 J Clin Aesthet Dermatol. 2023;16(4):43–52.
J Clin Aesthet Dermatol. 2023;16(4):43–52.
by Allyson Marshall-Hudson, PhD; Michael Tuley, PhD; Maureen Damstra, BA; Jonathan S. Dosik, MD; Matthew F. Myntti, PhD; Dianne Porral, BS; and Janel Palomo, BS, MBA
Drs. Marshall-Hudson, Tuley, and Dosik and Ms. Damstra are with TKL Research, Inc. in Fair Lawn, New Jersey. Ms. Porral, Ms. Palomo, and Dr. Myntti are with Next Science, LLC in Jacksonville, Florida.
FUNDING: Next Science, LLC, provided financial support for this study.
DISCLOSURES: Ms. Porral and Palomo are employees of Next Science, LLC. All other authors report no conflicts of interest relevant to the contents of this article.
ABSTRACT: Objective. The primary aim of this study was to assess the change in acne lesions and severity within all treatment groups over the course of a six-month study.
Methods. This was a six-month, multisite, randomized, double-blind, controlled study in female subjects with mild-to-moderate acne to assess the clinical and psychological outcomes of treatment with biofilm disrupting acne cream 2x, biofilm disrupting acne cream 1x, biofilm disrupting acne cream without salicylic acid, 2.5% benzoyl peroxide (BPO) gel, and placebo. Subjects applied the assigned product to their face twice daily and were evaluated for clinical acne and quality of life outcomes at baseline and after six, 12, 18, and 24 weeks of treatment.
Results. After 24 weeks of use, subjects treated with biofilm disrupting acne cream 2x had a significantly greater improvement in the Investigator Global Assessment (IGA), compared to those treated with 2.5% BPO gel. Based on dermatologic assessments, biofilm disrupting acne cream 2x, biofilm disrupting acne cream 1x, biofilm disrupting acne cream without salicylic acid, and placebo control were associated with less erythema and dryness, compared to 2.5% BPO gel.
Limitations. Assessments within this study had the potential for subjective differences due to variability between evaluators.
Conclusion. Biofilm disrupting acne cream 2x and biofilm disrupting acne cream 1x provided equivalent efficacy to 2.5% BPO gel with less of the adverse effects commonly associated with BPO, such as erythema and dryness. Both the biofilm disrupting acne cream without salicylic acid and the placebo control were associated with mild improvements to acne symptoms over the course of the 24-week study.
Trial Registry Information. ClinicalTrials.gov, NCT03106766.
Keywords: Acne vulgaris, biofilm, lesions, quality of life, Investigator’s Global Assessment, Cutibacterium acnes, sebaceous gland disease, skin disease
Acne vulgaris (AV) is the most common skin disease in the United States (US), affecting approximately 50 million people.1 The inflammatory skin disorder has a multifactorial pathogenesis, including Cutibacterium acnes (C. acnes) colonization, inflammation, sebum production, and keratinization.2 Acne is thought to begin with blockage of the opening of the pilosebaceous unit. Increased sebum production and sebaceous gland hypertrophy can lead to increased pressure and inflammation with growth of C. acnes and resultant further inflammation and rupture of the lesions.1,3 There are a variety of treatment options for facial acne, both topical and systemic, which target one or more of its pathogenic factors.
Bacterial biofilms are communities of bacteria that adhere to a wide variety of surfaces, held together by polymer matrices composed of polysaccharides, secreted proteins, and extracellular DNA.4,5 Burkhart et al6 report that C. acnes biofilms may penetrate into the sebum and act as an adhesive, promoting formation of microcomedones.7 The presence of biofilm in facial acne is emerging as a potentially important barrier to and target for treatment. On the skin, bacteria adhere to the surface of the pilosebaceous unit, secreting a protective physical polysaccharide barrier that provides resistance to antimicrobial therapies. Resistance to conventional antimicrobial treatment is not only due to the physical barrier created by the rapidly established biofilm, but also the expression of hundreds of new proteins.5,8 These proteins facilitate biofilm development by enabling bacterial surface attachment, as well as clustering and secretion of extracellular polysaccharides.
Next Science (Jacksonville, Florida) has developed acne cream products with formulations based on a material science approach that target both the biofilm and the bacteria entrenched within. These formulations are based on the previously clinically tested Next Science wound gel and Next Science acne gel. This novel biofilm-eradicating technology attacks biofilms in three ways:
- Breaks the ionic bridges that hold the biofilm together.
- Solubilizes the individual polymers, exposing the bacteria.
- Directly kills bacteria by cell lysis.
In a prospective, randomized, double-blind, vehicle control study, Next Science demonstrated that topical treatment with acne gel over three months significantly reduced the incidence of acne lesions and visibly improved dermal appearance, while having no negative impact on patients’ skin.9 Specifically, results showed a statistically and clinically significant improvement in inflammatory and noninflammatory acne lesions and Investigator Global Assessment (IGA) of facial acne when the acne gel was applied once per day in conjunction with daily, topical, nonprescription, standard of care acne treatment. Furthermore, several aspects of quality of life (QoL) assessments showed significant improvements.9 These outcomes are particularly important, as adolescents and young adults with acne may have a lower QoL due to emotional distress.10
In this study, the effectiveness of twice daily application of a biofilm disrupting acne cream 2x (Next Science acne cream 2x), biofilm disrupting acne cream 1x (Next Science acne cream 1x), Next Science acne cream with no salicylic acid, 2.5% benzoyl peroxide (BPO) gel, and a placebo control in the treatment of mild-to-moderate facial acne in female patients was evaluated over a six-month period.
Rationale
Benzalkonium chloride (BZK) is a surfactant used in the Next Science acne cream formulations to assist salicylic acid in the disruption of the biofilm and microbial cell membrane. Based on the mechanism of action of its ingredients and formulation against biofilm, it was hypothesized that regular use of the Next Science acne cream products would lead to improvement in facial acne lesions and would be more effective than the BPO gel product.
Objectives
The primary objective was to assess the change in inflammatory and noninflammatory acne lesions and IGA over the course of six months of treatment with Next Science acne cream 2x, Next Science acne cream 1x, Next Science acne cream with no salicylic acid, a marketed BPO product (Proactive® Repair 3 Repairing Treatment; Alchemee/Taro Pharmaceutical Industries Ltd, Mt Vernon, New York), and a placebo control.
The secondary objectives were to assess the mean percent change from baseline to Weeks 6, 12, 18, and 24 in dermatological treatment area evaluations and acne QoL questionniare responses.
Methods
Study design. This was a randomized, double-blind, multisite study intended to quantify the clinical and psychosocial effects of Next Science acne cream 2x, Next Science acne cream 1x, Next Science acne cream with no salicylic acid, 2.5% BPO gel, and a placebo control on facial acne. The study was conducted at three clinical sites: TKL Research, Inc.; KGL Skin Study Center; and Dermatology Consulting Services, PLLC. Subjects were randomized to use one study product and were provided a standard facial cleansing wash to use twice daily for the duration of the study. Subjects were provided with their assigned study product for twice daily facial use. A detailed description of the study procedures is presented in Table 1. Assessments were conducted by the same investigator or subinvestigator, whenever possible, to ensure consistent scoring.
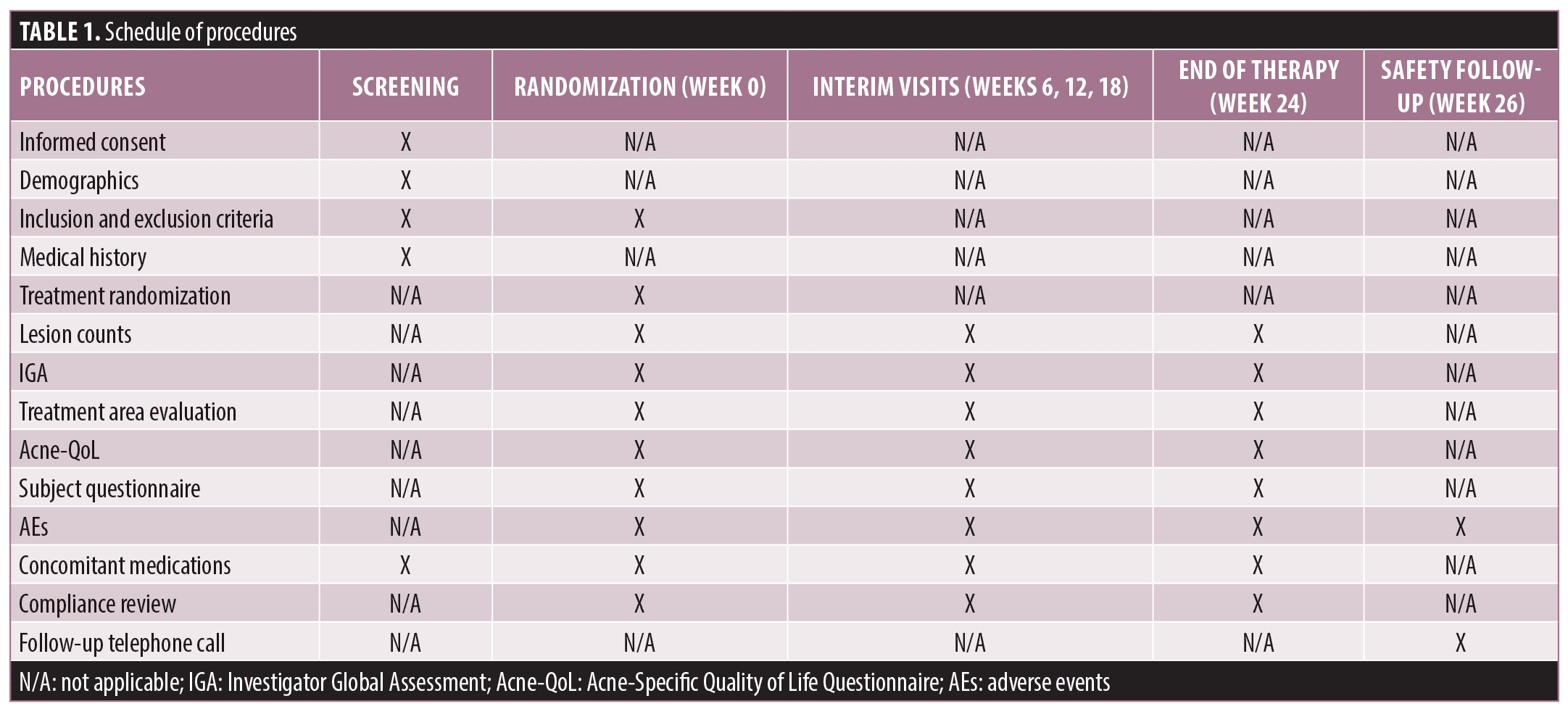
Study population. Healthy female subjects aged 13 to 35 years with mild-to-moderate inflammatory facial acne at randomization were eligible to participate. Mild-to-moderate inflammatory acne was defined as greater than 10 inflammatory lesions with two or fewer nodular/cystic lesions. Subjects were not permitted to use any other acne treatments for the duration of the study. The following washout periods were required prior to randomization: systemic medications primarily for the treatment of acne (including antibiotics and estrogens)—21 days; topical acne treatment—14 days; topical acne prescription—30 days; isotretinoin or oral retinoids—180 days; topical retinoids or alpha hydroxy/glycolic acid products, beta hydroxy, or salicylic acid products—14 days. No enrolled subjects were pregnant, planning to become pregnant, or breastfeeding a child at the time of enrollment. One subject was discontinued from the study due to pregnancy.
Materials. All subjects were provided with Equate Beauty Gentle Skin Cleanser (Walmart; Bentonville, Arkansas, US) to use as a standard facial cleanser, twice daily, for the duration of the study. Subjects were randomized to receive one of the five study treatments: Next Science acne cream 2x, Next Science acne cream 1x, Next Science acne cream with no salicylic acid, 2.5% BPO gel, or placebo control. Subjects used their assigned treatment twice daily on the face for the 24-week study and recorded product use for compliance purposes.
Lesion counts. The entire face from hairline to the mandibular line was assessed for acne lesions. All inflammatory and noninflammatory lesions in the treatment area were counted.
Investigator Global Assessment. The investigator used IGA as a static qualitative evaluation of overall facial acne severity.11
Treatment area evaluation. The following clinical signs/symptoms were assessed based on the subject’s presentation during the study visit and the subject’s report of these signs and symptoms for the period between study visits: erythema, dryness, erosion, and edema. A 4-point scoring system was used, in which 0 equals absent and 3 equals severe.
Subjective assessments. At each study visit, subjects were asked by clinical staff members if they experienced any reactions (erythema, dryness, erosion, edema) while using the study products and, if so, to rate the intensity.
Quality of life. Subjects self-reported their QoL using the Acne-Specific QoL Questionnaire (Acne-QoL). The Acne-QoL is a validated measure that contains 19 questions referring to the past week, organized into four domains: self-perception, role-social, role-emotional, and acne symptoms. Domain scores were calculated by adding the item scores within each domain, with higher scores reflecting better QoL determinations.12
Statistical analysis. Sample size was calculated by estimating the number of subjects required for 95-percent power to detect a statistically significant difference (p=0.05) between the inflammatory lesion count at the start of treatment versus the lesion count after 24 weeks of treatment, with a standard deviation(SD) of 23 percent and assuming a mean improvement of at least 38 percent over the treatment period.
Time zero counts, IGA scores, and level of improvement was compared among all treatment products.
Analysis of variance (ANOVA) calculations were performed to determine if there was a statistically significant difference in lesion reduction and IGA scores between treatment groups. Two-sided hypothesis testing was conducted for the primary outcome on all of the treatment subject populations. Resulting p-values less than or equal to 0.5 were considered statistically significant. Two-sided hypothesis testing was also conducted for subject-reported QoL and treatment area evaluations.
Ethics. This study was performed in compliance with Good Clinical Practice (GCP), including the archiving of essential study documents. The protocol and study materials were received and approved by an authorized institutional review board. Witten informed consent was obtained from all subjects prior to enrollment.
Results
Subject demographics and baseline characteristics. A total of 59 female subjects were enrolled, and 44 subjects (74.6%) completed the study. Subjects discontinued from the study due to the following reasons: adverse event (1 subject), voluntary withdrawal (10 subjects), loss to follow-up (3 subjects), and other (pregnancy, 1 subject). Eleven subjects were assigned to the Next Science Acne Cream 2x treatment group (Treatment A), 12 subjects were assigned to the Next Science Acne Cream 1x treatment group (Treatment B), 12 subjects were assigned to the Next Science without salicylic acid treatment group (Treatment C), 12 subjects were assigned to the 2.5% BPO gel treatment group (Treatment D), and 11 subjects were assigned to the placebo treatment group (Treatment E). Demographic and baseline characteristics of subjects are shown in Table 2.
Evaluation of overall assessments. Investigator Global Assessment. Figure 1 displays the IGA scores for each treatment group as change from baseline. There were improvements (negative change from baseline) in the IGA scores across the five treatment groups and timepoints, with the exception of a slight increase in IGA scores at the Week 24 visit for Treatment E, 2.5% BPO gel. There were statistically significant improvements in IGA scores at the Week 6 and Week 24 visits for Treatment A, Next Science Acne Cream 2x. No other changes from baseline were statistically significant.
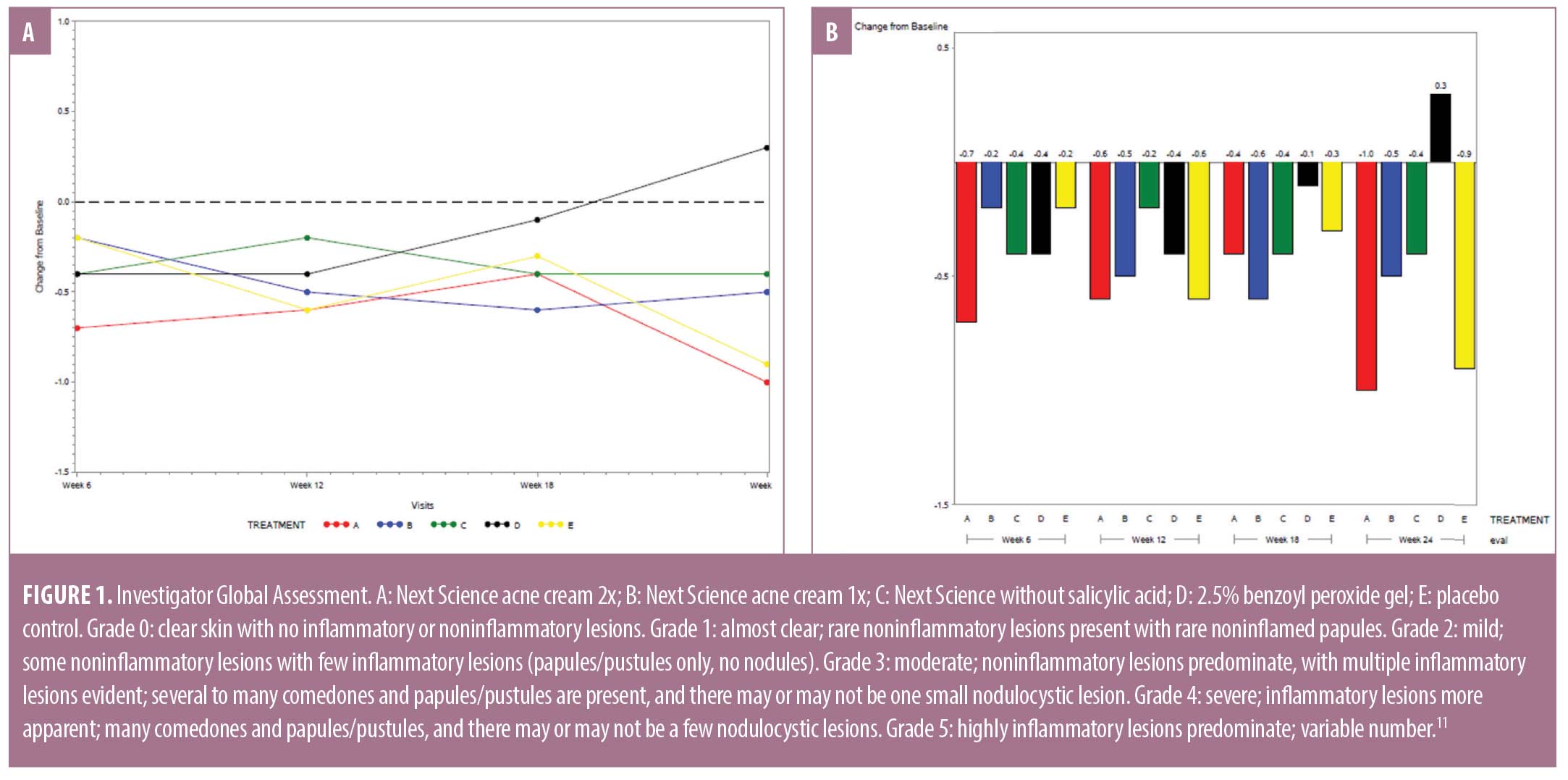
There was a significant difference in IGA scores between the five treatment groups at Week 24 (p=0.0474). The greatest improvement (39.3%) in IGA score was observed in subjects treated with Next Science acne cream 2x, followed by the placebo control (33.7%), Next Science acne cream 1x (21.1%), and Next Science acne cream without salicylic acid (16.6%). A 10.3-percent worsening of IGA score was observed in subjects treated with 2.5% BPO gel. A between-treatment analysis of covariance (ANCOVA) showed statistically significant differences in IGA score between treatment groups after 24 weeks of use, wherein Next Science acne cream 2x had the greatest improvement and 2.5% BPO gel had the least improvement of symptoms (p=0.0474).
Lesion counts. The entire face from the hairline to the mandibular line was assessed for acne lesions; all inflammatory and noninflammatory lesions (i.e., papules, pustules, comedomes, cysts/nodules) in the treatment area were counted, including the nose. Papules, comedomes, pustules, and cysts/nodules were counted and are presented as change from baseline (Figure 2 and Table 3).
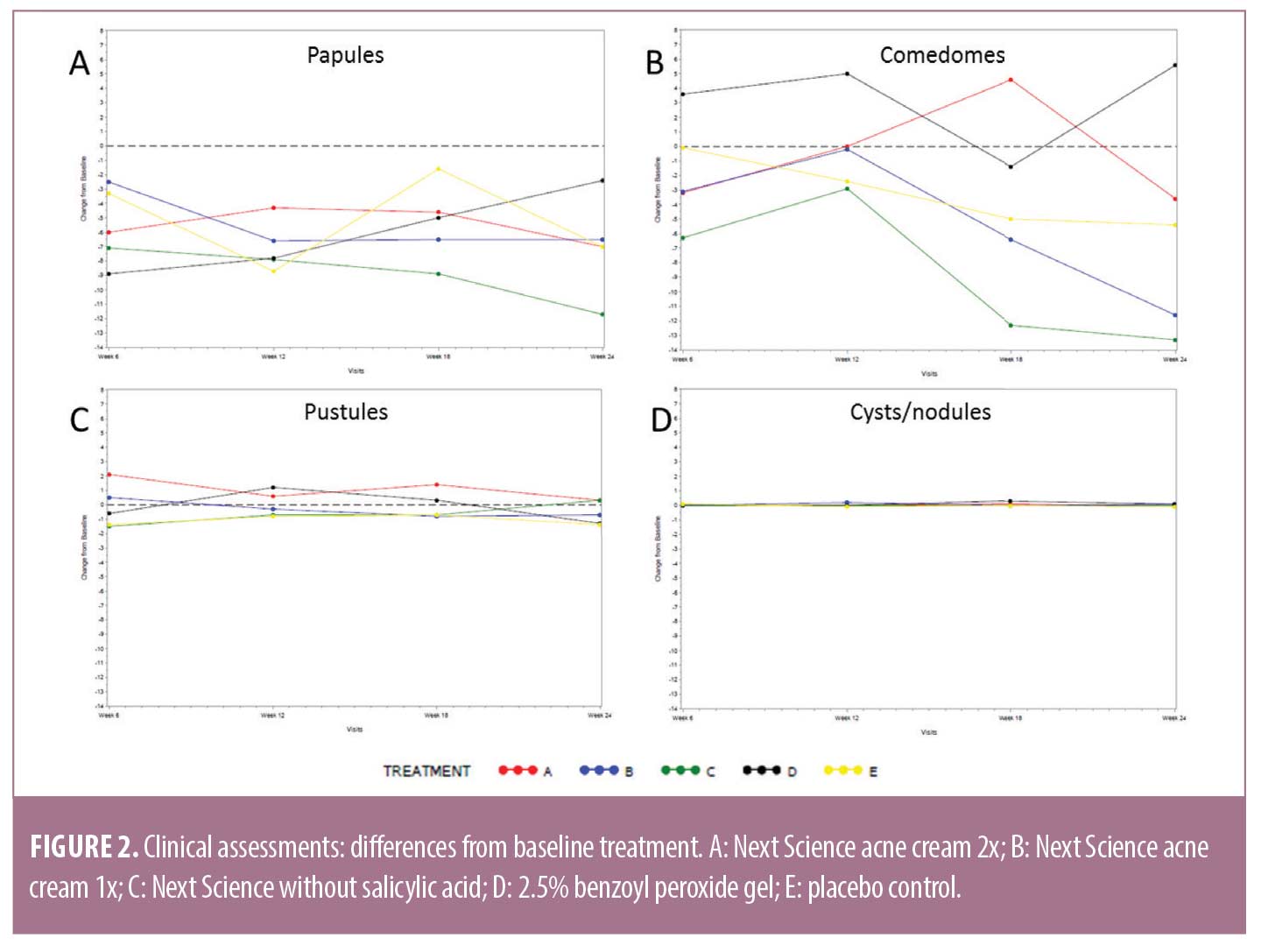
Across all timepoints and treatments, the number of facial papules were clinically reduced, compared to baseline. However, there were no significant differences between treatment groups, as calculated by ANCOVA (Figure 2A). Total lesion counts for comedomes, pustules, and cysts/nodules exhibited variability, with reductions observed inconsistently at some timepoints and with some treatments. However, no significant differences were calculated between treatment groups, as calculated by ANCOVA (Figures 2B–2D).
Total acne lesions are presented in Table 3. After 24 weeks of treatment, subjects using Next Science acne cream 2x showed a significant reduction in the number of facial papules (p=0.0039). Subjects using Next Science acne cream 1x and Next Science acne cream without salicylic acid showed a significant reduction in the number of facial papules (p=0.0020 and p=0.0156, respectively) and comedomes (p=0.0195 and p=0.0313, respectively) after 24 weeks of treatment. There were no significant differences between baseline and Week 24 in any facial lesion type for subjects treated with 2.5% BPO gel. Subjects treated with placebo control showed a significant reduction in the number of facial papules (p=0.0078) after 24 weeks of treatment. There were no statistically significant differences in papules, pustules, comedones, or nodules between treatment groups at Week 24, as calculated by ANCOVA (Table 3).
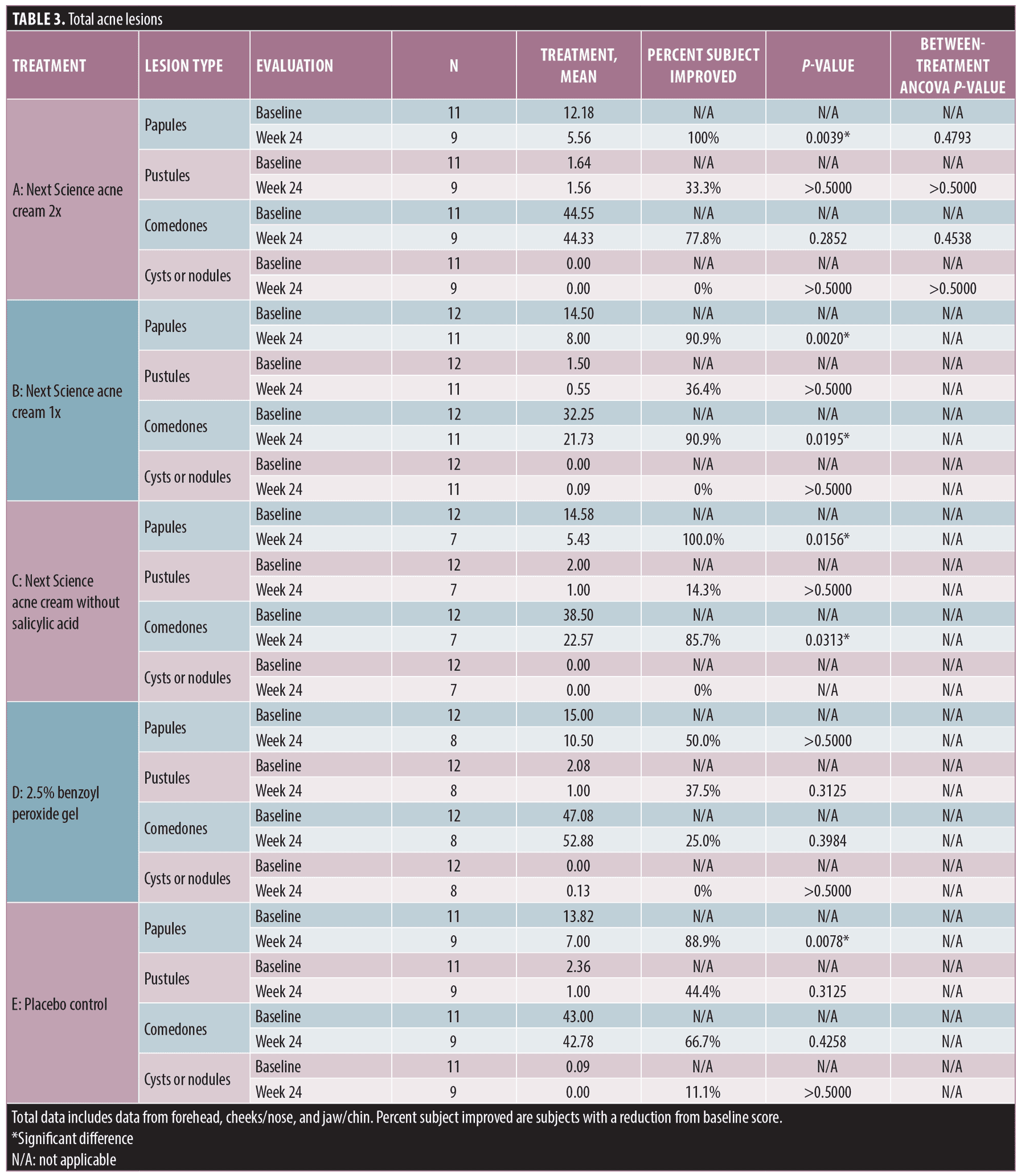
Dermatological assessments. The entire treatment area was evaluated for erythema, dryness, erosion, and edema. Clinical findings were assessed based on the subject’s presentation during study visits and the subject’s report of these signs and symptoms for the period between study visits (Figure 3).
At the Week 18 assessment, there was a significant between-treatment difference for erythema. Subjects treated with 2.5% BPO gel had significantly more erythema, compared to Next Science acne cream 2x, Next Science acne cream 1x, Next Science acne cream without salicylic acid, and placebo control (p=0.0074).
At the Week 24 assessment, there was a significant between-treatment difference for dryness. Subjects treated with 2.5% BPO gel and Next Science acne cream 1x had significantly more dryness, compared to Next Science acne cream 2x, Next Science acne cream without salicylic acid, and placebo control (p=0.0086).
Subjective assessments. At each study visit, subjects were asked by clinical staff members if they experienced any reactions (erythema, dryness, erosion, or edema) while using the study products and, if so, to rate the intensity. While the majority of subjects reported slight reductions in erythema, there were no statistically significant differences observed for erythema, dryness, erosion, or edema at any time across any study treatment (Figure 4).
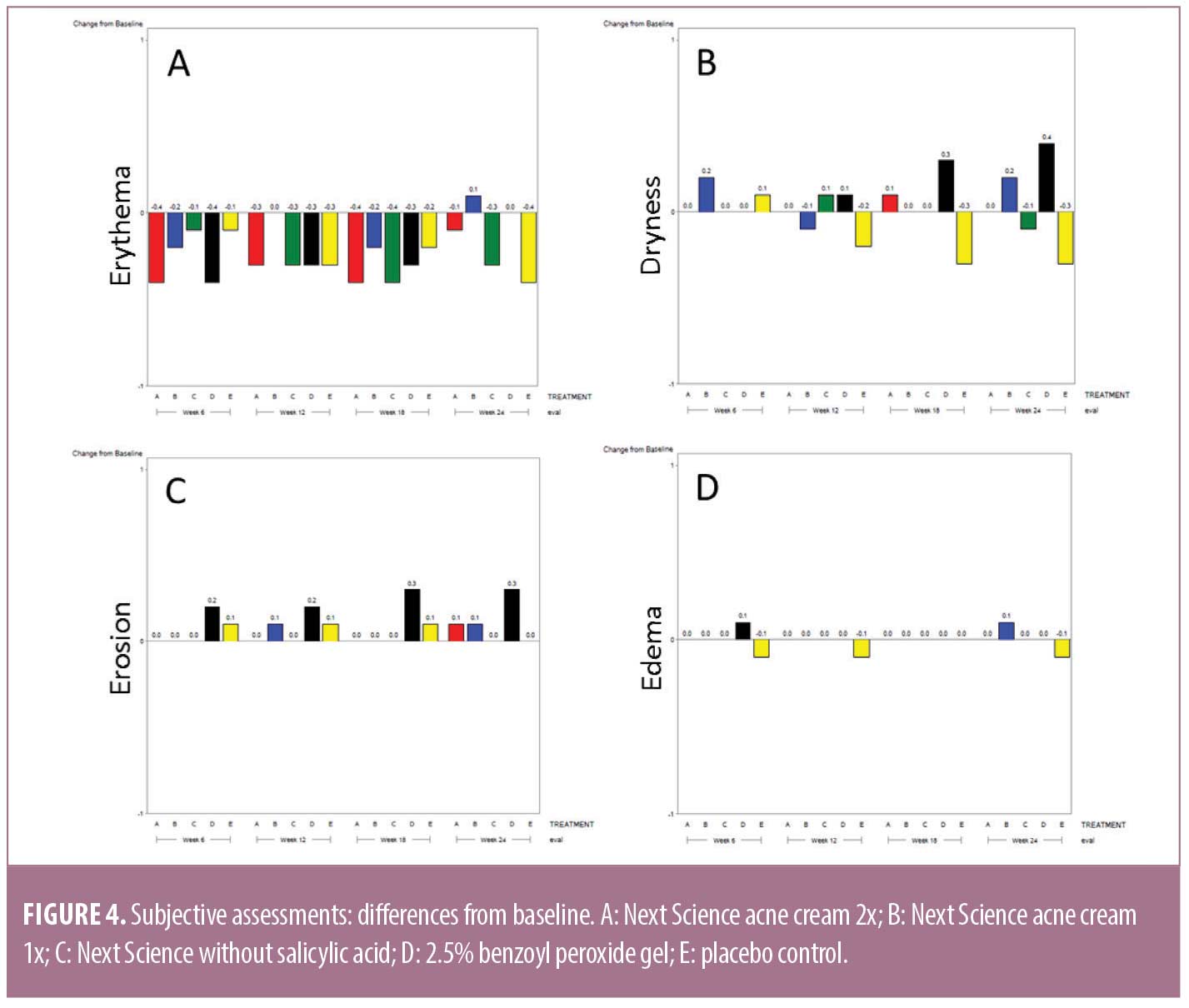
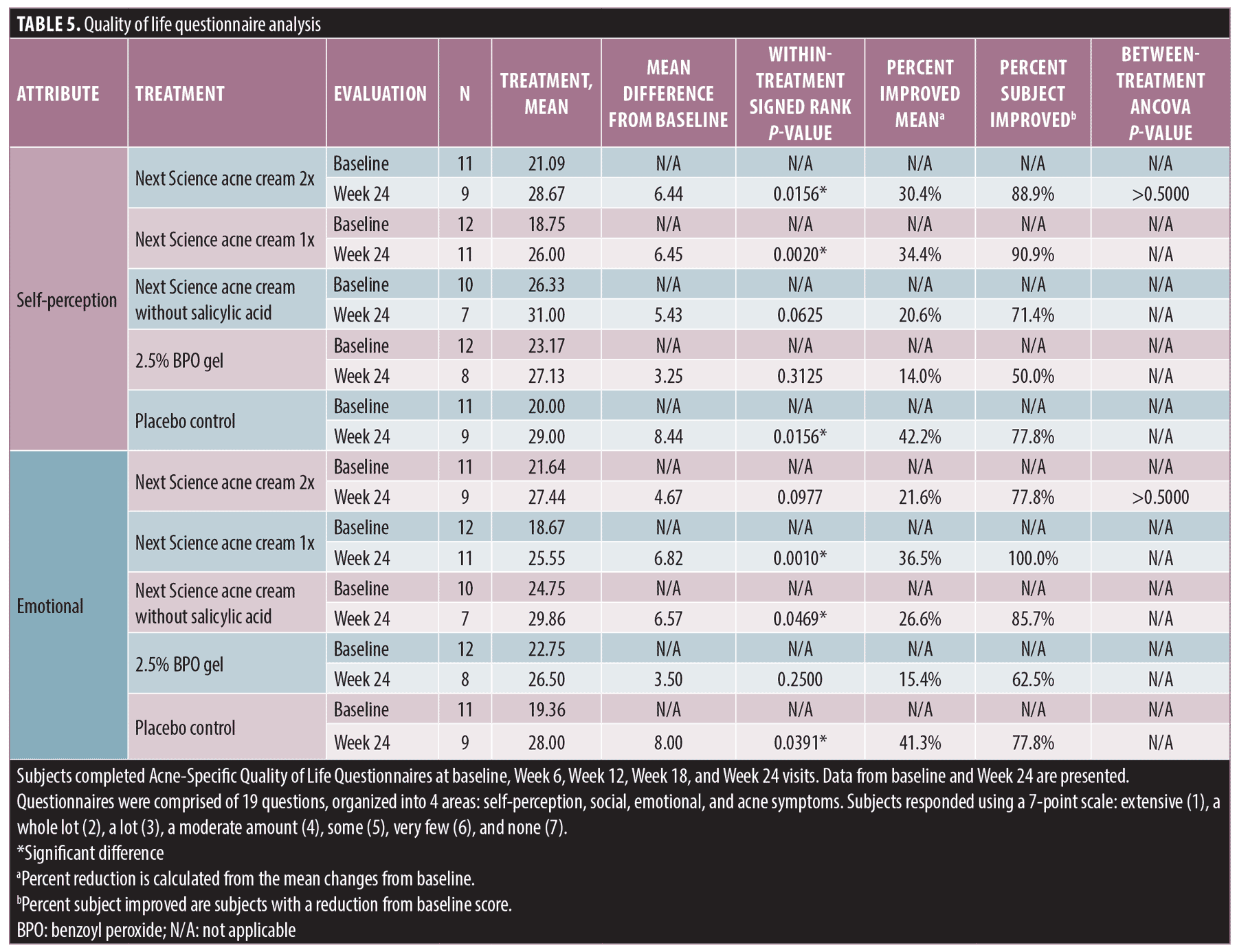
Subject-reported Acne-Specific Quality of Life Questionnaire. At baseline and each subsequent visit, subjects completed the Acne-QoL (Table 4).
Self-perception. Significant improvements in QoL attribute–self-perception were observed between baseline and Week 24 for the following treatments: Next Science acne cream 2x (p=0.0156), Next Science acne cream 1x (p=0.0020), and placebo control (p=0.0156). No significant differences between baseline and Week 24 were observed for subjects treated with Next Science acne cream without salicylic acid (p=0.0625) or 2.5% BPO gel (p=0.3125). The maximum percent of subject improvement was observed in subjects treated with Next Science acne cream 1x; 90 percent of subjects showed improvement in QoL attribute–self-perception. There was no between-treatment difference, as measured by ANCOVA (p>0.5).
Emotional. Significant improvements in QoL attribute–emotional were observed between baseline and Week 24 for the following treatments: Next Science acne cream 1x (p=0.0010), Next Science acne cream without salicylic acid (p=0.0469), and placebo control (p=0.0391). No significant differences between baseline and Week 24 were observed for subjects treated with Next Science acne cream 2x (p=0.0977) or 2.5% BPO gel (p=0.2500). The maximum percent of subject improvement was observed in subjects treated with Next Science acne cream 1x; 100 percent of subjects showed improvement in QoL attribute–emotional. There was no between-treatment difference, as measured by ANCOVA (p>0.5).
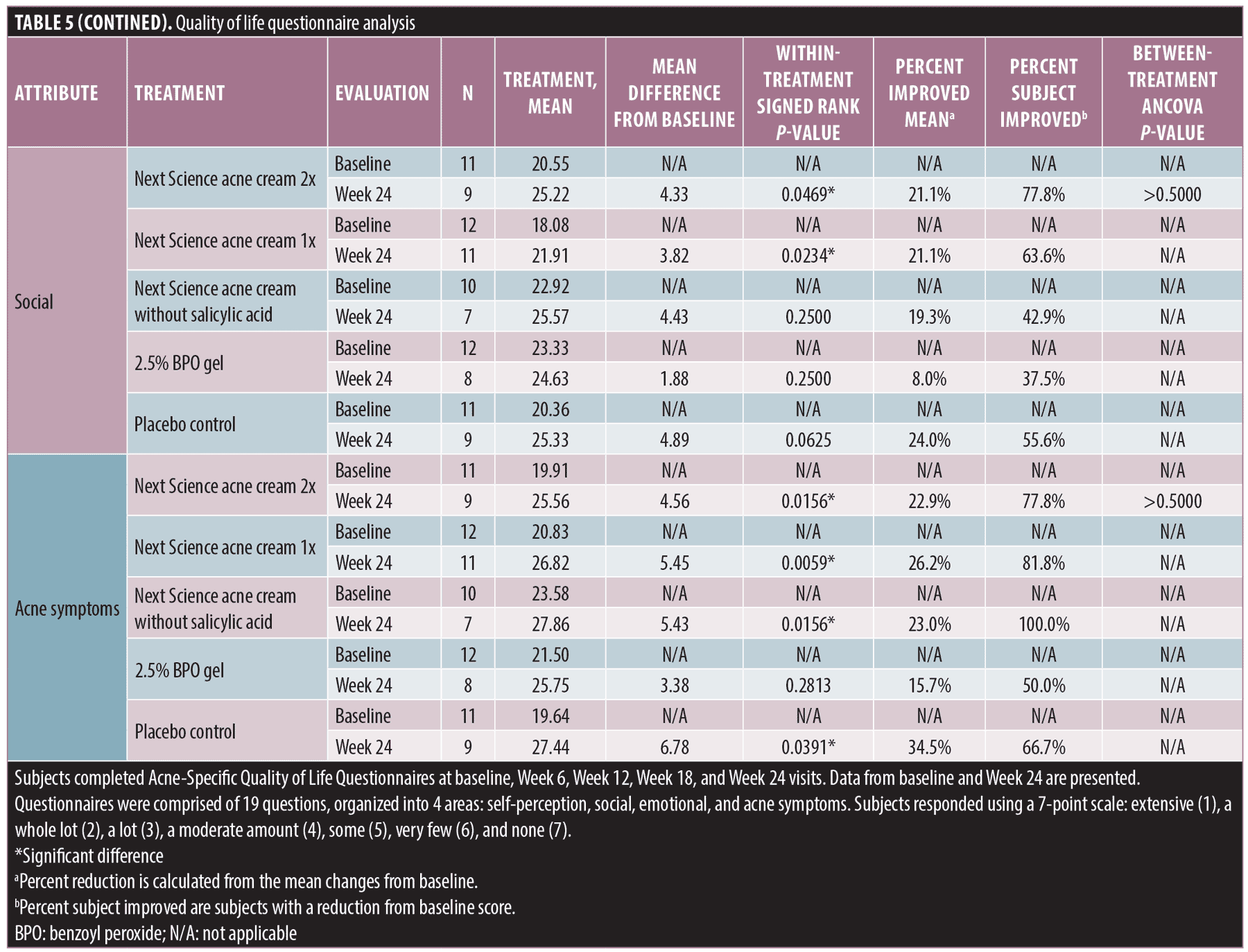
Social. Significant improvements in QoL attribute–social were observed between baseline and Week 24 for the following treatments: Next Science acne cream 2x (p=0.0469) and Next Science acne cream 1x (p=0.0234). No significant differences between baseline and Week 24 were observed for subjects treated with Next Science acne cream without salicylic acid (p=0.2500), 2.5% BPO gel (p=0.2500), or placebo control (p=0.0625). The maximum percent of subject improvement was observed in subjects treated with Next Science acne cream 2x; 78.8 percent of subjects showed improvement in QoL attribute–social. There was no between-treatment difference, as measured by ANCOVA (p>0.5).
Acne symptoms. Significant improvements in QoL attribute–acne symptoms were observed between baseline and Week 24 for the following treatments: Next Science acne cream 2x (p=0.0156), Next Science acne cream 1x (p=0.0059), Next Science acne cream without salicylic acid (p=0.0156), and placebo control (p=0.0391). No significant differences between baseline and Week 24 were observed for subjects treated with 2.5% BPO gel (p=0.2813). The maximum percent of subject improvement was observed in subjects treated with Next Science acne cream without salicylic acid; 100 percent of subjects showed improvement in QoL attribute–acne symptoms. There was no between-treatment difference, as measured by ANCOVA (p>0.5).
Discussion
C. acnes plays a complex role in the pathogenesis of acne, interacting with critical pathways, and leads to increased seborrhea, hyperkeratinization of the pilosebaceous unit, and inflammation. C. acnes biofilms have been reported more frequently in patients with acne. These biofilms have been hypothesized to act as a potential instigator of the inflammatory signaling cascade. Traditionally, acne treatments involve a combination of topical or systemic antibiotics with topical keratolytics and retinoids.9 These agents aim to reduce bacteria and downregulate the inflammatory process; limitations include patient compliance due to side effects and tolerability.1,3,8,9
BPO is a topical therapy indicated for mild-to-moderate AV at doses ranging from 2.5 to 10 percent in the form of a topical gel, wash, or cream. BPO typically demonstrates clinically visible improvements within three weeks and reaches maximum efficacy within 8 to 12 weeks of product use.1,14,15 BPO is classified as a nonantibiotic antibacterial agent that is bactericidal against C. acnes due to oxidization.15 It is often prescribed in combination with antibiotics, such as clindamycin and erythromycin. While effective alone or in combination with antibiotics, BPO has common adverse effects, including skin oxidation, hypersensitivity reactions, contact sensitization reactions, excessive erythema, burning, and peeling.3
Next Science created three formulations of a topical agent using BZK to disrupt C. acnes biofilm and provide therapeutic effectiveness for acne treatment. BZK is a biocide that exhibits good antibacterial activity against many pathogens and has been widely used as a disinfectant in the food industry.16,17 Quaternary ammonium compounds (QAC), such as BZK, bind to exposed anionic sites on the cell membranes, decreasing membrane fluidity and affecting the osmoregulatory and physiological functions of the cell membrane.18
The results from this efficacy and tolerability study show that the three formulations of Next Science acne cream have similar efficacy profiles, compared to 2.5% BPO gel, with regard to IGA. All three formulations performed better than 2.5% BPO gel at reducing facial papules after 24 weeks of treatment. There were trends toward lower levels of erythema, dryness, and erosion at the Week 24 assessments for Next Science gel formulations, compared to 2.5% BPO gel, indicating less overall irritation. Lastly, subjects treated with any of the Next Science cream preparations reported larger improvements to the QoL attributes of self-perception, emotional, social, and acne symptoms than subjects treated with 2.5% BPO gel.
Limitations. While this study suggests that Next Science gel formulations are comparable to 2.5% BPO gel in terms of improvement to IGA score and lesion counts, there are several limitations. First, the small sample size limited our ability to discern differences between treatments and timepoints. Second, the study was run at multiple clinical sites. The study attempted to reduce interevaluator differences in grading by requiring the same investigator or subinvestigator to complete assessments, whenever possible. However, due to the nature of the assessments, there was a potential for subjective differences in IGA, dermatological assessments, subjective assessments, and clinical assessments.
Our results show improvement of acne symptoms across all treatment groups, including the placebo control. A number of factors may have contributed to this, including enrollment requirements, subconscious evaluator bias, and sample size. During enrollment, subjects were required to have mild-to-moderate acne, defined as having 10 to 100 noninflammatory lesions, 10 to 50 inflammatory lesions, and an IGA score of 2 to 3. It is possible that a subject might have enrolled in the study during an acne flare, and their skin symptoms naturally improved, regardless of treatment, over the course of the study. Subjects with mild symptoms might have had borderline lesions counted during enrollment, when the same might not have been the case in subsequent visits. Furthermore, the evaluators were blind to treatment assignment; however, they were aware that subjects were receiving acne treatment for six, 12, 18, or 24 weeks. A subconscious bias may have been present in which the evaluator anticipated subjects’ skin to improve. In future studies, this could be evaluated by photographing the subjects’ faces and assessing the photographs for acne lesions and skin irritation; evaluators would be blinded to both treatment group and timepoint. Lastly, future studies may benefit from a larger sample size. The current study enrolled 11 to 12 subjects per treatment group. This sample size was calculated by estimating the number of subjects required for 95-percent power to detect a statistically significant difference (p=0.05) between the inflammatory lesion count at the start of treatment versus the lesion count after 24 weeks of treatment, with a standard deviation of 23 percent and assuming a mean improvement of at least 38 percent over the treatment period. A larger sample size would allow for the identification of smaller, but statistically significant, differences between treatment groups.
Conclusion
Overall, Next Science acne cream 2x, Next Science acne cream 1x, and Next Science acne cream without salicylic acid demonstrated a positive therapeutic response with limited irritation, compared to a 2.5% BPO gel treatment. Both the Next Science cream without salicylic acid and placebo control displayed mild improvements to acne symptoms over the course of the 24-week study.
Next Science acne cream 2x and Next Science acne cream 1x provided equivalent efficacy to 2.5% BPO gel with less of the adverse effects commonly associated with BPO, such as erythema and dryness. Both the Next Science cream without salicylic acid and placebo control displayed mild improvements to acne symptoms over the course of the 24-week study.
Acknowledgements
The authors thank the study sites TKL, KGL, and Dermatology Consulting Services, PLLC, and the volunteer subjects for their participation in this study.
References
- Zaenglein AI, Pathy AI, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945–973.e33.
- Kircik LH. The role of benzoyl peroxide in the new treatment paradigm for acne. J Drugs Dermatol. 2013;12(6):s73–s76.
- Ogé LK, Broussard A, Marshall MD. Acne vulgaris: diagnosis and treatment. Am Fam Physician. 2019;100(8):475–484.
- Muhammad MH, Idris AL, Fan X, et al. Beyond risk: bacterial biofilms and their regulating approaches. Front Microbiol. 2020;11(928):1–20.
- Tremblay YD, Lévesque C, Segers RP, Jacques M. Method to grow Actinobacillus pleuropneumoniae biofilm on a biotic surface. BMC Veterinary Research. 2013;9(213):1–7.
- Burkhart CG, Burkhart CN. Expanding the microcomedone theory and acne therapeutics: Propionibacterium acnes biofilm produces biological glue that holds corneocytes together to form plug. J Am Acad Dermatol. 2007;57(4):722–724.
- Platsidaki E, Dessinioti C. Recent advances in understanding Propionibacterium acnes (Cutibacterium acnes) in acne. F1000Res. 2018;7(F1000 Faculty Rev):1953:1–12
- Snyder S, Crandell I, Davis SA, Feldman SR. Medical adherence to acne therapy: a systemic review. Am J Clin Dermtol. 2014;15(2):87–94.
- Bernhardt MJ, Myntti MF. Topical treatment with an agent disruptive to P. acnes biofilm provides therapeutic response: results of a randomized clinical trial. J Drugs Dermatol. 2016;15(6):677–682.
- Kawashima M, Nahare T, Doi M. Clinical efficacy and safety of benzoyl peroxide for acne vulgaris: comparison between Japanese and Western patients. J Dermtol. 2017;44(11):1212–1218.
- US Department of Health and Human Services. Food and Drug Administration. Center for Disease Evaluation and Research. Acne vulgaris: establishing effectiveness of drugs intended for treatment. May 2018. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/acne-vulgaris-establishing-effectiveness-drugs-intended-treatment. Accessed 3 Mar 2023.
- Fehnel SE, McLeod LD, Brandman J, et al. Responsiveness of the Acne-Specific Quality of Life Questionnaire (Acne-QoL) to treatment for acne vulgaris in placebo-controlled clinical trials. Qual Life Res. 2002;11(8):809–816.
- Aschoff R, Möller S, Haase R, Kuske M. Tolerability and efficacy of clindamycin/tretinoin versus adapalene/benzoyl peroxide in the treatment of acne vulgaris. J Drugs Dermatol. 2021;20(3):277-283.
- Kircik LH. Advances in the understanding of the pathogenesis of inflammatory acne. J Drugs Dermatol. 2016;15(1):s7–s10.
- Hoffman LK, Bhatia N, Zeichner J. Topical vehicle formulations in the treatment of acne. J Drugs Dermatol. 2018;17(6):s6–s9.
- Jiang X, Jiang C, Yu T, et al. Benzalkonium chloride adaptation increases expression of the Agr system, biofilm formation, and virulence in Listeria monocytogenes. Front Microbiol. 2022;13:1–12.
- Pagedar A, Singh J. Evaluation of antibiofilm effect of benzalkonium chloride, iodophore and sodium hypochlorite against biofilm of Pseudomonas aeruginosa of dairy origin. J Food Sci Technol. 2015;52(8):5317–5322.
- Houari A, Di Martino P. Effect of chlorhexidine and benzalkonium chloride on bacterial biofilm formation. Letters in Appl Microbiol. 2007;45(6):652–656.

