 J Clin Aesthet Dermatol. 2021;14(4):E61–E67.
J Clin Aesthet Dermatol. 2021;14(4):E61–E67.
by Sanusi Umar, MD; Delphine J. Lee, MD, PhD; and Jenna J. Lullo, MD
Dr. Umar is with the Department of Medicine at the University of California at Los Angeles in Los Angeles, California; the Division of Dermatology, Harbor-UCLA Medical Center in Torrance, California; and the Dr. U Hair and Skin Clinic in Manhattan Beach, California. Dr. Lee is with the Department of Medicine at the University of California at Los Angeles in Los Angeles, California and the Division of Dermatology, Harbor-UCLA Medical Center in Torrance, California. Dr. Lullo is with the Division of Dermatology, Harbor-UCLA Medical Center in Torrance, California.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Background.Although many treatments are available for acne keloidalis nuchae (AKN), no systematic classification scheme exists to evaluate the outcomes of these treatments.
Objective. This study aimed to propose an AKN classification scheme.
Methods. A retrospective data analysis of several parameters, including lesion distribution, lesion type, and scalp disease association, was conducted in 108 men diagnosed with AKN between July 2009 and November 2020 in an outpatient dermatology setting. A three-tier classification system was developed as follows: Tier 1, lesion distribution relative to an area demarcated by two horizontal lines on the occipital prominences and tips of the mastoid processes and lesion sagittal width defined using Classes I through IV; Tier 2, lesion types including papules/nodules (discrete/merged), plaques, and tumorous masses; and Tier 3, the presence or absence of folliculitis decalvans (FD) or dissecting cellulitis (DC).
Results. All patients were non-white men, with most being of African (58%) or Hispanic (37%) descent. The most prevalent Tier 1 AKN presentation was Class II (58%). The mean sagittal width for Classes I through III were 2.4cm (I), 4.5cm (II), and 8.0cm (III), with Class IV characterized by widespread scalp disease. Plaques were most common in Tier 2-type lesions. FD or DC was found in seven percent of the study participants. Patients of African descent had a greater tendency to develop tumorous masses (p<0.02).
Limitations. The retrospective study design and possible selection bias.
Conclusion. We proposed an AKN classification scheme as a tool for objectively describing AKN lesions and evaluating treatment outcomes.
KEYWORDS: Acne keloidalis nuchae, AKN, acne keloidalis nuchae classification, treatment
Acne keloidalis nuchae (AKN) is a chronic debilitating condition that typically affects the occipital region or nape of the neck and produces scarring folliculitis if left untreated. Although it is conventionally thought that AKN mainly affects men of African descent,1–7 studies with other demographics suggest that other ethnic groups are significantly affected.8,9
The name of this condition suggests that it is a keloid, but growing evidence supports that it is not.10 Unlike true keloids, procedures for AKN that eliminate lesional hair follicles through surgery, laser, or radiation resulted in permanent remission without the need for keloid preventive treatments.2,3,8–20
To date, there has been no systematic classification scheme for AKN. The terms “early” and “late” as classification criteria are highly subjective8,12,16,17 since not all papules become plaques and masses, with some remaining as discrete papules permanently. The lack of an AKN classification has resulted in success rates of new therapies varying considerably because the efficacy of any chosen treatment will be affected by the type of AKN lesion treated. The absence of a proper scoring scheme has been cited as a limiting factor in AKN study designs.17
We performed a retrospective analysis of AKN patients with the following study objectives:
- Determine the frequency of ethnic groups diagnosed with AKN
- Determine dermatologic disease associations, including keloids, folliculitis decalvans (FD), dissecting cellulitis (DC), acne vulgaris, razor bumps of the beard area (pseudofolliculitis barbae), and hidradenitis suppurativa
- Establish a classification system based on the analysis of all available data from patients that can be used by clinicians and researchers to evaluate treatment outcomes
Methods
Study design and population. All patients diagnosed with AKN seen between July 2009 and November 2020 in a dermatology clinic located in Los Angeles, California, were included in this retrospective analysis. An institutional review board exemption was obtained from the Western Institutional Review Board.
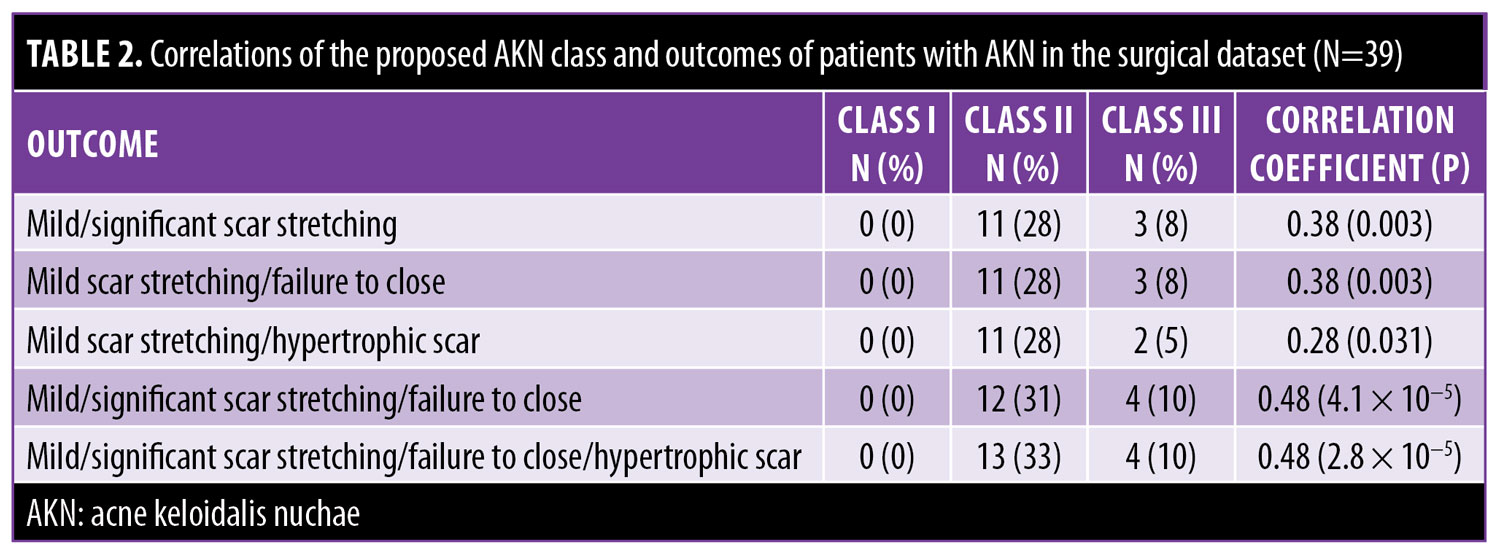
Data collection. A descriptive analysis of the following data for the entire population was conducted: patient age and race; frequency of clinically confirmed DC/FD, misdiagnosed DC/FD, keloids, acne vulgaris, pseudofolliculitis barbae, and hidradenitis suppurativa; and previous nonsurgical treatments (Table 1). A subset of the population who underwent surgery (“surgical dataset”; n=39) was also analyzed to determine the relationship of the complications of scar stretching (mild or significant), hypertrophic scarring, and failure to close (secondary intention healing) with the proposed classification scheme (Table 2).
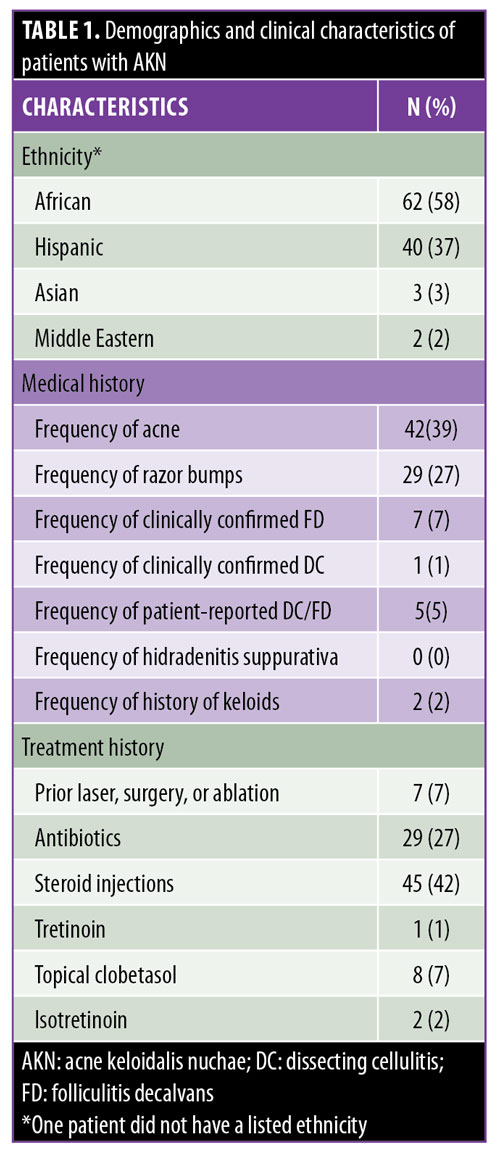
Classification scheme. The proposed three-tier AKN classification was based on the lesion type and sagittal width (Figure 1), incorporating data on lesion distribution, primary lesion type, and associated scalp disease. The first-tier classification criteria were created by using the distribution of predominant lesions relative to an area that lies between two horizontal lines drawn at the level of the occipital prominence and the inferior tips of the mastoid processes (Figure 1A). The occipital prominence was used as a superior landmark because breaching it removes the AKN lesion from the generally concave zone that is naturally present in the nape-neck axis. Lesions confined to concave zones heal better when excised lesions are allowed to heal by secondary intention healing21 (the favored treatment approach for AKN2,13,22–24). The determination of class categories was based on clinical experience and results of the complication analysis of the surgical dataset (Table 2). Class I sagittal width is small enough to minimize the occurrence of a wide scar to the degree that scars are typically less than 0.5cm wide after surgical excision, Class II generally tolerates the possibility of minor scar widening (0.5–2.5cm), and Class III increases the possibility of major scar stretching of greater than 2.5cm and the risk of failure of the wound to close. Widespread scalp disease (Class IV) completes this categorization scheme. A summary of the Tier 1 classes is as follows:
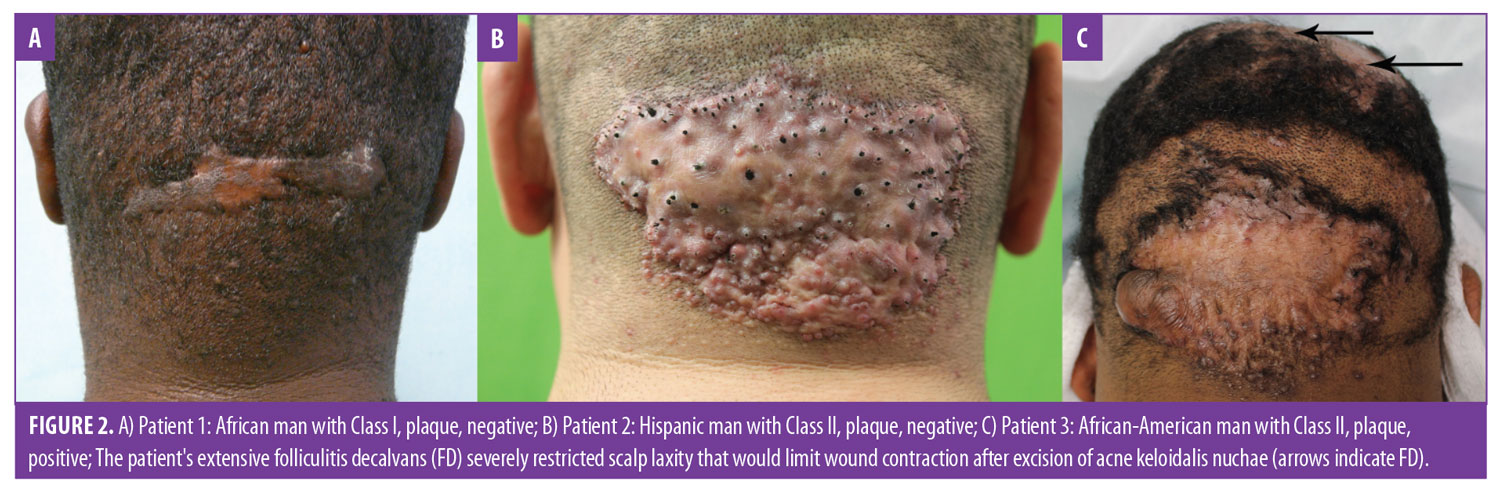
- Class I lesions are confined to a 3-cm or less sagittal width within the area defined by the aforementioned clinical demarcation lines (Figure 2A).
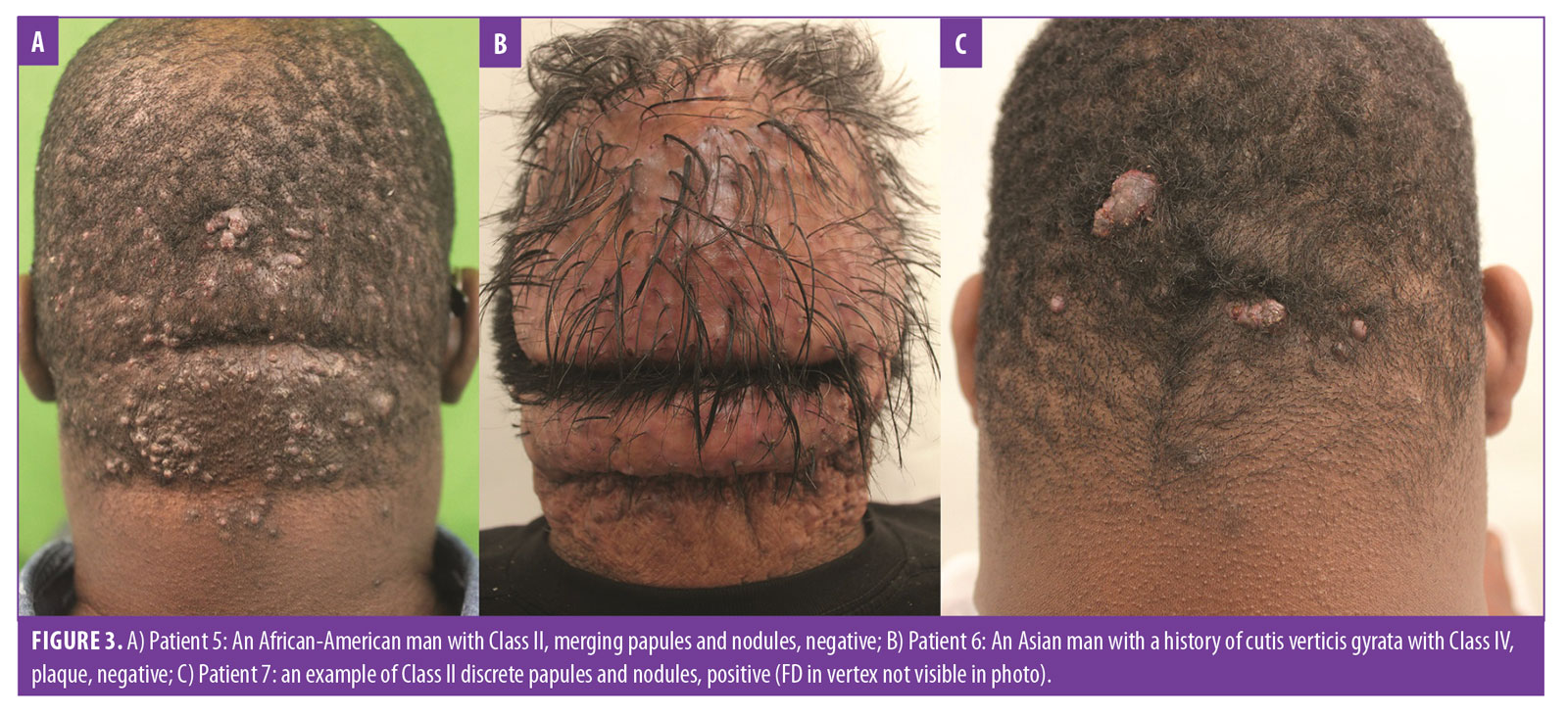
- Class II lesions are confined to a sagittal width of more than 3cm but less than 6.5cm, not breaching the demarcation lines (Figures 2B, 2C, 3A, and 3C).
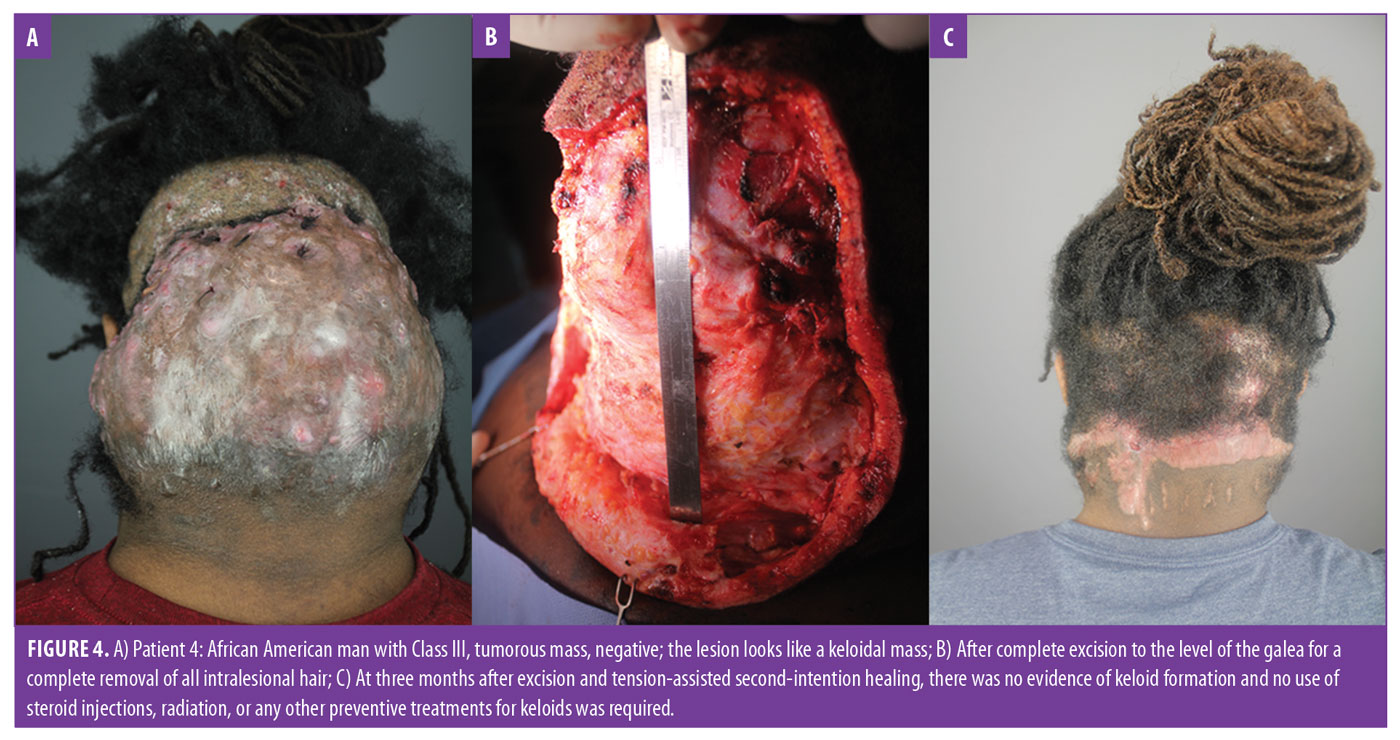
- Class III lesions are greater than 6.5cm in size or breach clinical demarcation lines but are generally confined to the nuchal area (Figure 4A).
- Class IV lesions are widespread and significantly exceed the nuchal area (Figure 3B).
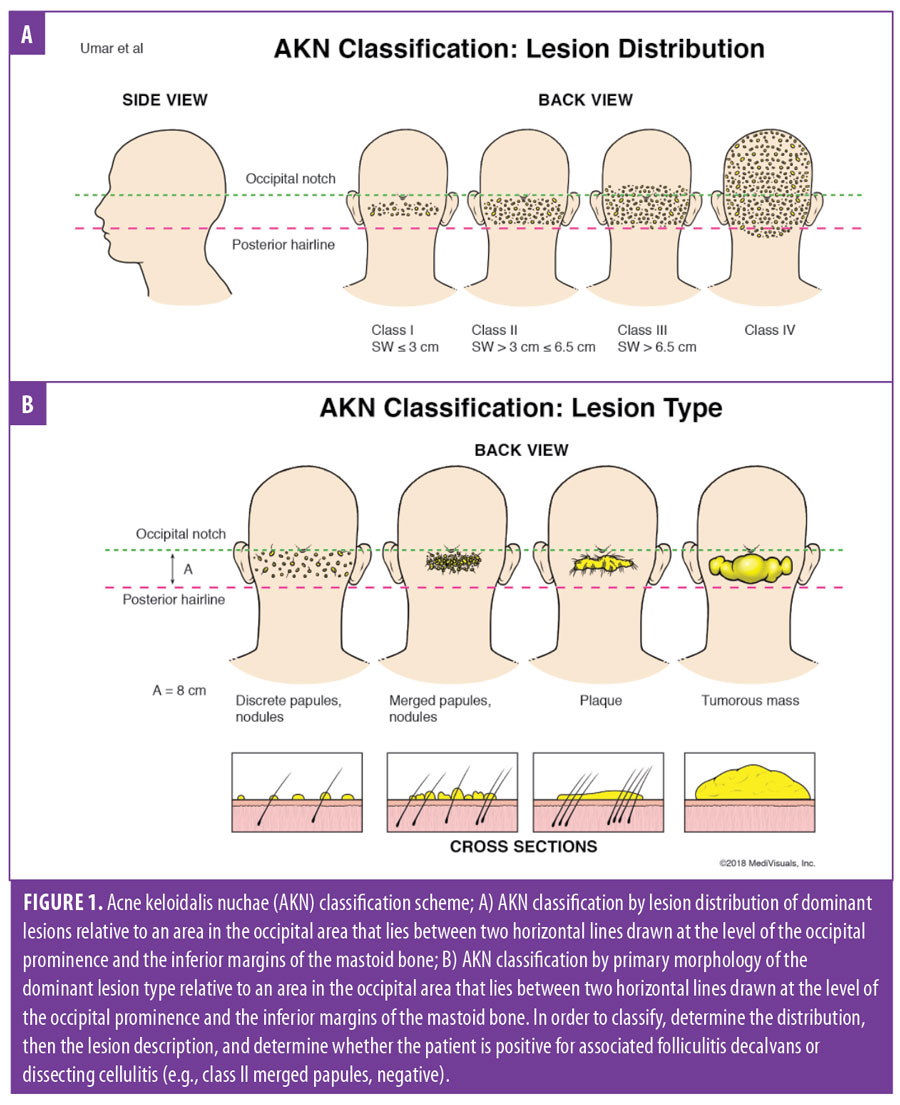
Next, Tier 2 classification criteria were created to incorporate the primary morphology of the predominant lesions (Figure 1B): discrete papules or nodules (Figure 3C), merged papules or nodules (Figure 3A), plaque (Figures 1A–C and 3B), and tumorous masses (Figure 4A). These descriptions include all of the primary morphologies observed in the cohort, which could affect curative treatment paths.2,3,8–20
Finally, Tier 3 classification criteria (scalp disease criteria) were added as either positive if AKN was associated with chronic scalp folliculitis—either DC or FD (Figure 2C)—or negative if there was no association. These criteria were necessitated by the frequent association of AKN with these conditions6,24,25 and the potential for them to compound AKN management.13
Statistical analysis. Descriptive data of the entire population are expressed as mean and standard deviation (SD) for continuous variables and number (percentage) for categorical variables. Associations between variables were analyzed using the Cramer V or Kendall tau-b. The surgical dataset was also analyzed for available complications and the proposed class (Table 3).
The relationship between progressively larger sagittal lesion widths (breakpoints of ?3cm, 3–6.5cm, and >6.5cm) and three classes (I–III) was analyzed using logistical regression analysis to determine the odds ratio of each of the three sagittal width categories being of a particular AKN class. Also, the relationship between scarring complications and three classes (I–III) was analyzed using cross-tabulations and Kendall’s tau-b to determine the odds ratio of each complication category being of a particular AKN class.
Two-sided p-values of less than 0.05 were considered statistically significant. PASW 25 (IBM Corporation, Armonk, New York) was used to perform all statistical analyses.
Results
Analysis of the population and disease associations. One hundred and eight men diagnosed with AKN were enrolled in this study (mean age: 35.8 years; SD: 8.57 years; range: 21–62 years). Most (58%) were of African descent, followed by Hispanic (37%), and other non-white ethnicities (Asian: 3%; Middle Eastern: 2%). Both the frequencies of acne (39%) and pseudofolliculitis barbae (27%) were high, and the incidence of patient-reported DC/FD was lower (5%) than the clinically confirmed pathology rate (7%). Only three in eight patients diagnosed in the clinic had a previous diagnosis, while two in five patients with a prior diagnosis of DC/FD were incorrectly diagnosed (p<0.003), with all FD occurring in the crown-vertex area. No patient reported or showed clinical signs of hidradenitis supprativum. In addition, only two of 108 patients (2%) reported a history of keloidal scarring, with one showing clinical evidenced in the earlobe and the other in the shoulder. Finally, many patients (42%) received steroid injections and antibiotics (27%) before presentation.
Analysis of the acne keloidalis nuchae classification system. There was a relationship (Table 3) between progressively larger sagittal lesion width and Classes I, II, and III (p<0.001). Analysis of the surgical dataset (Table 2) showed that only Class I case had no scar stretching, hypertrophy, or failure to close (p<0.001). Class II lesions had a 55-percent less chance of scar stretching or hypertrophic scarring in the surgical dataset as compared with class III lesions (p<0.003). Class III lesions had a seven-fold greater chance of a wide scar (typically ?2.5 cm) or failure of the wound to close relative to Class II lesions (p=0.086).
AKN class II was the most prevalent (58%) class, with plaques being the most common lesion type (Table 3). The mean sagittal width of the principal lesion in all patients was 4.8cm. The mean sagittal width was markedly different between AKN classes as follows: I, 2.4cm (SD: 0.67); II, 4.5cm (SD: 0.81); and III, 8.1cm (SD: 2.55). Additionally, there was a trend (p=0.056) in that the proportion of tumorous masses that steadily increased with the class (I: 0%; II: 22%; III: 42%).
An association between lesion type and race was also noted for patients of African descent versus those of other descents as follows discrete papules and nodules, one in 62 (2%) versus one in 45 (2%); merged papules and nodules, 24 in 62 (39%) versus 16 in 45 (36%), plaques, 23 in 62 (37%) versus 25 in 45 (56%); and tumorous mass, 14 in 62 (23%) versus three in 45 (7%) (p=0.02).
The complications in the surgical dataset (n=39) were mild scar stretching (n=12; 31%), significant scar stretching (n=2; 5%), failure to close (n=2; 5%), and hypertrophic scarring (n=3; 8%). Table 3 shows the correlations between the proposed class and combinations of different complications.
Summary of the proposed three-tier classification of acne keloidalis nuchae. With demarcation lines defined as two horizontal lines drawn at the level of the occipital prominence and the inferior tips of the mastoid processes (Figure 1A), the classification system is presented below.
Tier 1 (primary). Class I lesions are confined to a sagittal width of 3cm or less within the area defined by the aforementioned clinical demarcation lines. Class II lesions are confined to a sagittal width of between 3cm and 6.5cm but without breaching the demarcation lines. Class III lesions are greater than 6.5cm or breach clinical demarcation lines but are generally confined to the nuchal area. Finally, Class IV lesions are widespread and significantly exceed the nuchal area.
Tier 2 (secondary). Discrete papules or nodules: scattered papules and nodules separate from one another. Merged papules or nodules: papules and nodules that coalesced or abutting one another. Interval skin is mostly diseased. Plaque: Continuous lesions that could be raised, atrophic, or simply indurated. Tumorous mass: smooth, dome-shaped mass(es)
Tier 3 (tertiary). Associated scalp disease is defined as positive if AKN is associated with either DC or FD (Figure 2C), or negative if there is no association.
How to use the three-tier AKN classification terminology. Consider the example of patient classification of the patients in Figure 2C using the three-tier system: the patient has a lesion distribution confined to borders of the demarcation lines and a sagittal width of less than 6.5cm, making him a Tier 1, Class II case; his lesion is a plaque (Tier 2), and he is positive for FD (Tier 3). Thus, his three-tier classification is AKN Class II, plaque, positive.
Discussion
Although men of African descent made up almost two-thirds of our study population, a substantial proportion was primarily of Hispanic descent (37%). Our study cohort might reflect the demographics of the population surrounding our clinic location, which includes the greater Los Angeles area in which Hispanics are the largest minority population, in contrast to a similar study in Jamaica that found AKN exclusively in people of African heritage.6 This finding challenges the common perception that the disease is mostly confined to individuals of African descent.1–7 Moreover, the reporting of AKN studies in other parts of the world8,9 suggests that understanding AKN is important in the context of other non-white ethnicities. For example, our data suggest that men of African descent are more likely to develop keloid-like tumorous masses and less likely to develop discrete papules and nodules than men of other races. Our study found no ethnic whites or involvement of female sex, confirming the rarity of AKN in these populations.6,25–28 Finally, the mean age of our patients (35.8 years) also correlates closely with that reported in other studies of AKN.2,3,9
Both the frequencies of acne (39%) and pseudofolliculitis barbae (27%) were high (Table 1), with rates more than twice those reported by East-Innis et al.6 While co-existence between acne vulgaris or pseudofolliculitis barbae and AKN can be observed, it is unknown whether there is a causal association or shared common pathology between them.4,6,29 Although Alexis et al30 reported that both conditions are unrelated, our data suggest that it is plausible that both conditions may have factors in common depending upon the mechanisms involved. We did not observe hidradenitis suppurativa in any of the patients with AKN, which is consistent with the lack of reports of this association in the existing literature. More recently, evidence is accumulating that both hypertension and metabolic syndrome may be risk factors for AKN.6,28,31
AKN is often mischaracterized as a keloidal condition. Although the nonkeloidal nature of AKN was noted as early as 1972 by Cosman and Wolff,10 many practitioners have approached treatment for AKN as they would a keloid with the expectation of keloidal scar formation after excision. It is also implied by the name that the patient tends to develop keloids elsewhere on their bodies. However, in a retrospective analysis of 37 patients with AKN, we previously showed that neither AKN nor keloids would develop at excision sites if all lesional hair follicles were removed during the treatment process.13 This result is supported by several other smaller case studies2,3,13–15 and holds true even in patients with large keloid-like masses13 (Figures 4A–4C). This finding in patients with AKN contrasts with reported postexcision recurrence rates of true keloids of 45 to 100 percent for the entire body,32 but it is consistent with a very low keloid rate of 0.8 percent following head or neck surgery reported in a general African-American population.33 Our data also suggest that patients with AKN are not especially prone to developing keloids elsewhere; in our AKN patients of African descent, only 2 of 48 (4%) had a history of developing keloids elsewhere, which is similar to the 3 to 6-percent prevalence observed in the general population of a comparable demographic.34,35 Additionally, one of these two patients underwent bat excision with tension suture-assisted secondary intention healing and did not develop keloids as of his seven-month follow-up vist after surgery.13 Overall, these facts suggest there is no association between AKN and keloids. Therefore, keloids were not considered in the final classification system that emerged.
Prior studies have suggested that chronic scalp folliculitis (either DC or FD) is frequently associated with AKN.6,7,36 Of five patients with a prior diagnosis of chronic scalp folliculitis, we were able to clinically confirm DC and FD in three (all in the crown-vertex area) and diagnosed five patients who did not have a prior diagnosis (Table 1). This suggests that there may be an association of DC/FD with AKN (7% in our data, with East-Innis et al6 reporting a rate of 7%), indicating that our data agree with the literature. Additionally, all the clinically-evident FD manifested as coexisting plaques in the crown-vertex area. This suggests that crown-vertex plaques in AKN patients should be assessed for FD before they are assumed to be AKN lesions as well. Finally, a previous study reported the occurrence of DC or FD as a cause for wider scars or failure of wounds to close after excision surgeries,13 with the likely mechanism being the impairment of scalp laxity by tissue changes due to DC or FD.
The ultimate objective of this study was to create an AKN classification scheme that can be used by clinicians and researchers to evaluate treatment and study outcomes. After studying several parameters in our patient cohort that could affect patients’ management course and outcomes, we primarily used the area demarcated by the occipital notch and posterior hairline, sagittal width of the lesion, lesion location, lesion type, and association with DC or FD to create four primary classes. There is no objective AKN lesion severity scale currently used in assessing treatment outcomes.2,8,9,11,12,15,17,20 Success rates of new therapies have varied considerably because the efficacy of any chosen treatment may be affected by the type of AKN lesion treated, a factor that has been cited as a challenge in objectively evaluating AKN treatment outcome studies.17 Many studies have resorted to using the nonspecific term “early” and “late,” often attributing the term “early” to papular lesions and “late” to AKN masses, respectively. This view is based in part on the belief that AKN papules would merge to become large masses at a later stage. However, AKN lesions do not always transition from papules to masses. Many lesions that start as papules and nodules will remain as papules and never form AKN masses. Additionally, not all large AKN masses are formed by the coalition of papules. Our analyses suggest that the principles used in the proposed AKN classification can successfully differentiate lesions based on findings from a clinical examination.
Limitations. The main strengths of our study are its size (given the low prevalence of AKN) and the development of a classification scheme for evaluating AKN therapies. One limitation is its retrospective nature. Another limitation is possible selection bias in the surgical dataset, which excludes some patients who elected to undergo treatments other than the recommended surgery because of financial and other reasons. Large prospective studies will help to validate this AKN classification scheme.
Conclusion
In summary, we developed an AKN classification system as a parameter for evaluating AKN therapies and a standard for clinicians and researchers to use in objectively characterizing patient presentations and treatment outcomes in future studies. The finding that AKN has no relationship with true keloid formation or tendency to correlate has favorable implications for treatment outcomes of the disease. Our study also establishes AKN to be a disease of people of color that substantially affects non-white ethnicities, including people of African and Hispanic descent, with patients of African descent showing a relatively higher tendency to develop AKN masses.
Acknowledgment
The authors would like to thank Dr. Marissa Carter (Strategic Solutions, Bozeman, Montana) for performing the statistical analysis.
References
- Knable AL Jr., Hanke CW, Gonin R. Prevalence of acne keloidalis nuchae in football players. J Am Acad Dermatol. 1997;37(4):570–574.
- Glenn MJ, Bennett RG, Kelly AP. Acne keloidalis nuchae: treatment with excision and second-intention healing. J Am Acad Dermatol. 1995;33(2 pt 1):243–246.
- Gloster HM. The surgical management of extensive cases of acne keloidalis nuchae. Arch Dermatol. 2000;136(11):1376–1379.
- Salami T, Omeife H, Samuel S. Prevalence of acne keloidalis nuchae in Nigerians. Int J Dermatol. 2007;46(5):482–484.
- Ogunbiyi A. Acne keloidalis nuchae: prevalence, impact, and management challenges. Clin Cosmet Investig Dermatol. 2016;9:483–489.
- East-Innis AD, Stylianou K, Paolino A, Ho JD. Acne keloidalis nuchae: risk factors and associated disorders – a retrospective study. Int J Dermatol. 2017;56(8):828–832.
- Ayanlowo OO. Scalp and hair disorders at the dermatology outpatient clinic of a tertiary hospital. Port Harcourt Med J. 2017;11(3):127–33.
- Tawfik A, Osman MA, Rashwan I. A novel treatment of acne keloidalis nuchae by long-pulsed Alexandrite laser. Dermatol Surg. 2018;44(3):413–420.
- Gamil HD, Khater EM, Khattab FM, Khalil MA. Successful treatment of acne keloidalis nuchae with erbium:YAG laser: a comparative study. J Cosmet Laser Ther. 2018;20
(7–8):419–423. - Cosman B, Wolff M. Acne keloidalis. Plast Reconstr Surg. 1972;50(1):25–30.
- Pestalardo CM, Cordero A Jr, Ansorena JM, et al. Acne keloidalis nuchae. Tissue expansion treatment. Dermatol Surg. 1995;21(8): 723–724.
- Maranda EL, Simmons BJ, Nguyen AH, et al. Treatment of acne keloidalis nuchae: a systematic review of the literature. Dermatol Ther (Heidelb). 2016;6(3):363–378.
- Umar S, David CV, Castillo JR, et al. Innovative surgical approaches and selection criteria of large acne keloidalis nuchae lesions. Plast Reconstr Surg Glob Open. 2019;7(5):e2215.
- Umar S, Castillo JR, David CV, Sandhu S. Patient selection criteria and innovative techniques for improving outcome and cosmesis in acne keloidalis nuchae lesion excision and primary closure. JAAD Case Rep. 2018;5(1):24–28.
- Kanthak FF, Cullen ML. Skin graft in the treatment of chronic furunculosis of the posterior surface of the neck (folliculitis keloidalis). South Med J. 1951;44(12): 1154–1157.
- Quarles FN, Brody H, Badreshia S, et al. Acne keloidalis nuchae. Dermatol Ther. 2007;20(3):128–132.
- Woo DK, Treyger G, Henderson M, et al. Prospective controlled trial for the treatment of acne keloidalis nuchae with a long-pulsed neodymium-doped yttrium-aluminum-garnet laser. J Cutan Med Surg. 2018;22(2):236–238.
- Umar S. Selection criteria and techniques for improved cosmesis and predictable outcomes in laser hair removal treatment of acne keloidalis nuchae. JAAD Case Rep. 2019;5(6):529–534.
- Hurkudli DS, Sarvajnamurthy S, Suryanarayan S, Chugh VS. Novel uses of skin biopsy punches in dermatosurgery. Indian J Dermatol. 2015;60(2):170–175.
- Millan-Cayetano JF, Repiso-Jimenez JB, Del Boz J, de Troya-Martin M. Refractory acne keloidalis nuchae treated with radiotherapy. Australas J Dermatol. 2017;58(1):e11–e13.
- Hinrichsen N, Birk-Sørensen L, Gottrup F, Hjortdal V. Wound contraction in an experimental porcine model. Scand J Plast Reconstr Surg Hand Surg. 1998;32(3): 243–248.
- Califano J, Miller S, Frodel J. Treatment of occipital acne keloidalis by excision followed by secondary intention healing. Arch Facial Plast Surg. 1999;1(4):308–311.
- Bajaj V, Langtry JA. Surgical excision of acne keloidalis nuchae with secondary intention healing. Clin Exp Dermatol. 2008;33(1):53–55.
- Etzkorn JR, Chitwood K, Cohen G. Tumor stage acne keloidalis nuchae treated with surgical excision and secondary intention healing. J Drugs Dermatol. 2012;11(4):540–541.
- Azurdia RM, Graham RM, Weismann K, et al. Acne keloidalis in Caucasian patients on cyclosporin following organ transplantation. Br J Dermatol. 2000;143(2):465–467.
- Kelly AP. Pseudofolliculitis barbae and acne keloidalis nuchae. Dermatol Clin. 2003;21(4):645–653.
- Loayza E, Cazar T, Uraga V, et al. Acne keloidalis nuchae in Latin American women. Int J Dermatol. 2015;54(5):e183–e185.
- Na K, Oh SH, Kim SK. Acne keloidalis nuchae in Asian: a single institutional experience. PLoS One. 2017;12(12):e0189790.
- Sperling LC, Homoky C, Pratt L, Sau P. Acne keloidalis is a form of primary scarring alopecia. Arch Dermatol. 2000;136(4): 479–484.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae. Dermatol Clin. 2014;32(2):183–191.
- Verma SB, Wollina U. Acne keloidalis nuchae: another cutaneous symptom of metabolic syndrome, truncal obesity, and impending/overt diabetes mellitus? Am J Clin Dermatol. 2010;11(6):433–436.
- Mustoe TA, Cooter RD, Gold MH, et al. International clinical recommendations on scar management. Plast Reconstr Surg. 2002;110(2):560–571.
- Young WG, Worsham MJ, Joseph CL, et al. Incidence of keloid and risk factors following head and neck surgery. JAMA Facial Plast Surg. 2014;16(5):379–380.
- Glass DA 2nd. Current understanding of the genetic causes of keloid formation. J Investig Dermatol Symp Proc. 2017;18(2):S50–553.
- Velez Edwards DR, Tsosie KS, Williams SM, et al. Admixture mapping identifies a locus at 15q21.2-22.3 associated with keloid formation in African Americans. Hum Genet. 2014;133(12):1513–1523.
- Harries MJ, Paus R. The pathogenesis of primary cicatricial alopecias. Am J Pathol. 2010;177(5):2152–2162.

