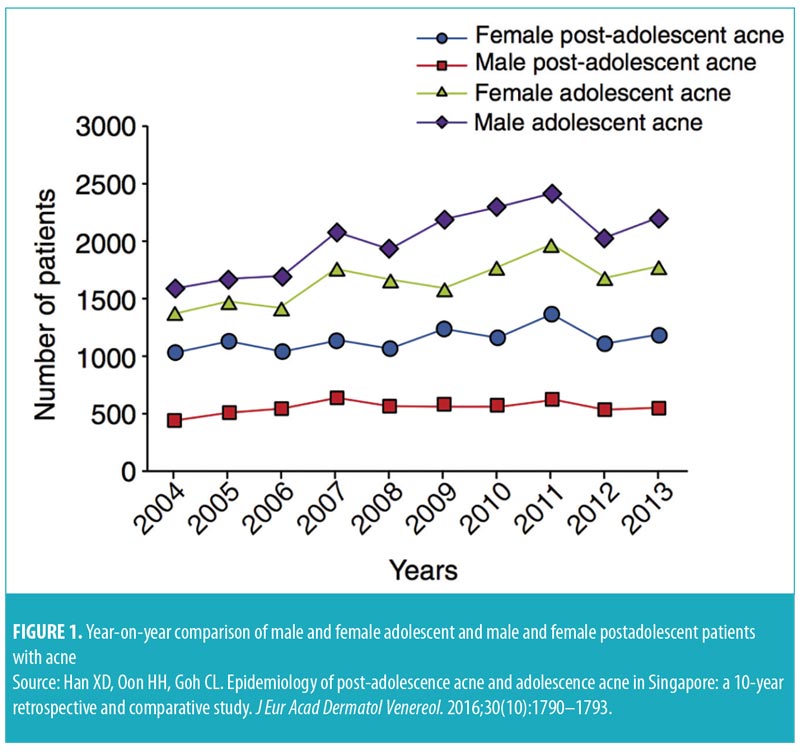
In a community-based study in Singapore, acne was found in about 88 percent of adolescents aged 13 to 19 years.4 In another study, 41 percent of adults treated at the National Skin Centre in Singapore had experienced acne since adolescence, although the majority presented with adult-onset acne. Comedonal acne is more prevalent in adolescents, while cystic acne is seen more often in adults.5 Additionally, adolescent acne is more common in men (61%), while postadolescent acne is more common in women (69%) (Figure 1).5,6 Pruritus is common (70%) in patients with acne and negatively affects mood in about 55 percent of these patients.7 Postacne scarring and dyspigmentation are also prevalent in patients with acne. In a cohort of 40 patients, 58 percent of patients with mild acne had macular erythema or hyperpigmentation; in those with moderate acne, 54 percent had postacne scarring and 14 percent had hypertrophic/keloid scars.8 Acne is also likely to be a significant contributor to psychological distress, particularly in adolescents. About half of patients reported that they are “rarely” or “never” comfortable with their acne. Twenty-eight percent of these patients reported self-esteem concerns related to their acne, while more than 25 percent reported that they felt “depressed.” A majority (60%) shared feelings of concern due to scarring caused by acne.4 These statistics highlight the importance of effective and timely treatment of acne.
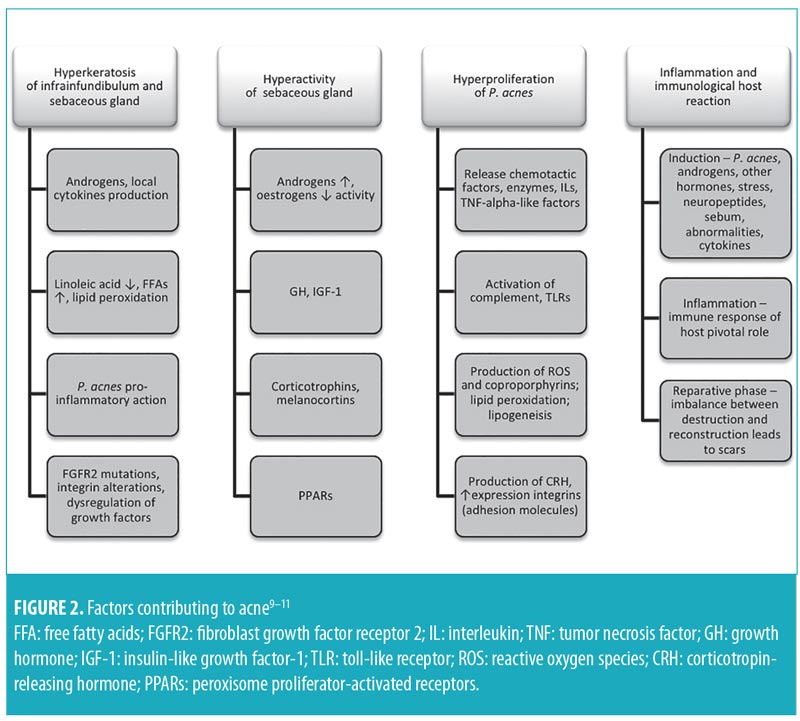
Similar to the general population, the course of acne in Singaporeans is influenced by hormonal and genetic factors, although exogenous elements (e.g., physical, chemical, environmental, dietary) can precipitate flares.9 Propionibacterium acnes (P. acnes) is central to inflammation (Figure 2).9–12
Methods
The Dermatological Society of Singapore (DSS) Acne Advisory Board members represent various institutions and the private sector in Singapore and were nominated by the DSS Executive Committee for a period of one year. Patient views were sought informally from patients, relatives, friends, and staff of the board members.
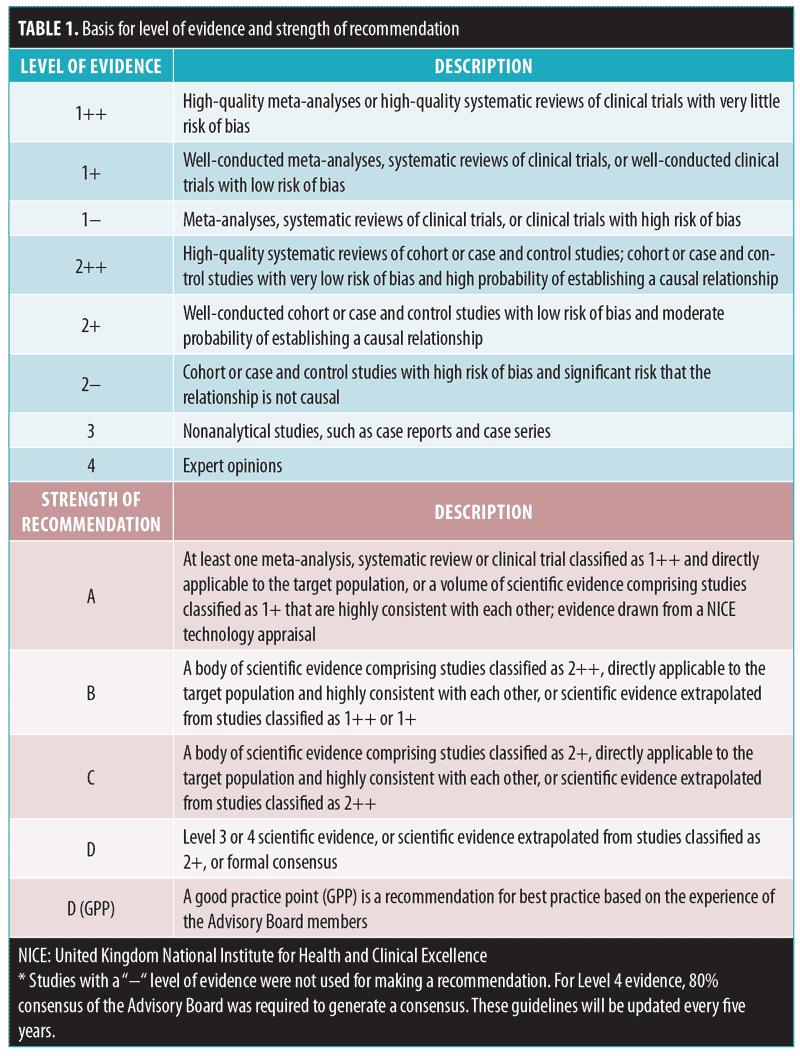
Management recommendations were developed based on the Appraisal of Guidelines for Research and Evaluation (AGREE) II. The references include meta-analyses and current guidelines (e.g., S3, Canadian, Global Alliance for Acne, South-East Asia Study Alliance [SASA], Malaysia and Philippine Guidelines, and the National Skin Centre Management Guidelines for Acne).13–18 A literature search was conducted on PubMed to identify relevant research from the date of its inception until December 31, 2014. These recommendations were appraised based on the United Kingdom National Institute for Health and Clinical Excellence (NICE) and Scottish Intercollegiate Guidelines Network (SIGN) guidelines (Table 1).
Disclaimer. While these guidelines are evidence-based, they cannot be substituted for clinical judgement, and patients should be assessed and managed on an individualized basis. These recommendations were derived from critically appraised data at the time of this document’s preparation. While a “user pays” healthcare system allows for more flexibility in implementing these guidelines in clinical practice, it also poses a barrier to patients who are cost-conscious. As costs varies, these guidelines will not address costs specifically, but rather provide general recommendations regarding the costs of laboratory investigations, medications, and cosmeceuticals.
Diagnosis and Assessment
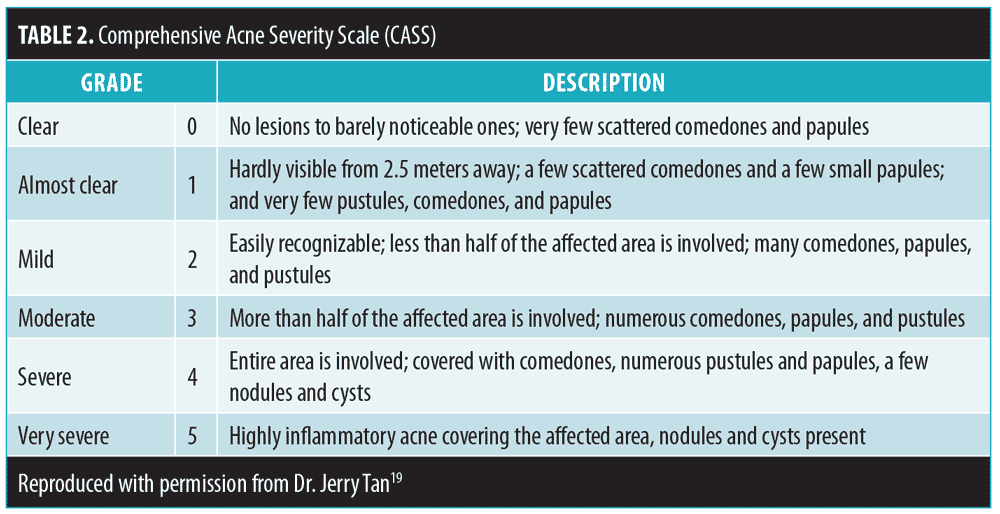
The Comprehensive Acne Severity Scale (CASS) can be used to qualitatively estimate acne severity by assessing inflammatory and noninflammatory lesions, as well as to evaluate patient response to treatment (Table 2).19 Aggravating or causative factors (e.g., acnegenic products, occlusion-causing agents, medications, stress, diet, smoking, obesity, occupation, sports, and other lifestyle habits) and systemic disorders (e.g., Cushing syndrome, androgen-secreting tumors) should be assessed. In women, clinicians should assess for signs of hyperandrogenism (i.e., menstrual irregularity, hirsutism, and androgenetic alopecia).20–22 Previous acne treatments and response, as well as specific adverse effects and adherence issues, should be evaluated. While assessing patient awareness, obtaining previous treatment history and psychosocial effects data are essential. The use of validated questionnaires (e.g., Acne-specific Quality of Life) might be advantageous, but are not essential.23
Diet, Obesity, and Acne
Diet modification as an adjunct to acne treatment should be based on good-quality evidence ti avoid unnecessary prohibitions that might lead to nutritional deficiency.24 Due to strong biochemical, histopathologic, and clinical evidence, a low glycemic index (GI) diet is encouraged for patients with acne (Level 2, Grade B). The GI ranks food according to the effects of its carbohydrate content on blood glucose level. For instance, high-GI food (GI>70) results in rapid rise in blood glucose, while low-GI food causes little change (Table 3).15,25–29
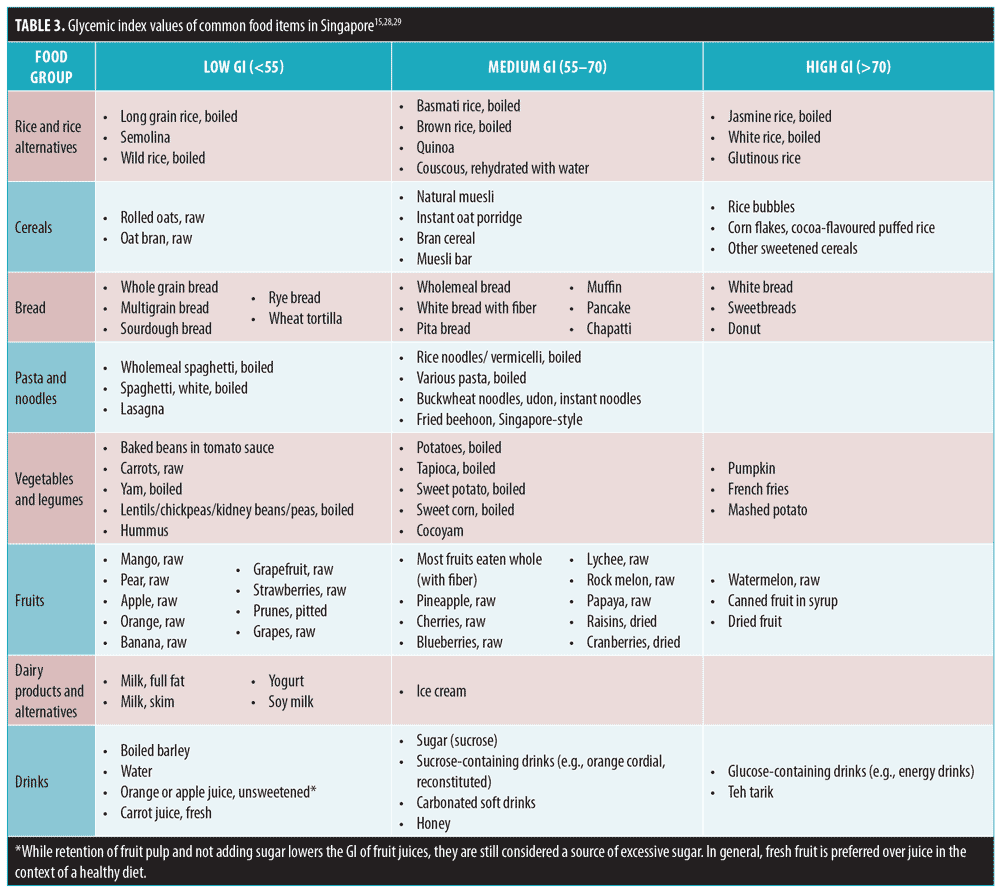
Factors that influence GI include amount of soluble fiber (high fiber has lower GI); fat and protein content; type of sugar (e.g., fructose has a lower GI and glucose has higher GI); type of starch; amount of cooking/processing (i.e. cooking/processing increases GI); food form (e.g., small particle size is more quickly digested and absorbed); and acidity (delays absorption).
Chocolate and oily/fatty foods are commonly implicated in acne. Recent studies have shown a correlation between chocolate consumption and acne, and thus chocolate should be avoided (Level 2-, Grade D). However, there is generally insufficient evidence supporting the withdrawal of oily or fatty food.30–32 Observational studies suggest that milk, particularly skim milk, exacerbates acne. 33–35 Whey has also been associated with new onset or exacerbation of acne. This effect was more prominent in women and in individuals without current acne and/or no family history of acne.36 Further clinical trials are required before any restrictions on whey consumption can be recommended.37
Obesity is associated with acne, particularly inflammatory acne. School children with a body mass index (BMI) of less than 18.5kg/m2 had a lower prevalence of acne, while those with a BMI-for-age in the 95th percentile or above had a significantly higher rate of acne development. This is most likely due to the peripheral hyperandrogenism that accompanies obesity.38
Topical Therapies
Topical antimicrobial therapy. P. acnes has shown a growing antibiotic resistance in Singapore.4,18,39 Worldwide, the highest rates are seen in those who previously used topical erythromycin and clindamycin.40 Antibiotic resistance in P. acnes confers a potential reduction in treatment efficacy, and transfer of resistant organisms through close contact is possible. Additionally, antibiotic resistance (P. acnes) can potentially promote resistance within other bacterial pathogens. De novo antibiotic resistance is an ongoing problem; thus, topical antibiotic monotherapy is not recommended.
Combination therapy is preferred, not only because monotherapy is less effective, but because topical antibiotic monotherapy is associated with rapid antibiotic resistance.41,42 Consider alternative antibacterials, such as BPO, salicylic acid, or dermocosmetics.
Topical BPO (2.5%, 5%) is effective against P. acnes and efficacious in inflammatory acne. Its use is encouraged over topical antibiotics to reduce development of antibiotic resistance, but widespread use is limited by irritation. The combination therapy of BPO and adapalene is more effective than adapalene or BPO alone.43 A summary of recommendations regarding topical therapies is provided in Table 4.
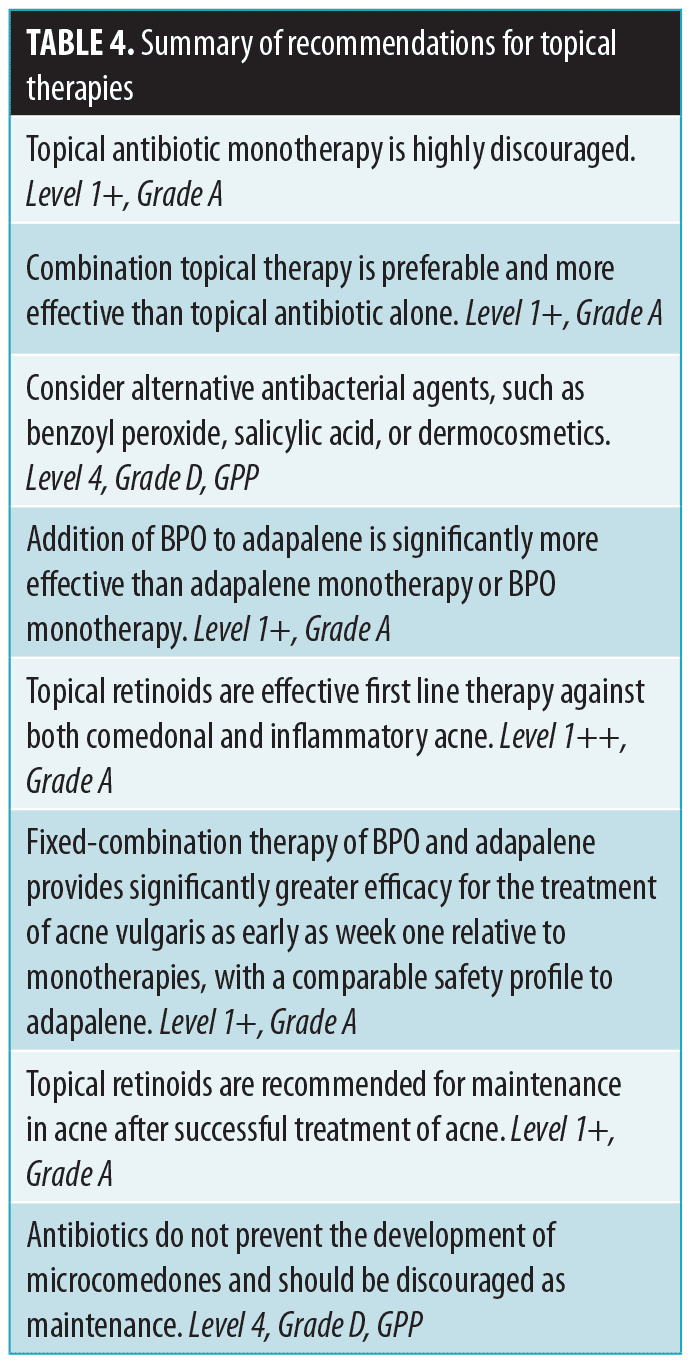
Topical retinoids. Topical retinoids are an effective first-line therapy against comedonal and inflammatory acne.44 These agents have demonstrated, in vivo, anti-inflammatory activity.45 They reduce microcomedones and mature comedones, promote desquamation of follicular epithelium, and reduce inflammatory and noninflammatory lesions.
Prescription retinoids (e.g., tretinoin) have established rejuvenation effects; skin texture is improved via activation of retinoid receptors.46 OTC retinoid esters (e.g., retinol, retinyl palmitatem, retinyl propionate) also demonstrate the same effects but to a lesser extent. These products are not available in some countries.
Regarding sensitivity, adapalene has the least irritating effect.47 There was more erythema, desquamation, dryness, stinging, pruritus, and transepidermal water loss with tretinoin than adapalene. Tolerability was lowest among Chinese patients, followed by Indian, Malay, and Caucasian patients.48
Fixed-combination BPO and adapalene. Fixed-combination BPO and adapalene provides significantly greater efficacy as early as the first week of treatment, relative to monotherapies, with a comparable safety profile to adapalene alone.43
In three 12-week trials in patients 12 years of age or older with moderate acne, success rates were significantly higher with adapalene 0.1%/BPO 2.5% gel than with adapalene 0.1% gel or BPO 2.5% gel alone, and combination therapy had an earlier onset of action. A rapid onset of action was observed using this combination treatment, reducing lesions from the first week. Additionally, this combination therapy clears both inflammatory and non-inflammatory lesions, targets three out of four pathogenic causes of acne, is antibiotic-free (removing the risk of antibiotic resistance) and has shown long-term efficacy and tolerability.49
Maintenance treatment. Acne typically recurs soon after cessation of active treatment, making maintenance treatment necessary. About 28 percent of sections of normal-appearing skin from patients with acne show histologic features of microcomedones, the subclinical precursors to both inflammatory and noninflammatory acne lesions, and biopsy of papules demonstrates the presence of microcomedones in 52 percent of patients with acne.50 Maintenance anticomedogenic agents have been shown to effectively control acne and prevent relapses and minimize sequelae.50
Retinoids are recommended for maintenance.51–53 Antibiotics do not prevent the development of microcomedones and should not be used for maintenance.
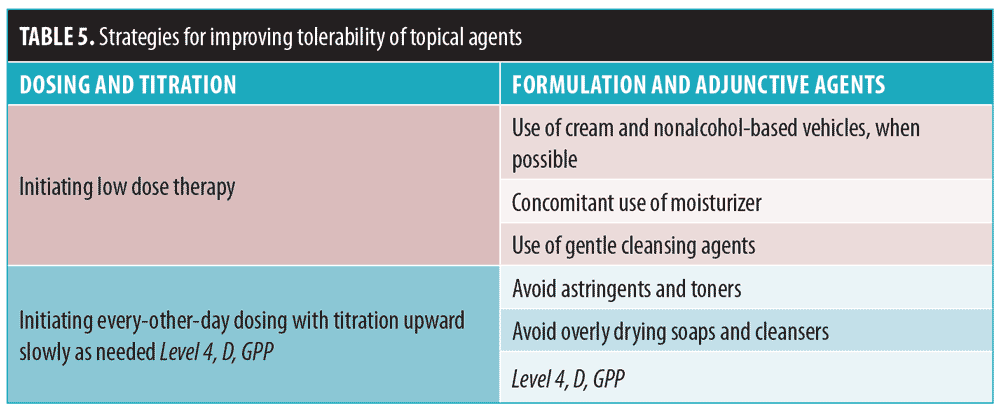
Topical therapy-induced skin irritation. The most common side effect of these therapies is irritation, including dryness, erythema, scaling, stinging/burning, and itching. These result from the disruption of the skin barrier due to external (e.g., inflammatory process, harsh cleansing) and internal factors (e.g., sebum overproduction, altered ceramides/free fatty acids/cholesterol). To mitigate irritation, the skin barrier should be restored.54,55 Dosing, titration, formulation strategies, and adjunctive agents can be employed to improve tolerability of topical agents (Table 5).
Miscellaneous. Topical dapsone is not currently available in Singapore. Cases of methemoglobinemia that have resulted in hospitalization were reported in association with twice-daily use of dapsone 5% gel in postmarketing surveys.56 This occurs frequently in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency, a condition that is common in Singapore.57
Systemic Antibiotics
Systemic antibiotics are indicated for moderate-to-severe papulopustular acne, in conjunction with the appropriate topical agents (Table 6). Antibiotics work by reducing P. acnes levels, inhibiting of bacterial lipases, and anti-inflammatory activities. Among the spectrum of activities are inhibition of neutrophil leukotaxis, reduction in cytokine secretion, and decrease in matrix metalloproteinase activity.58–60 The evidence for their efficacy is extremely well-established and, thus, additional elaboration will not be provided.13,61–63
In Singapore, there has been a reported increase in resistance rates of P. acnes from eight percent in 1999 to 14.9 percent in 2007.4,39 Further, Tan et al4 documented resistant P. acnes in school-attending adolescents who had not been previously treated for acne. The concern over antibiotic resistance warrants judicious use. Patient education and adherence are essential to ensure good outcomes while minimizing the risk of resistance.
There is a general consensus that oral antibiotic therapy should not exceed 3 to 4 months and that a minimum duration of six weeks is commonly required to see clinical improvement. Oral antibiotics should not be used as a single agent or with another topical antibiotic and should be combined with other recommended topical agents. The characteristics of systemic antibiotics commonly used in acne are seen in Table 7.
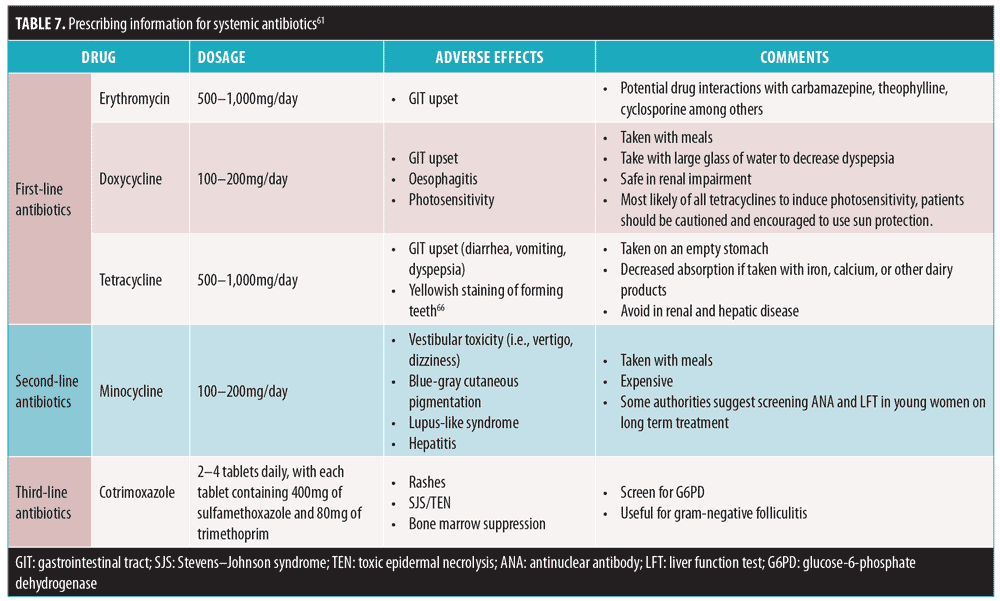
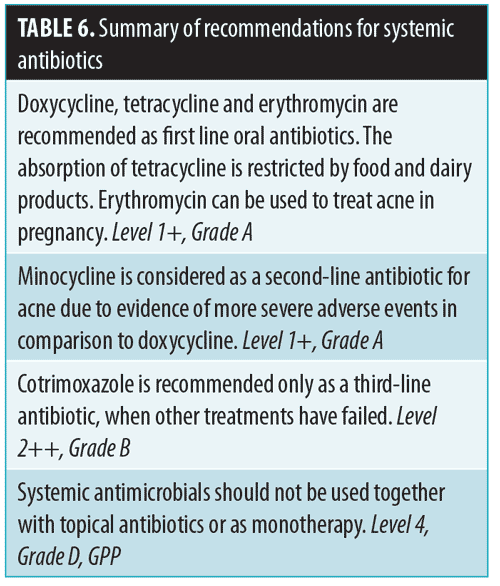
First-line antibiotics. Doxycycline and erythromycin are recommended first-line oral antibiotics.13,61,63 Doxycycline is contraindicated in children under eight years of age and in pregnant and lactating women. Erythromycin is as effective as tetracycline in the treatment of inflammatory acne and is effective and safe for use in younger patients. Erythromycin can also be considered for the treatment of severe acne in pregnancy.64 Tetracycline can be used as an alternative to doxycycline; however, tetracycline must be taken on an empty stomach and in the absence of dietary iron and calcium, and is therefore not generally considered for use as first-line therapy.13,65,66
Second-line antibiotics. Studies have shown that minocycline is as effective as tetracycline and doxycycline in the quantitative reduction of inflammatory acne.67 Early research has indicated that minocycline tends to produce a more rapid clinical improvement when compared to tetracycline. However, subsequent controlled trials did not support the superiority of any one of these agents above the rest.62 The rationale for recommending minocycline as second-line therapy is based on a systematic review of case reports that showed more severe adverse events occurring with minocycline treatment.63 Minocycline is associated with more central nervous system side effects and has been linked to lupus and autoimmune hepatitis.68
Third-line antibiotics. Cotrimoxazole is recommended only as a third-line treatment when other options have failed.61,69,70 It is effective, but has the potential for serious adverse events (e.g., Stevens-Johnson syndrome/toxic epidermal necrolysis, bone marrow suppression).61,70 It is also contraindicated among individuals who are G6PD-deficient.61 The potential risks versus benefits should be discussed with the patient.
Currently, long-term use of other systemic antibiotics for acne, such as penicillin and clindamycin, is not favored by the Advisory Board. One particular complication of long-term systemic antibiotic therapy with tetracyclines or erythromycin is the development of gram-negative folliculitis resulting from an overgrowth of lactose-fermenting gram-negatives. Its management involves shifting to an antibiotic specific to gram-negative bacteria, such as amoxicillin clavulanate or ciprofloxacin.
Overall, the current best practice regarding the use of systemic antibiotics is an individualized approach. When choosing oral antibiotics, factors such as cost, patient preference, efficacy of the medication, and risk-benefit profile must be taken into account. Physicians should also use the recommended nonantibiotic topical agents (e.g., retinoids, BPO). Systemic and topical antimicrobials should not be used together or as monotherapy.71
Systemis Isotretinoin
The efficacy of oral isotretinoin in cases of severe acne has been established (Table 8). However, treatment regimens are highly variable among expert groups. Systemic isotretinoin is indicated for use in severe nodulocystic acne, severe acne after having failed 6 to 8 weeks of oral antibiotics in combination with topical retinoids and BPO, and in patients with severe scarring or psychological and/or physical distress as a result of acne.13,72
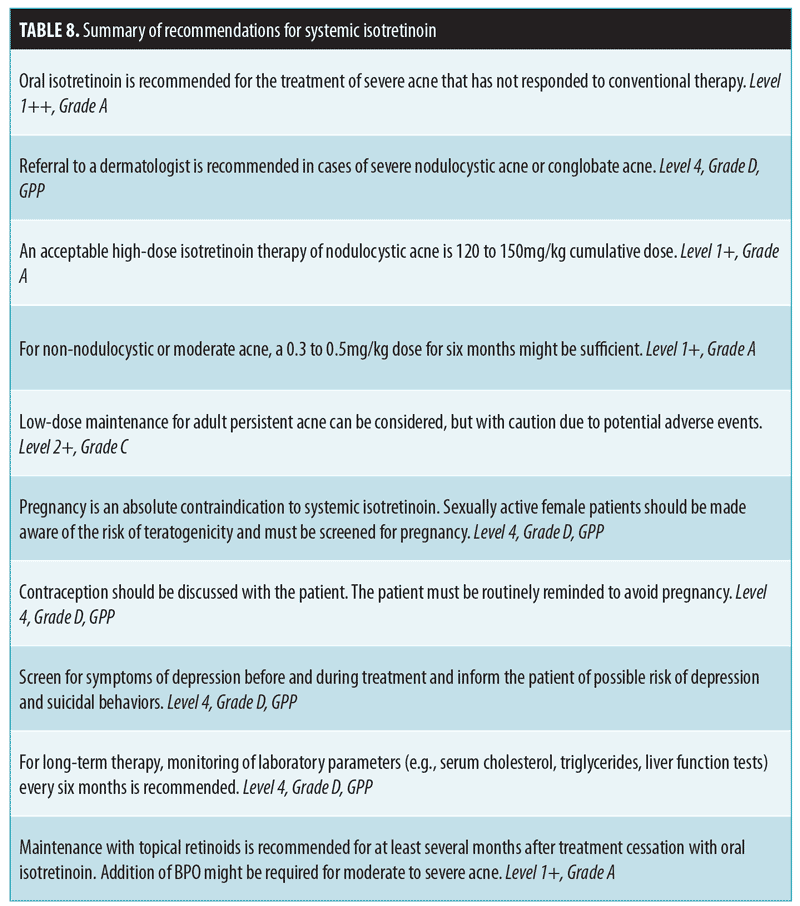
For severe nodulocystic acne or conglobate acne, referral to a dermatologist is recommended. For high-dose isotretinoin therapy of nodulocystic acne, a 120 to 150mg/kg cumulative dose is acceptable.13,72 For non-nodulocystic or moderate acne, 0.3 to 0.5mg/kg for six months will likely be sufficient.13,73–75 A daily dose of 0.25 to 0.5mg/kg can be started and adjusted as tolerated. Pulse therapy (every 1 to 3 weeks) is not recommended due to higher relapse rates.76 Low-dose maintenance for persistent acne in adults can be considered, but with caution due to the potential for adverse events (e.g., teratogenicity, hepatotoxicity, hyperlipidemia).77–79 Lastly, combination with oral tetracyclines should be avoided due to the risk of pseudotumour cerebri.80,81
Monitoring. Pregnancy. Pregnancy is an absolute contraindication to systemic isotretinoin. Physicians must ensure that female patients are not pregnant and are aware of the risk of teratogenicity before starting therapy. Contraception (e.g., hormonal, intrauterine device, sterilization, barrier, or abstinence) should be discussed with female patients when considering systemic isotretinoin. Treatment can be withheld until commencement of the next menstrual period. The administration of a pretreatment pregnancy test is at the doctor’s discretion or done in accordance with the region’s medical regulations. During follow-up, the patient must be routinely reminded to avoid pregnancy (e.g., by documenting last menstrual period at every visit).
Depression. The causal link between isotretinoin and depression is controversial. Rates range from 1 to 11 percent across trials with similar rates in oral antibiotic control groups (some demonstrated trend towards fewer or less severe depressive symptoms after treatment).13 Physicians should screen patients for symptoms of depression before and during treatment and inform them of the possible risks of depression and suicidal behavior while taking isotretinoin.
Laboratory testing. Laboratory parameters, such as liver function tests and serum cholesterol and triglycerides should be checked at pretreatment13,72 and again after medication initiation (e.g., after 6 to 8 weeks of treatment or earlier if necessary).72 For long-term therapy, monitoring every six months is recommended.
Relapse/recurrence. On average, relapse rates following systemic isotretinoin treatment can vary between 21 and 30 percent.82 Risk factors for recurrence include male sex, age younger than 16 years, residence in urban areas, cumulative oral retinoid dose greater than 2,450mg, and treatment duration longer than 121 days.83 Maintenance with topical retinoids is recommended for at least several months after the cessation of oral isotretinoin; the addition of BPO might be required for moderate-to-severe acne.51,72,84
Hormonal Therapy and Other Systemic Therapies
Combined oral contraceptives. Combined oral contraceptives (COCs) are effective in the treatment of both noninflammatory and inflammatory acne.85 Evidence comparing COCs with systemic antibiotic therapy is scarce and conflicting. Minocycline shows comparable efficacy to ethinyl estradiol combined with cyproterone acetate (EE-CPA); EE-CPA shows superior efficacy compared to tetracycline; and combining EE-CPA and tetracycline shows no superior efficacy compared to EE-CPA alone.13
Although antibiotics appear superior at three months, oral contraceptives are equivalent to antibiotics at six months in reducing acne lesions and might be a better first-line alternative to systemic antibiotics for long-term acne management in women.86 Indications include moderate-to-severe papulopustular acne in women, signs of hyperandrogenism, need for effective contraception (e.g., during oral isotretinoin use), and as adjuvant therapy to topical and systemic therapies.
Choice of hormonal therapy. The COCs registered in Singapore are listed in Table 9.87 A Cochrane review of 31 trials of COCs supported their efficacy in reducing inflammatory and noninflammatory facial acne lesions. A few important and consistent differences were found between COC types in their effectiveness for treating acne,88 as follows:
- A levonorgestrel COC was more effective than placebo in decreasing total, inflammatory, and noninflammatory lesion counts and led to a clinician assessment of “clear” or “almost clear” lesions and participant self-assessment of improved acne lesions.
- For two combined trials of a drospirenone COC, the investigator’s assessment of “clear” or “almost clear” skin favored the drospirenone group versus placebo.
- In one trial, the drospirenone COC group showed a greater percentage of changes for total, inflammatory, and noninflammatory lesion counts, as well as papule and closed comedone counts compared to the placebo.
- COCs that contained chlormadinone acetate or cyproterone acetate demonstrated greater improvement in acne than levonorgestrel. Also, a cyproterone acetate COC demonstrated better outcomes than desogestrel COC, but the studies produced conflicting results.
- Levonorgestrel demonstrated slightly better outcomes in acne than desogestrel, but the results were not consistent.
- A drospirenone COC appeared to be more effective in treating acne than norgestimate or nomegestrol acetate plus 17-beta-estradiol, but less effective than cyproterone acetate.
In a 2012 Cochrane review by Bhate et al,85 the authors concluded that there was no evidence that COCs containing cyproterone were more effective than other COCs for the treatment of acne. More trials comparing COCs to each other and other acne treatments are needed.
Progestogen-only contraceptives often worsen acne, as they bind androgen receptors, and so should be avoided in women who have no contraindications to estrogen-containing preparations. Third-generation progestogens, such as desogestrel, norgestimate, and gestodene, bind more selectively to the progesterone receptor than do the second-generation progestogens (e.g., levonorgestrel and norethisterone), but at the cost of an increased risk of venous thromboembolism (VTE).
A recent updated Cochrane review on the use of spironolactone* in hirsutism and acne concluded that there is no evidence for its effectiveness in acne. *Addendum dated April 13, 2016: the current DSS guidelines are based on a literature review until December 31, 2014. This updated article now lists spironolactone as an alternative therapy for moderate and severe acne.27
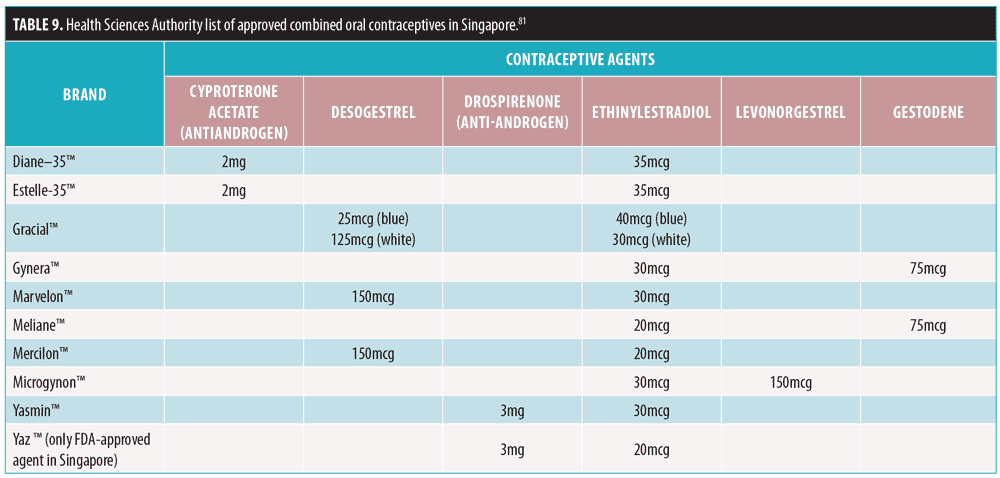
Risks/contraindications. It is necessary to identify risks/contradindications for certain conditions (Table 10) associated with COC use.89
Dosing and administration. Dosing should start on the first day of menstrual cycle as follows: one tablet daily for 21 days, stop for seven days, then repeat. The duration of treatment is expected to be within a 6- to 12-month course.90 There is no increase in efficacy from combining oral antibiotics and oral contraceptives.
Adverse effects. Blood pressure should be documented upon initial consult and monitored at follow-up visits. Adverse effects of COCs include nausea, vomiting, breast tenderness, headaches, menstrual disturbance, fluid retention, and venous thrombosis. The association of VTE and COCs is known, with an estrogen dose-dependent relationship. During a woman’s reproductive years, her risk for VTE with ethinyl estradiol is 6 to 8 times higher than in nonusers.89 The evidence regarding COCs and stroke is unclear. In healthy, young women, the risk of ischemic stroke is low, but concomitant COC use and smoking increases the risk significantly. As for malignancy, the evidence demonstrating a link between breast cancer and hormone exposure is marginal and controversial. For cervical cancer, there might be an association; however, the association is reduced with cessation of a COC, and, by 10 years, the risk of cervical cancer is similar to that of those who have never taken COCs.89 Recently, the Singapore Health Promotions Board recommended decreasing cervical screenings to once every three years after the onset of sexual activity or beginning at the age of 25.91 It is acceptable to start COCs with succeeding consultation that includes a Pap smear.
In summary, COCs are effective for noninflammatory and inflammatory acne (Level 1++, A). They may be considered alternatives to systemic antibiotics or systemic retinoids for moderate-to-severe papulopustular acne in women, in combination with topical retinoids with or without BPO. Identifying risk for certain conditions associated with COC use is a necessity, and patients should be advised.
Adjunctive Therapy
Adjunctive acne therapy can provide further clinical effect in addition to conventional therapy. Recommendations are summarized in Table 11.
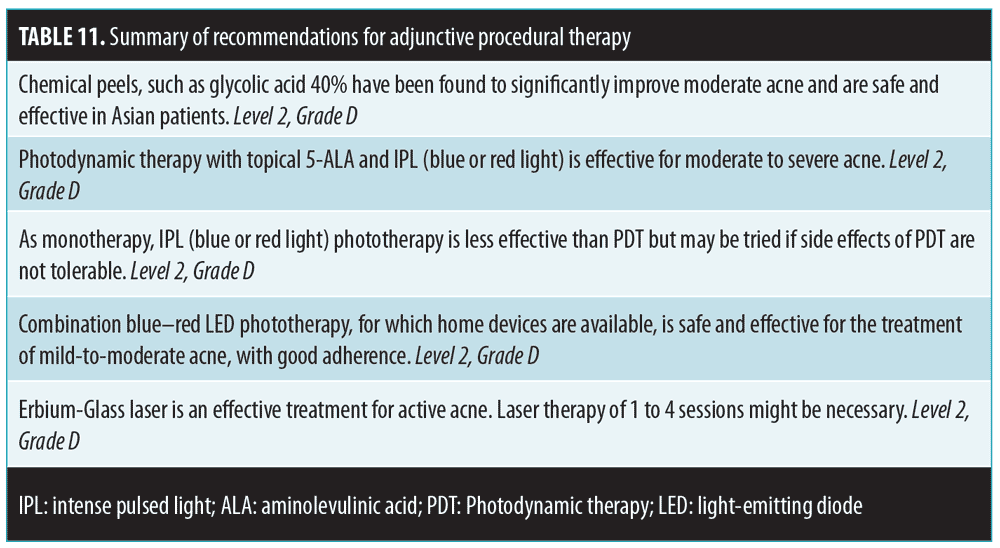
Chemical peels. Chemical peels for acne and acne scars in Asian patients have been shown to be safe and effective.92,93 More trials with better study designs and higher numbers of subjects are needed to further establish the role of chemical peels in Asian patients with acne.92 For instance, glycolic acid 40% has been found to significantly improve moderate acne.93
Light devices. Photodynamic therapy with topical 5-aminolevulinic acid and intense pulsed light (IPL) and other light sources have been shown to be effective in moderate-to-severe acne.94,95 In combination with fractional CO2 laser treatments, IPL has been shown to reduce the inflammatory lesion and atrophic scar scores compared to at baseline. Subsequent fractional CO2 laser treatments further decreased the atrophic scar score. Around 90 percent of the patients experienced significant or moderate overall improvement, and almost 80 percent patients rated their results as “excellent” or “good.” The melanin index (MI), erythema index (EI), and skin sebum level all significantly decreased after IPL treatments, and the EI and sebum level were still low when assessed at the three-month follow-up, although the MI had increased again by this point. The adverse effects of both treatments were transient and bearable.94,95
Combination blue–red light-emitting diode (LED) phototherapy at home has been shown to reduce sebum production, attenuate inflammatory cell infiltrations, and decrease sebaceous gland size.96
Lasers. Papules, pustules, and nodules respond well to therapy using the 1,550-nm Erbium–Glass laser. The sebaceous gland size decreased significantly, hence the long remission period.97 Treatment efficacy is due to the antibacterial effect on P. acnes and the ablation of sebaceous glands. Although cost might be an issue, laser therapy can induce remission in acne long term. Pain, when present, is tolerable.
Other energy devices. Fractional radiofrequency microneedle therapy has been demonstrated in one trial to have sebosuppressive effects from a single treatment, but its therapeutic efficacy requires further evaluation.98
Adjuvant Therapy: Cosmeceuticals/Dermocosmetics
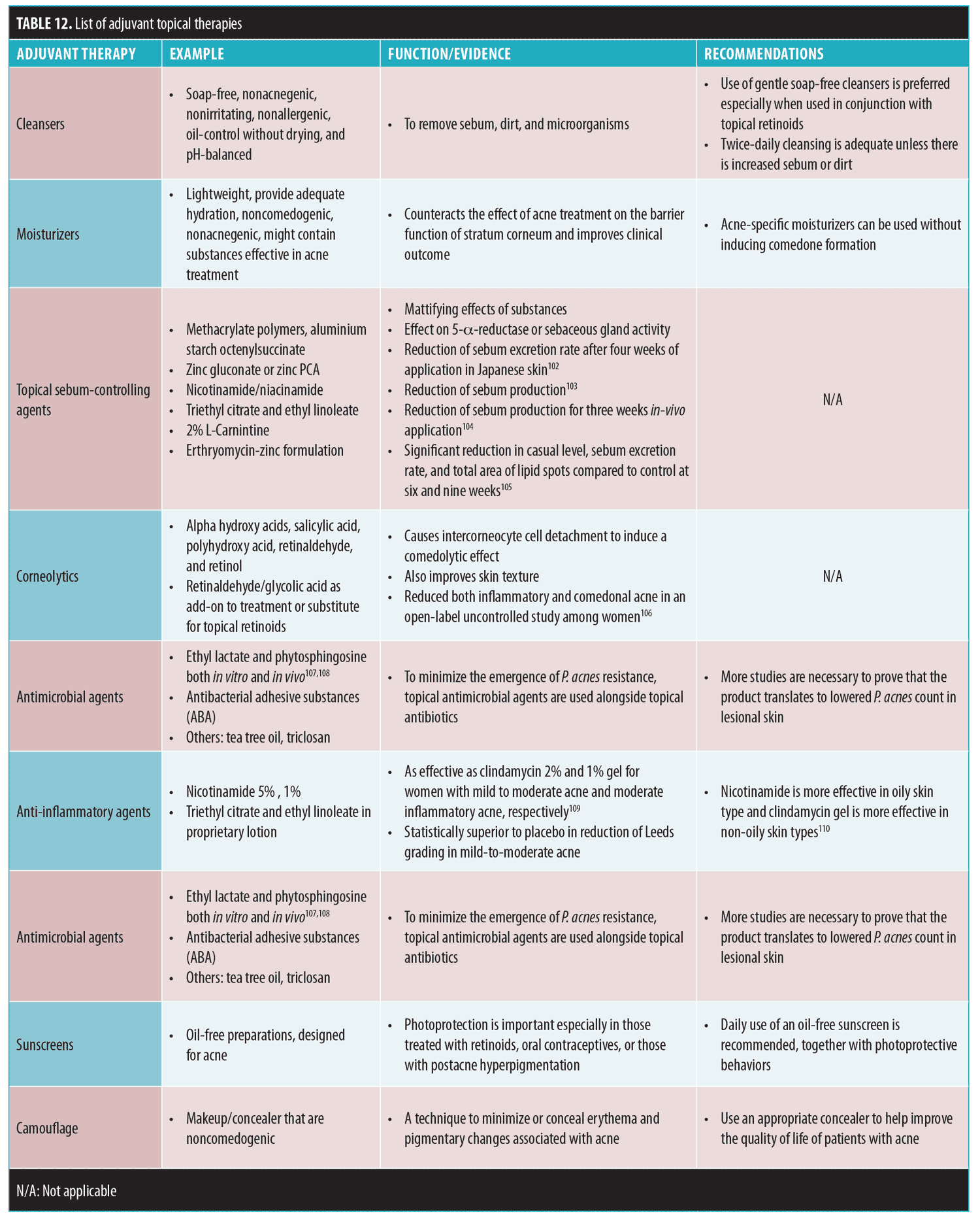
The term cosmeceutical is defined as “a topical preparation that is sold as a cosmetic but [which] has performance characteristics that suggest pharmaceutical action.”99 The term is used interchangeably with dermocosmetics. In existing resources on dermocosmetics, many use trade names, extrapolate efficacy of single ingredients from studies using proprietary combination products, contain biases, and overstate the efficacy of proprietary products. These methods can leave patients confused. Cosmeceuticals are not always necessary (i.e., the simpler the regimen, the easier it might be for the patient to adhere to it), but can be added as an adjuvant. The role of adjuvant therapy is to help reduce side effects that might be associated with treatment (e.g., irritation and dryness), provide a synergistic effect when used together with conventional treatment, reduce adverse sequelae (e.g., postinflammatory hyperpigmentation [PIH] or scarring), and improve quality of life.100,101
The range of cosmeceutical agents for the management of acne is listed in Table 12.55,102–110 Studies investigating cosmeceutical products tend to involve a combination of agents, making it difficult to provide a level of evidence for a single agent. Hence, only a few cosmeceutical agents are listed and without an assocciated level of evidence.
Management of Mild, Moderate, and Severe Acne
These management guidelines draw heavily from pre-existing, evidence-based guidelines and reviews. Expert opinions, particularly those that are relevant to local Singapore patients and conditions, have also been considered. Management options in terms of acne severity are summarized in Table 13.
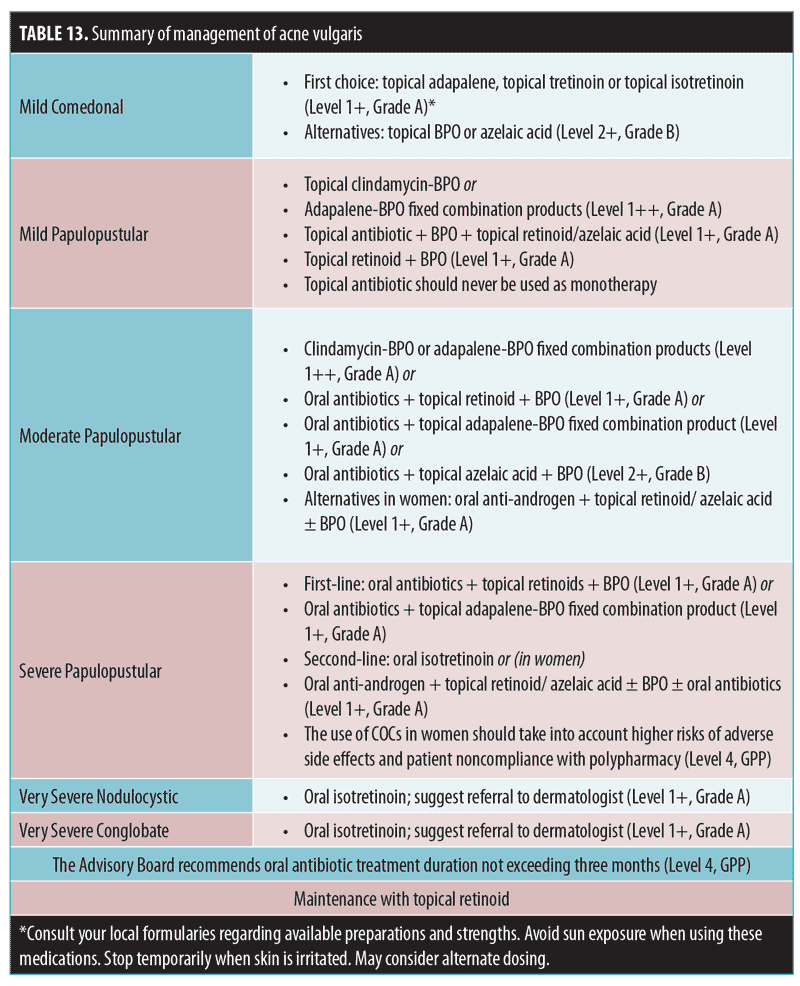
Mild acne. Comedonal acne. Limited data suggest that increasing concentrations of tretinoin cream (i.e., 0.01%, 0.025%, 0.05%, and 0.1%) are associated with increased efficacy but with an increased rate of side effects.111 In comparison, adapalene 0.1% gel or cream causes less irritation versus tretinoin and is as efficacious as tretinoin cream.48,112 Topical isotretinoin gel 0.05% application for acne is not associated with any significant systemic levels of the drug and is effective and well-tolerated.113,114 Topical isotretinoin preparations are not available in some countries.
Azelaic acid 20% is a mild comedolytic with anti-inflammatory activity.115 It is safe and effective as a treatment and maintenance option for women with adult acne with noninferior efficacy to adapalene (0.1%) in the control of inflammatory acne.116 It can be used during pregnancy117 and can be useful in patients with acne and PIH, as it induces hypopigmentation in darker skin.118 BPO (2.5%, 5%, and 10%) has anti-inflammatory, antibacterial, and mild comedolytic activities and does not induce bacterial resistance.119 Lower concentrations of BPO (2.5% or 5%) are preferred, as there is increased irritation with increasing concentrations of BPO and without a significant increase in efficacy between the 2.5% and 10% concentrations.120
Papulopustular acne. Recommended treatment for mild papulopustular acne includes fixed-combination products containing BPO (with clindamycin or adapalene) with or without topical retinoid/azelaic acid. BPO should be applied in the morning, and the topical retinoid should be administered at night. It is advised to avoid simultaneous BPO and tretinoin application due to potential oxidation of the tretinoin cream.121 The topical fixed-combination products are effective in the treatment of mild-to-moderate papulopustular acne and can be used as monotherapy applied once-daily.122–125 Systematic reviews have noted the superiority of combination products against individual components alone. These options also generally have good tolerability profiles and have now become the standard of care for mild-to-moderate papulopustular acne.13,72
Moderate acne (papulopustular). Treatment recommendations for moderate papulopustular acne include fixed combinations of antibiotics and/or adapalene/BPO and/or topical azelaic acid. The choice of medication depends on several factors, including cost, convenience, adherence, and patient preference. Treatment duration with systemic antibiotics should not exceed 3 to 4 months. At Week 6 of therapy, the patient’s response to treatment should be assessed. The use of oral antiandrogens as an alternative second-line treatment might be suitable for some women with moderate papulopustular acne. The various contraindications should always be considered.
Severe acne. Papulopustular acne. Treatment recommendations include the use of oral antibiotics, topical retinoid/azelaic acid, BPO, and oral antiandrogen combinations. Topical treatment alone is not recommended. Patients should be followed closely to monitor treatment response, and second-line therapy should be considered when treatment response is inadequate after a suitable amount of time. Some doctors combine oral contraceptives with systemic antibiotics; however, there is insufficient data on the potentially superior efficacy of this approach. Consideration of risks should be included in clinical decision making before prescribing COCs.
Nodulocystic and conglobate acne. For these types of acne, oral isotretinoin is the recommended treatment and its use should be managed by a dermatologist.
Acne in Adult Women
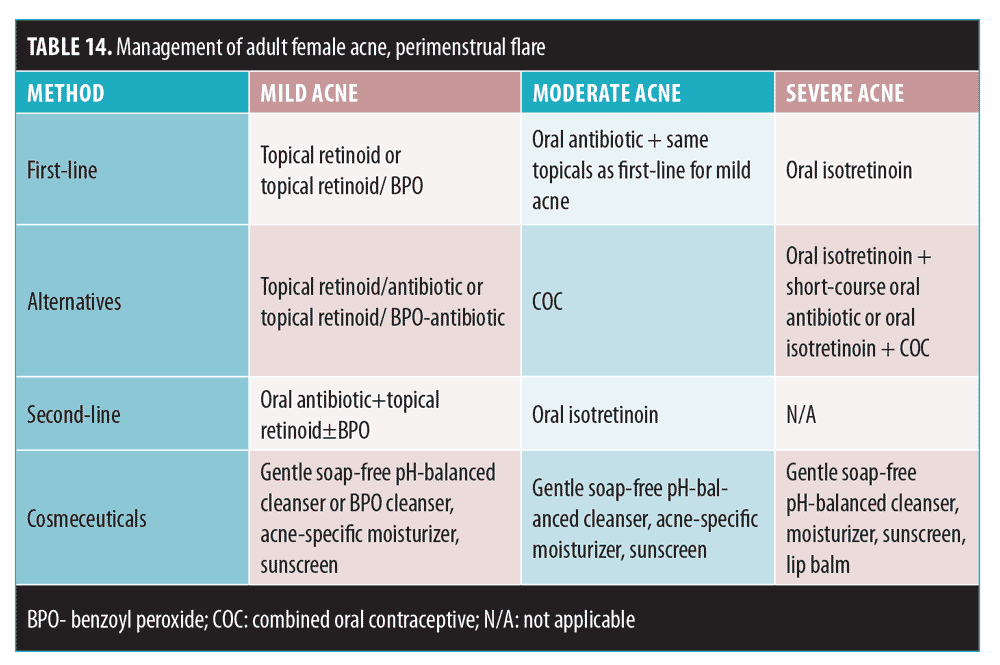
A large scale international study of 374 women from Europe, the Americas, and Asia evaluated the significance of acne in this population.126 Adult acne can be defined as the presence of acne lesions after the age of 25 years. It might be an extension or relapse of adolescent acne (persistent acne) or can be a new occurrence (late-onset acne). More than 50 percent of individuals in their 30s reported acne, and late onset is more common in women. There are two distinct clinical presentations of acne in adult women: one is similar to adolescent acne (almost 90%) and the other is a mild, inflammatory/nodular acne localized to the mandibular region. The pathogenesis might be different (i.e., androgens), and recognition of this entity is needed. The majority (93.7%) of women had facial comedones. Scarring occurred in 59.4 percent, and PIH in 50.4 percent of the women in this study. Acne in adult women represents a psychosocial burden and affects quality of life.
Acne in adults is largely mild-to-moderate in severity and can be refractory to treatment.127 Subgroup analyses of recent large-scale controlled clinical trials have shown that many adult women respond well to standard first-line acne therapy. Refractory cases might require long-term treatment and referral to a dermatologist. Treatment choices are outlined in Table 14.
Special Populations
Pregnant/lactating women. Treatment of acne in this population requires safety considerations for both the mother and fetus/infant (Table 15).117,128–132 As discussed, hormonal therapy, tetracyclines, cotrimoxazole, and both oral and topical retinoids should be avoided. Treatment choices are outlined in Table 16. In cases of mild-to-moderate acne, consider topical antibiotics used in combination with topical acne agents.133 Topical BPO and adapalene are generally safe. For moderate-to-severe acne, previously mentioned topical agents can be used with oral antibiotics. Although some studies suggest that the use of topical retinoids on a limited body surface area is likely safe, most experts do not recommend the use of topical retinoids in pregnant or lactating patients.128
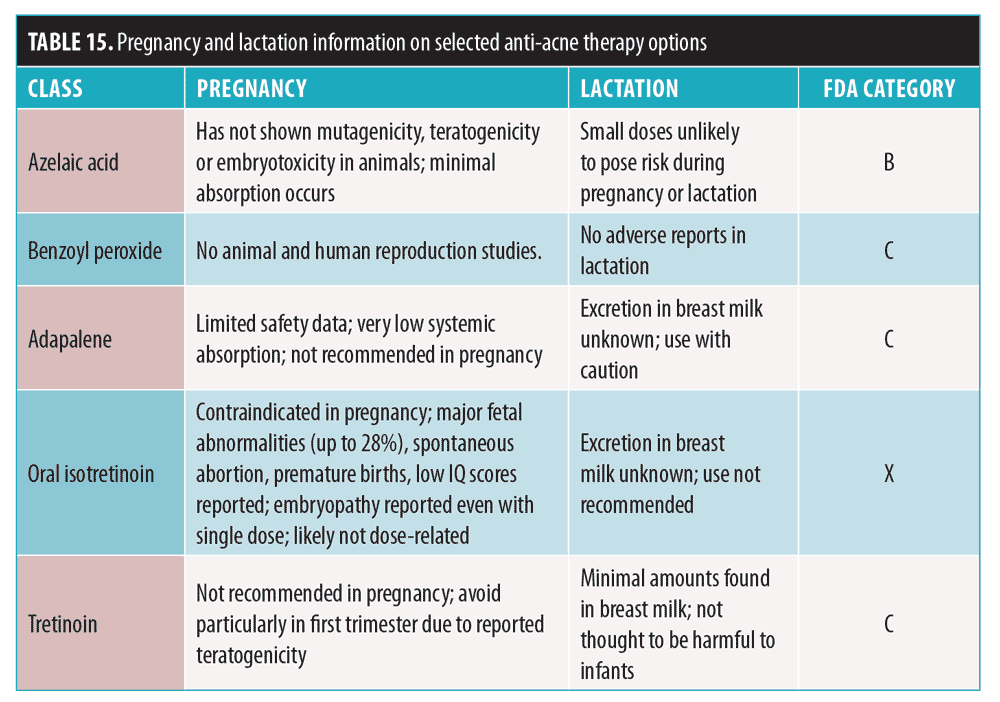
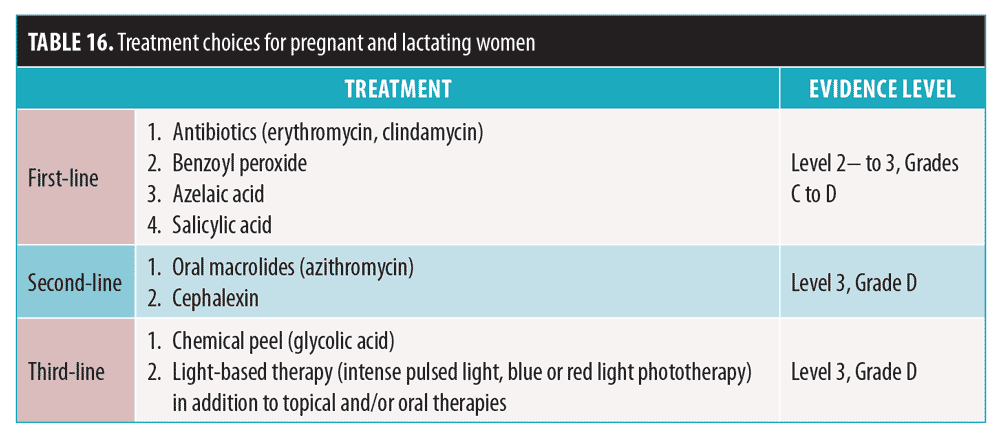
Pediatric patients. For children 1 to 7 years old with significant acne, evaluation for systemic associations is warranted, with referral to a pediatric endocrinologist to rule out a gonadal/ovarian pathology.134,135 Recommendations are summarized in Table 17. A low starting BPO concentration is recommended, due to increased risk of irritation; this can minimize antibiotic resistance when used with antibiotics.135 Topical retinoids may be used as monotherapy or in combination products and in regimens of care for all types and severities of acne in children and adolescents of all ages.135 If topical antibiotic treatment is extended beyond a few weeks, topical BPO should be added or used in combination products.135
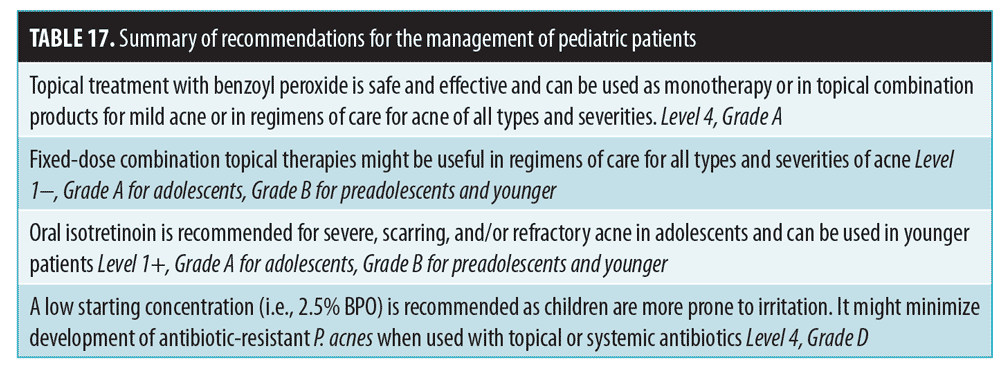
Oral antibiotics are appropriate for moderate-to-severe inflammatory acne at any age.135 Tetracycline derivatives can be used once the child has acquired full dentition. Second-generation tetracyclines (e.g., doxycycline, minocycline) are sometimes preferred due to ease of use, fewer problems with absorption, and less frequent dosing.135 Patient education and monitoring for adverse events should be practiced. Extensive counseling is recommended when using certain agents. Previously mentioned issues regarding the use of isotretinoin and COCs should be discussed. Hormonal therapy with COCs can be useful as second-line therapy in regimens of care in pubertal women with moderate to severe acne. Tobacco use and family history of thrombotic events should be assessed (Level 4, D, GPP).135
Pityrosporum folliculitis can complicate acne treatment and has a predilection for adolescents. Both folliculitis and acne can be aggravated by occupation, sports, and the humid tropical climate of Singapore.136 Retinoid dermatitis or photosensitivity from topical retinoids can be worsened by tropical sun exposure. Such exposure also results in prolonged postacne erythema and increased risk of PIH. Patients with acne should be advised accordingly.
Treatment of Acne Scars
Scars refer to the imperfect repair of the skin following skin inflammation. There is dystrophy of the epidermis and dermis that results in focal skin atrophy or hypertrophy. This is in contrast to dyspigmentation due to postinflammation without any skin dystrophy. Such cases present as erythema or PIH and heal spontaneously without leaving any dystrophic scars. This type of dyspigmentation should not be classified as scars and instead referred to as pseudoscars.
Postacne scars cannot be reversed, but can be made less noticeable and more easily covered with makeup. Post-acne scars can be physically disabling and psychologically disturbing.137
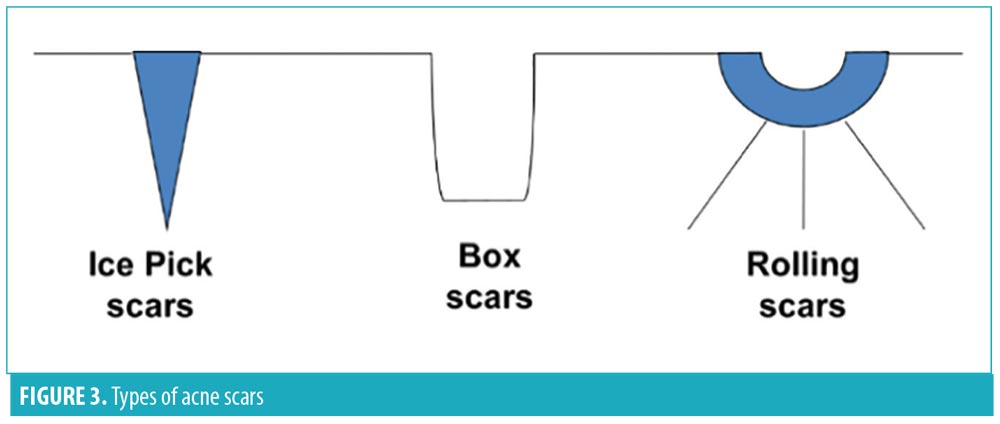
There are in general three morphologies of acne scars (Figure 3): ice pick, box car, and rolling.138 Asian skin has peculiar postacne scarring morphologies, in that some patients jawline keloids, or soft papular scars that resemble sebaceous hyperplasia on the nose and chin, and dumbbell or nodular keloids on the chest.138
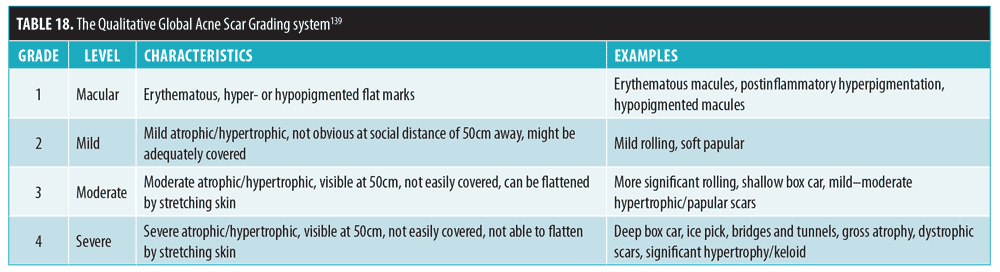
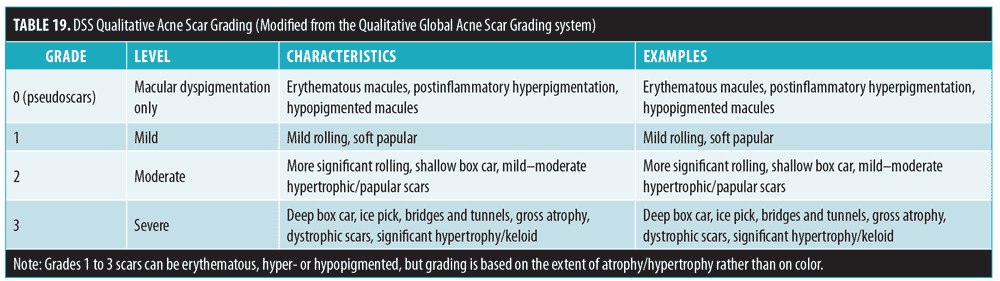
The Qualitative Global Acne Scar Grading139 can be used for rating of the severity of acne scarring (Table 18). Grade 1 severity (macular dyspigmentation) can cause patient distress but is only temporary and is generally not considered true acne scarring. Hence, DSS has adopted a modified version of this scale in which macular dyspigmentation is instead considered to be grade 0 (Table 19).
The choice and extent of treatment modalities for acne scars will depend on their morphology and severity. Summary recommendations are enumerated in Table 20. Laser resurfacing is another option and includes ablative laser resurfacing or photothermolysis (AP), nonablative laser resurfacing or photothermolysis (NP), and fractional resurfacing or photothermolysis (FP). A systematic review comparing AP to NP reported a short-term improvement (26 to 83% for AP and 26 to 50% for NP) in acne scars based on both subjective and objective measurements.140 FP is the current gold standard of laser treatment, due to less downtime. It uses thermally induced coagulation and produces a columnar-shaped microthermal zone with diameters of less than 250µm and covering up to 10 to 43 percent of total skin. There is no significant difference in efficacy between different laser settings despite adjusting fluences or densities.141 The PIH from FP is usually mild and short-lived, lasting no longer than three months, even in individuals with darker skin types. PIH can be reduced by conservative treatment densities in Fitzpatrick Skin Types IV to VI.142,143

A new modality of erbium-doped yttrium aluminium garnet (Er:YAG) laser combining short-pulsed and dual-mode laser was reported to be safe and effective in treating atrophic facial acne scars in Asian patients with darker skin tones.144 Fractional Er:YAG and CO2 lasers provided comparable outcomes for scar treatment, but Er:YAG laser was associated with less treatment discomfort.145
Fractional radiofrequency (RF) therapy and bipolar RF energy therapy have also been shown to be safe and effective.146 Fractional RF and fractional erbium-doped glass are similar in effectiveness.147 Use of fractional laser with RF followed by fractional RF was shown to be safe and effective for treatment of acne scars, with a modest improvement and low PIH rate comparable to other resurfacing techniques in this Asian case series.148
ActiveFX fractional CO2 laser therapy (Lumenis, San Jose, California, USA) is a new technology for the treatment of facial acne scars, using a diffractive lens array and 755-nm picosecond laser.149 This laser produced improvement in appearance and texture of acne scars at three months after the last treatment, with objective findings similar to those of fractional ablative laser treatments. Histologic findings suggest that improvement in scarring from this treatment goes beyond collagen remodeling.150
Microneedling with dermaroller, also known as collagen induction therapy (CIT), is a simple and inexpensive modality for acne scars, demonstrating satisfactory results with little downtime, but with occasional side effects in Asian skin (e.g., PIH, tram-track scarring).151 Additional studies are needed to confirm their efficacy. In Singapore, microneedling is included under the Ministry of Health List B Aesthetic Procedures and should only be performed as part of a clinical trial. These options are adjunctive and should never be used as first-line therapy.
References
- Rathi SK. Acne vulgaris treatment: the current scenario. Indian J Dermatol. 2011;56(1):7–13.
- Tan JKL, Tang J, Fung K, et al. Prevalence and severity of facial and truncal acne in a referral cohort. J Drugs Dermatol. 2008;7(6):551–556.
- Shen Y, Wang T, Zhou C, et al. Prevalence of acne vulgaris in Chinese adolescents and adults: a community-based study of 17,345 subjects in six cities. Acta Derm Venereol. 2012; 92(1):40–44.
- Tan HH, Tan AWH, Barkham T, et al. Community-based study of acne vulgaris in adolescents in Singapore. Br J Dermatol. 2007;157(3):547–551.
- Oon H. Personal communication. December 31, 2015.
- Han XD, Oon HH, Goh CL. Epidemiology of post-adolescence acne and adolescence acne in Singapore: a 10-year retrospective and comparative study. J Eur Acad Dermatol Venereol. 2016;30(10):1790–1793.
- Lim YL, Chan YH, Yosipovitch G, Greaves MW. Pruritus is a common and significant symptom of acne. J Eur Acad Dermatol Venereol. 2008;22(11):1332–1336.
- Wong SN. Presented at: the South-East Asia Study Alliance (SASA). In: Prevalence of scarring in mild to moderate acne vulgaris. 2012.
- Whiting DA. Acne. West J Med. 1979;131(6):551–557.
- Knor T. The pathogenesis of acne. Acta Dermatovenerol Croat. 2005;13(1):44–49.
- Toyoda M, Morohashi M. Pathogenesis of acne. Med Electron Microsc. 2001;34(1):29–40.
- Tan HH, Goh CL, Yeo MG, Tan ML. Antibiotic sensitivity of Propionibacterium acnes isolates from patients with acne vulgaris in a tertiary dermatological referral centre in Singapore. Ann Acad Med Singapore. 2001;30(1):22–25.
- Nast A, Dreno B, Bettoli V, et al. European evidence-based (S3) guidelines for the treatment of acne. J Eur Acad Dermatol Venereol. 2012;26 Suppl 1:1–29.
- Thiboutot D, Gollnick H, Bettoli V, et al. New insights into the management of acne: an update from the Global Alliance to Improve Outcomes in Acne group. J Am Acad Dermatol. 2009;60(5 Suppl):S1–S50.
- Ministry of Health, Malaysia. Management of Acne—Quick Reference Guide for Healthcare Providers. 2012.
- Asai Y, Baibergenova A, Dutil M, et al. Management of acne: Canadian clinical practice guideline. CMAJ. 2016;188(2):118–126.
- Philippine Dermatological Society. Guidelines for acne treatment. 2011:1–5.
- Goh CL, Abad-Casintahan F, Aw DCW, et al. South-East Asia study alliance guidelines on the management of acne vulgaris in South-East Asian patients. J Dermatol. 2015;42(10):945–953.
- Tan JKL, Tang J, Fung K, et al. Development and validation of a comprehensive acne severity scale. J Cutan Med Surg. 2007;11(6):211–216.
- Lolis MS, Bowe WP, Shalita AR. Acne and Systemic Disease. Med Clin North Am. 2009;93(6):1161–1181.
- Lowenstein EJ. Diagnosis and management of the dermatologic manifestations of the polycystic ovary syndrome. Dermatol Ther. 2006;19(4):210–223.
- Jabbour SA. Cutaneous manifestations of endocrine disorders: a guide for dermatologists. Am J Clin Dermatol. 2003;4(5):315–331.
- Martin AR, Lookingbill DP, Botek A, et al. Health-related quality of life among patients with facial acne—assessment of a new acne-specific questionnaire. Clin Exp Dermatol. 2001;26(5):380–385.
- Ferdowsian HR, Levin S. Does diet really affect acne?. Skin Therapy Lett. 2010;15(3):1–2, 5.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92(3):241–246.
- Smith RN, Mann NJ, Braue A, et al. The effect of a high-protein, low glycemic-load diet versus a conventional, high glycemic-load diet on biochemical parameters associated with acne vulgaris: a randomized, investigator-masked, controlled trial. J Am Acad Dermatol. 2007;57(2):247–256.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945–73.e33.
- Diabetes Society of Singapore. The highs and lows of glycaemic index. Diabetes Singapore. 2011:19–21.
- The University of Sydney. The Glycaemic Index [Internet]. Available from: http://www.glycemicindex.com/. Accessed January 24, 2017.
- Caperton C, Block S, Viera M, et al. Double-blind, placebo-controlled study assessing the effect of chocolate consumption in subjects with a history of acne vulgaris. J Clin Aesthet Dermatol. 2014;7(5):19–23.
- Delost GR, Delost ME, Lloyd J. The impact of chocolate consumption on acne vulgaris in college students: a randomized crossover study. J Am Acad Dermatol. 2016;75(1):220–222.
- Vongraviopap S, Asawanonda P. Dark chocolate exacerbates acne. Int J Dermatol. 2016;55:587–591.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. Milk consumption and acne in teenaged boys. J Am Acad Dermatol. 2008;58(5):787–793.
- Adebamowo CA, Spiegelman D, Danby FW, et al. High school dietary dairy intake and teenage acne. J Am Acad Dermatol. 2005;52(2):207–214.
- Ismail NH, Manaf ZA, Azizan NZ. High glycemic load diet, milk and ice cream consumption are related to acne vulgaris in Malaysian young adults: a case control study. BMC Dermatol. 2012;12:13.
- Pontes T de C, Fernandes Filho GMC, Trindade A de SP, Sobral Filho JF. Incidence of acne vulgaris in young adult users of proteinIncidence of acne vulgaris in young adult users of protein-calorie supplements in the city of Joao Pessoacalorie supplements in the city of Joao Pessoa—PB. An Bras Dermatol. 2013;88(6):907–912.
- Bronsnick T, Murzaku EC, Rao BK. Diet in dermatology: part I. Atopic dermatitis, acne, and nonmelanoma skin cancer. J Am Acad Dermatol. 2014;71(6):1039.e1–1039.e12.
- Tsai MC, Chen W, Cheng YW, et al. Higher body mass index is a significant risk factor for acne formation in schoolchildren. Eur J Dermatol. 2006;16(3):251–253.
- Tan HH, Goh CL, Yeo M. Antibiotic sensitivity of Propionibacterium acnes isolates studied in a skin clinic in Singapore. Arch Dermatol. 1999; 135(6):723.
- Ross JI, Snelling AM, Carnegie E, et al. Antibiotic-resistant acne: lessons from Europe. Br J Dermatol. 2003;148(3):467–478.
- Gold LS. Efficacy and tolerability of fixed-combination acne treatment in adolescents. Cutis. 2013;91(3):152–159.
- Wolf JEJ, Kaplan D, Kraus SJ, et al. Efficacy and tolerability of combined topical treatment of acne vulgaris with adapalene and clindamycin: a multicenter, randomized, investigator-blinded study. J Am Acad Dermatol. 2003;49(3 Suppl):S211–S217.
- Thiboutot DM, Weiss J, Bucko A, et al. Adapalene-benzoyl peroxide, a fixed-dose combination for the treatment of acne vulgaris: results of a multicenter, randomized double-blind, controlled study. J Am Acad Dermatol. 2007;57:791–799.
- Thielitz A, Sidou F, Gollnick H. Control of microcomedone formation throughout a maintenance treatment with adapalene gel, 0.1%. J Eur Acad Dermatol Venereol. 2007;21(6): 747–753.
- Hensby C, Cavey D, Bouclier M, et al. The in vivo and in vitro anti-inflammatory activity of CD271: a new retinoid-like modulator of cell differentiation. Agents Actions. 1990;29(1–2):56–58.
- Hubbard BA, Unger JG, Rohrich RJ. Reversal of skin aging with topical retinoids. Plast Reconstr Surg. 2014;133(4):481e–90e.
- Greenspan A, Loesche C, Vendetti N, et al. Cumulative irritation comparison of adapalene gel and solution with 2 tazarotene gels and 3 tretinoin formulations. Cutis. 2003;72(1):76–81.
- Goh CL, Tang MBY, Briantais P, Kaoukhov A, Soto P. Adapalene gel 0.1% is better tolerated than tretinoin gel 0.025% among healthy volunteers of various ethnic origins. J Dermatolog Treat. 2009;20(5):282–288.
- Keating GM. Adapalene 0.1%/benzoyl peroxide 2.5% gel: a review of its use in the treatment of acne vulgaris in patients aged ? 12 years. Am J Clin Dermatol. 2011;12(6):407–420.
- Thielitz A, Helmdach M, Ropke EM, Gollnick H. Lipid analysis of follicular casts from cyanoacrylate strips as a new method for studying therapeutic effects of antiacne agents. Br J Dermatol. 2001;145(1):19–27.
- Thiboutot DM, Shalita AR, Yamauchi PS, et al. Adapalene gel, 0.1%, as maintenance therapy for acne vulgaris: a randomized, controlled, investigator-blind follow-up of a recent combination study. Arch Dermatol. 2006;142(5): 597–602.
- Zhang JZ, Li LF, Tu YT, Zheng J. A successful maintenance approach in inflammatory acne with adapalene gel 0.1% after an initial treatment in combination with clindamycin topical solution 1% or after monotherapy with clindamycin topical solution 1%. J Dermatolog Treat. 2004;15(6):372–378.
- Alirezai M, George SA, Coutts I, et al. Daily treatment with adapalene gel 0.1% maintains initial improvement of acne vulgaris previously treated with oral lymecycline. Eur J Dermatol. 2007;17(1):45–51.
- Bergfeld WF. The pathophysiology of acne vulgaris in children and adolescents, part 1. Cutis. 2004;74(2):92–97.
- Draelos ZD, Ertel KD, Berge CA. Facilitating facial retinization through barrier improvement. Cutis. 2006;78(4):275–281.
- Aczone Full Prescribing Information [Internet]. Available from: https://www.allergan.com/assets/pdf/aczone7-5_pi. Acessed August 6, 2018.
- Joseph R, Ho LY, Gomez JM, et al. Mass newborn screening for glucose-6-phosphate dehydrogenase deficiency in Singapore. Southeast Asian J Trop Med Public Health. 1999;30 Suppl 2:70–71.
- Ianaro A, Ialenti A, Maffia P, et al. Anti-inflammatory activity of macrolide antibiotics. J Pharmacol Exp Ther. 2000;292(1):156–163.
- Cazalis J, Tanabe S, Gagnon G, Sorsa T, Grenier D. Tetracyclines and chemically modified tetracycline-3 (CMT-3) modulate cytokine secretion by lipopolysaccharide-stimulated whole blood. Inflammation. 2009;32(2):130–137.
- Castro MM, Kandasamy AD, Youssef N, Schulz R. Matrix metalloproteinase inhibitor properties of tetracyclines: therapeutic potential in cardiovascular diseases. Pharmacol Res. 2011;64(6):551–560.
- Tan AW, Tan HH. Acne vulgaris: a review of antibiotic therapy. Expert Opin Pharmacother. 2005;6(3):409–418.
- Ozolins M, Eady EA, Avery AJ, et al. Comparison of five antimicrobial regimens for treatment of mild to moderate inflammatory facial acne vulgaris in the community: randomised controlled trial. Lancet (London, England). 2004;364(9452): 2188–2195.
- Smith K, Leyden JJ. Safety of doxycycline and minocycline: a systematic review. Clin Ther. 2005;27(9):1329–1342.
- Bleeker J, Hellgren L, Vincent J. Effect of systemic erythromycin stearate on the inflammatory lesions and skin surface fatty acids in acne vulgaris. Dermatologica. 1981;162(5):342–349.
- Reisner RM. Antibiotic and anti-inflammatory therapy of acne. Dermatol Clin. 1983;1(3): 385–397.
- Sanchez AR, Rogers RS 3rd, Sheridan PJ. Tetracycline and other tetracycline-derivative staining of the teeth and oral cavity. Int J Dermatol. 2004;43(10):709–715.
- Hubbell CG, Hobbs ER, Rist T, White JWJ. Efficacy of minocycline compared with tetracycline in treatment of acne vulgaris. Arch Dermatol. 1982;118(12):989–992.
- Elkayam O, Yaron M, Caspi D. Minocycline-induced autoimmune syndromes: an overview. Semin Arthritis Rheum. 1999;28(6):392–397.
- Cotterill JA, Cunliffe WJ, Forster RA, et al. A comparison of trimethoprim-sulphamethoxazole with oxytetracycline in acne vulgaris. Br J Dermatol. 1971;84(4):366–369.
- Bhambri S, Del Rosso JQ, Desai A. Oral trimethoprim/sulfamethoxazole in the treatment of acne vulgaris. Cutis. 2007; 79(6):430–434.
- Dreno B, Thiboutot D, Gollnick H, et al. Antibiotic stewardship in dermatology: limiting antibiotic use in acne. Eur J Dermatol. 2014;24(3):330–334.
- Thiboutot D, Gollnick H, Bettoli V, et al. New insights into the management of acne: An update from the Global Alliance to Improve Outcomes in Acne Group. J Am Acad Dermatol. 2009;60(5 Suppl):S1–S50.
- Lee JW, Yoo KH, Park KY, et al. Effectiveness of conventional, low-dose and intermittent oral isotretinoin in the treatment of acne: a randomized, controlled comparative study. Br J Dermatol. 2011;164(6):1369–1375.
- Bagatin E, Guadanhim LRS, Enokihara MMSS, et al. Low-dose oral isotretinoin versus topical retinoic acid for photoaging: a randomized, comparative study. Int J Dermatol. 2014;53(1):114–122.
- Rasi A, Behrangi E, Rohaninasab M, Nahad ZM. Efficacy of fixed daily 20 mg of isotretinoin in moderate to severe scar prone acne. Adv Biomed Res. 2014;3:103.
- Goulden V, Clark SM, McGeown C, Cunliffe WJ. Treatment of acne with intermittent isotretinoin. Br J Dermatol. 1997;137(1):106–108.
- Palmer RA, Sidhu S, Goodwin PG. “Microdose” isotretinoin. Br J Dermatol. 2000;143(1):205–206.
- Rademaker M. Isotretinoin: dose, duration and relapse. What does 30 years of usage tell us?. Australas J Dermatol. 2013;54(3):157–162.
- Thielitz A, Krautheim A, Gollnick H. Update in retinoid therapy of acne. Dermatol Ther. 2006; 19(5):272–279.
- Wolverton SE. Comprehensive Dermatologic Drug Therapy. 3rd edition. Philadelphia, PA: Elsevier; 2013.
- Dhir R, Gehi NP, Agarwal R, More YE. Oral isotretinoin is as effective as a combination of oral isotretinoin and topical anti-acne agents in nodulocystic acne. Indian J Dermatol Venereol Leprol. 2008;74(2):187.
- Wessels F, Anderson AN, Kropman K. The cost-effectiveness of isotretinoin in the treatment of acne. Part 1. A meta-analysis of effectiveness literature. S Afr Med J. 1999;89(7 Pt 2):780–784.
- Azoulay L, Oraichi D, Berard A. Isotretinoin therapy and the incidence of acne relapse: a nested case-control study. Br J Dermatol. 2007; 157(6):1240–1248.
- Strauss JS, Krowchuk DP, Leyden JJ, et al. Guidelines of care for acne vulgaris management. J Am Acad Dermatol. 2007;56(4):651–663.
- Bhate K, Williams HC. What’s new in acne?. An analysis of systematic reviews published in 2011–2012. Clin Exp Dermatol. 2014;39(3):273–278.
- Koo EB, Petersen TD, Kimball AB. Meta-analysis comparing efficacy of antibiotics versus oral contraceptives in acne vulgaris. J Am Acad Dermatol. 2014;71(3):450–459.
- Singapore Health Sciences Authority. PZ4970 Inforsearch—Register of Therapeutic Products [Internet]. Available from: http://eservice.hsa.gov.sg/prism/common/enquirepublic/SearchDRBProduct.do?action=load. Accessed January, 9 2017.
- Arowojolu AO, Gallo MF, Lopez LM, Grimes DA. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012;(7):CD004425.
- Lam C, Zaenglein AL. Contraceptive use in acne. Clin Dermatol. 2014;32(4):502–515.
- Zouboulis CC, Rabe T. [Hormonal antiandrogens in acne treatment]. J Dtsch Dermatol Ges. 2010;8 Suppl 1:S60–S74.
- Society for Colposcopy and Cervical Pathology of Singapore. SCCPS Scientific Committee Position Paper on HPV Vaccination. 2016.
- Handog EB, Datuin MSL, Singzon IA. Chemical peels for acne and acne scars in asians: evidence based review. J Cutan Aesthet Surg. 2012; 5: 239–46.
- Kaminaka C, Uede M, Matsunaka H, et al. Clinical evaluation of glycolic acid chemical peeling in patients with acne vulgaris: a randomized, double-blind, placebo-controlled, split-face comparative study. Dermatol Surg. 2014;40(3):314–322.
- Mei X, Shi W, Piao Y. Effectiveness of photodynamic therapy with topical 5-aminolevulinic acid and intense pulsed light in Chinese acne vulgaris patients. Photodermatol Photoimmunol Photomed. 2013;29(2):90–96.
- Wat H, Wu DC, Rao J, Goldman MP. Application of intense pulsed light in the treatment of dermatologic disease: a systematic review. Dermatol Surg. 2014;40(4):359–377.
- Kwon HH, Lee JB, Yoon JY, et al. The clinical and histological effect of home-use, combination blue-red LED phototherapy for mild-to-moderate acne vulgaris in Korean patients: a double-blind, randomized controlled trial. Br J Dermatol. 2013;168(5):1088–1094.
- Moneib H, Tawfik AA, Youssef SS, Fawzy MM. Randomized split-face controlled study to evaluate 1550-nm fractionated erbium glass laser for treatment of acne vulgaris—an image analysis evaluation. Dermatol Surg. 2014;40(11):1191–1200.
- Lee KR, Lee EG, Lee HJ, Yoon MS. Assessment of treatment efficacy and sebosuppressive effect of fractional radiofrequency microneedle on acne vulgaris. Lasers Surg Med. 2013;45(10):639–647.
- Kligman D. Cosmeceuticals. Dermatol Clin. 2000;18(4):609–15.
- Goh C, Noppakun N, Micali G, et al. Meeting the challenges of acne treatment in Asian Patients: a review of the role of dermocosmetics as adjunctive therapy. J Cutan Aesthet Surg. 2016;9(2):85–92.
- The role of dermocosmetics in modern acne treatment. In: Dermatology: Acne. 2014: 234–251.
- Draelos ZD, Matsubara A, Smiles K. The effect of 2% niacinamide on facial sebum production. J Cosmet Laser Ther. 2006;8(2):96–101.
- Charakida A, Charakida M, Chu AC. Double-blind, randomized, placebo-controlled study of a lotion containing triethyl citrate and ethyl linoleate in the treatment of acne vulgaris. Br J Dermatol. 2007;157(3):569–574.
- Peirano RI, Hamann T, Dusing HJ, et al. Topically applied L-carnitine effectively reduces sebum secretion in human skin. J Cosmet Dermatol. 2012;11(1):30–36.
- Pierard-Franchimont C, Goffin V, Visser JN, et al. A double-blind controlled evaluation of the sebosuppressive activity of topical erythromycin-zinc complex. Eur J Clin Pharmacol. 1995;49(1–2):57–60.
- Dreno B, Nocera T, Verriere F, et al. Topical retinaldehyde with glycolic acid: study of tolerance and acceptability in association with anti-acne treatments in 1,709 patients. Dermatology. 2005;210 Suppl:22–29.
- Pavicic T, Wollenweber U, Farwick M, Korting HC. Anti-microbial and -inflammatory activity and efficacy of phytosphingosine: an in vitro and in vivo study addressing acne vulgaris. Int J Cosmet Sci. 2007;29(3):181–190.
- Prottey C, George D, Leech RW, et al. The mode of action of ethyl lactate as a treatment for acne. Br J Dermatol. 1984;110(4):475–485.
- Shahmoradi Z, Iraji F, Siadat AH, Ghorbaini A. Comparison of topical 5% nicotinamid gel versus 2% clindamycin gel in the treatment of the mild-moderate acne vulgaris: a double-blinded randomized clinical trial. J Res Med Sci. 2013;18(2):115–117.
- Khodaeiani E, Fouladi RF, Amirnia M, et al. Topical 4% nicotinamide vs. 1% clindamycin in moderate inflammatory acne vulgaris. Int J Dermatol. 2013;52(8):999–1004.
- Webster G, Cargill DI, Quiring J, Vogelson CT, Slade HB. A combined analysis of 2 randomized clinical studies of tretinoin gel 0.05% for the treatment of acne. Cutis. 2009;83(3):146–154.
- Irby CE, Yentzer BA, Feldman SR. A review of adapalene in the treatment of acne vulgaris. J Adolesc Health. 2008;43(5):421–424.
- Chen C, Jensen BK, Mistry G, et al. Negligible systemic absorption of topical isotretinoin cream: implications for teratogenicity. J Clin Pharmacol. 1997;37(4):279–284.
- Chalker DK, Lesher JLJ, Smith JGJ, et al. Efficacy of topical isotretinoin 0.05% gel in acne vulgaris: results of a multicenter, double-blind investigation. J Am Acad Dermatol. 1987;17(2 Pt 1):251–254.
- Sieber MA, Hegel JKE. Azelaic acid: properties and mode of action. Skin Pharmacol Physiol. 2014;27 Suppl 1:9–17.
- Thielitz A, Lux A, Wiede A, et al. A randomized investigator-blind parallel-group study to assess efficacy and safety of azelaic acid 15% gel vs. adapalene 0.1% gel in the treatment and maintenance treatment of female adult acne. J Eur Acad Dermatol Venereol. 2015;29(4):789–796.
- Kong YL, Tey HL. Treatment of acne vulgaris during pregnancy and lactation. Drugs. 2013;73(8): 779–787.
- Yin NC, McMichael AJ. Acne in patients with skin of color: practical management. Am J Clin Dermatol. 2014;15(1):7–16.
- Mills OHJ, Kligman AM, Pochi P, Comite H. Comparing 2.5%, 5%, and 10% benzoyl peroxide on inflammatory acne vulgaris. Int J Dermatol. 1986;25(10):664–667.
- Tanghetti EA, Popp KF. A current review of topical benzoyl peroxide: new perspectives on formulation and utilization. Dermatol Clin. 2009;27(1):17–24.
- Handojo I. The combined use of topical benzoyl peroxide and tretinoin in the treatment of acne vulgaris. Int J Dermatol. 1979;18(6):489–496.
- Kawashima M, Hashimoto H, Alio Saenz AB, et al. Clindamycin phosphate 1.2%-benzoyl peroxide 3.0% fixed-dose combination gel has an effective and acceptable safety and tolerability profile for the treatment of acne vulgaris in Japanese patients: a phase III, multicentre, randomised, single-blinded, active-controlled, parallel-group study. Br J Dermatol. 2015;172(2):494–503.
- Eichenfield LF, Alio Saenz AB. Safety and efficacy of clindamycin phosphate 1.2%-benzoyl peroxide 3% fixed-dose combination gel for the treatment of acne vulgaris: a phase 3, multicenter, randomized, double-blind, active- and vehicle-controlled study. J Drugs Dermatol. 2011;10(12):1382–1396.
- Brodell RT, Schlosser BJ, Rafal E, et al. A fixed-dose combination of adapalene 0.1%-BPO 2.5% allows an early and sustained improvement in quality of life and patient treatment satisfaction in severe acne. J Dermatolog Treat. 2012;23(1):26–34.
- Jacobs A, Starke G, Rosumeck S, Nast A. Systematic review on the rapidity of the onset of action of topical treatments in the therapy of mild-to-moderate acne vulgaris. Br J Dermatol. 2014;170(3):557–564.
- Dreno B, Thiboutot D, Layton AM, et al. Large-scale international study enhances understanding of an emerging acne population: adult females. J Eur Acad Dermatol Venereol. 2015;29(6):1096–1106.
- Dreno B, Layton A, Zouboulis CC, et al. Adult female acne: a new paradigm. J Eur Acad Dermatol Venereol. 2013;27(9):1063–1070.
- Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation: Part I. Pregnancy. J Am Acad Dermatol. 2014;70(3):401.e1-14; quiz 415.
- Lynde C, Tan J, Andriessen A, et al. A consensus on acne management focused on specific patient features. J Cutan Med Surg. 2014;18(4):243–255.
- Ng S, Ho S, Oon H. NSC guideline on drugs used in pregnancy and lactation. 2015.
- Willhite CC, Sharma RP, Allen PV, Berry DL. Percutaneous retinoid absorption and embryotoxicity. J Invest Dermatol. 2017;95(5):523–529.
- Autret E, Berjot M, Jonville-Béra AP, et al. Anophthalmia and agenesis of optic chiasma associated with adapalene gel in early pregnancy. Lancet. 2017;350(9074):339.
- Pugashetti R, Shinkai K. Treatment of acne vulgaris in pregnant patients. Dermatol Ther. 2013;26(4):302–311.
- Lee KC, Lio PA. Evidence-based recommendations for the diagnosis and treatment of paediatric acne. Arch Dis Child Educ Pract Ed. 2014;99(4):135–137.
- Eichenfield LF, Krakowski AC, Piggott C, et al. Evidence-based recommendations for the diagnosis and treatment of pediatric acne. Pediatrics. 2013;131 Suppl: S163–S186.
- Foo CCI, Goon ATJ, Leow YH, Goh CL. Adverse skin reactions to personal protective equipment against severe acute respiratory syndrome—a descriptive study in Singapore. Contact Dermatitis. 2006; 55(5):291–294.
- Goodman G. Commentary on “comparison of a fractional microplasma radiofrequency technology and carbon dioxide fractional laser for the treatment of atrophic acne scars: a randomized split-face clinical study”. Dermatol Surg. 2013;39(4):567–570.
- Jacob CI, Dover JS, Kaminer MS. Acne scarring: a classification system and review of treatment options. J Am Acad Dermatol. 2001;45(1): 109–117.
- Goodman GJ, Baron JA. Postacne scarring: a qualitative global scarring grading system. Dermatologic Surg. 2006;32(13):1458–1466.
- Ong MWS, Bashir SJ. Fractional laser resurfacing for acne scars: a review. Br J Dermatol. 2012; 166(6):1160–1169.
- Yuan XH, Zhong SX, Li SS. Comparison study of fractional carbon dioxide laser resurfacing using different fluences and densities for acne scars in Asians: a randomized split-face trial. Dermatol Surg. 2014;40(5):545–552.
- Chan HHL, Manstein D, Yu CS, et al. The prevalence and risk factors of post-inflammatory hyperpigmentation after fractional resurfacing in Asians. Lasers Surg Med. 2007;39(5):381–385.
- Kono T, Chan HH, Groff WF, et al. Prospective direct comparison study of fractional resurfacing using different fluences and densities for skin rejuvenation in Asians. Lasers Surg Med. 2007;39(4):311–314.
- Lee SJ, Kang JM, Chung WS, et al. Ablative non-fractional lasers for atrophic facial acne scars: a new modality of erbium:YAG laser resurfacing in Asians. Lasers Med Sci. 2014;29(2):615–619.
- Manuskiatti W, Iamphonrat T, Wanitphakdeedecha R, Eimpunth S. Comparison of fractional erbium-doped yttrium aluminum garnet and carbon dioxide lasers in resurfacing of atrophic acne scars in Asians. Dermatol Surg. 2013;39(1 Pt 1):111–120.
- Gold MH, Biron JA. Treatment of acne scars by fractional bipolar radiofrequency energy. J Cosmet Laser Ther. 2012;14(4):172–178.
- Rongsaard N, Rummaneethorn P. Comparison of a fractional bipolar radiofrequency device and a fractional erbium-doped glass 1,550-nm device for the treatment of atrophic acne scars: a randomized split-face clinical study. Dermatol Surg. 2014;40(1):14–21.
- Yeung CK, Chan NPY, Shek SYN, Chan HHL. Evaluation of combined fractional radiofrequency and fractional laser treatment for acne scars in Asians. Lasers Surg Med. 2012;44(8):622–630.
- Huang L. A new modality for fractional CO2 laser resurfacing for acne scars in Asians. Lasers Med Sci. 2013;28(2):627–632.
- Brauer JA, Kazlouskaya V, Alabdulrazzaq H, et al. Use of a picosecond pulse duration laser with specialized optic for treatment of facial acne scarring. JAMA Dermatol. 2015;151(3):278–284.
- Dogra S, Yadav S, Sarangal R. Microneedling for acne scars in Asian skin type: an effective low cost treatment modality. J Cosmet Dermatol. 2014;13(3):180–187.

