J Clin Aesthet Dermatol. 2025;18(9):58–64.
by Dharm Sodha, MD; Atithi Patel, BS; and Peter Lio, MD
Dr. Sodha and Ms. Patel are with the University of Illinois College of Medicine in Chicago, Illinois. Dr. Lio is with Medical Dermatology Associates of Chicago and the Feinberg School of Medicine at Northwestern University in Chicago, Illinois.
FUNDING: No funding was provided for this article.
DISCLOSURES: Dr. Lio reports being on the speaker’s bureau for AbbVie, Arcutis, Eli Lilly, Galderma, Hyphens Pharma, Incyte, La Roche-Posay/L’Oreal, Pfizer, Pierre-Fabre Dermatologie, Regeneron/Sanofi Genzyme, Verrica; reports consulting/advisory boards for Alphyn Biologics (stock options), AbbVie, Almirall, Amyris, Arcutis, ASLAN, Bristol-Myers Squibb, Burt’s Bees, Castle Biosciences, Codex Labs (stock options), Concerto Biosci (stock options), Dermavant, Eli Lilly, Galderma, Janssen, LEO Pharma, Lipidor, L’Oreal, Merck, Micreos, MyOR Diagnostics, Regeneron/Sanofi Genzyme, Sibel Health, Skinfix, Suneco Technologies (stock options), Theraplex, UCB, Unilever, Verdant Scientific (stock options), Verrica, Yobee Care (stock options). In addition, Dr. Lio has a patent pending for a Theraplex product with royalties paid and is a Board member and Scientific Advisory Committee Member emeritus of the National Eczema Association. Dr. Lio was also part of the Joint Task Force guideline committee and is an author on the JTF guidelines document. The remaining authors have no conflicts of interest to disclose.
Abstract: Introduction: In 2023, the American Academy of Allergy, Asthma, and Immunology (AAAAI)/American College of Allergy, Asthma, and Immunology Joint Task Force (JTF) updated their guidelines for management of atopic dermatitis from their last update in 2012. The American Academy of Dermatology (AAD) also updated their own guidelines from 2014 for the diagnosis, assessment, safety, and efficacy of treatments in 2024. In this review, we outline the key changes from the prior guidelines and highlight the major differences between the two recommendations. Results: The majority of the guidelines and recommendations are similar in their overall recommendations with small stylistic differences including application frequency and combination of therapies. Key differences between the two recommendations include topical PDE-4 Inhibitors, topical JAK Inhibitors, systemic JAK inhibitors, azathioprine, methotrexate, and mycophenolate. The final difference between the AAD and JTF guidelines is that AAD categorizes evidence and comments on the certainty of association for each group of comorbidities, while JTF makes general statements regarding comorbidities. Keywords: atopic dermatitis, treatment guidelines, topical corticosteroids, bleach baths, systemic
Introduction
Atopic dermatitis (AD) is a chronic, pruritic inflammatory skin condition characterized by recurrent eczematous lesions and intense itching. The condition affects up to 20 percent of children and 10 percent of adults with growing prevalence.1–3 It is commonly treated by dermatologists, allergists, pediatricians, and primary care providers. Despite advancements in systemic therapy for AD, topical approaches remain the mainstay of treatment due to their favorable safety profile and proven efficacy.
The management of AD often addresses a broad spectrum of issues, from managing acute exacerbations to modifying environmental exposures. Different groups of clinicians will usually approach AD in their unique ways, as Saavedra et al4 demonstrated that allergists were more likely to prescribe elimination diets and dietary change alone compared to dermatologists. The American Academy of Dermatology (AAD) previously released guidelines for the management of AD in 2014. Since then, a plethora of new evidence and treatments has emerged, and the AAD has provided new guidelines as recently as February 2024. In addition, the Asthma and Immunology Joint Task Force on Practice Parameters (JTF) has also updated its guidelines on AD, which were last released in 2012. These updated guidelines will assist physicians in managing AD and utilizing newly developed approaches. In this review, we compare the guidelines provided by these two organizations and highlight key differences along with significant updates since the last guidelines. These differences and changes will elucidate the unique approaches in management between allergists and dermatologists that may highlight biases and the subtypes of patients with AD.
Methods
The following organizations were reviewed for their recently submitted guidelines:
- American Academy of Dermatology5–7
- Joint Task Force on Practice Parameters (JTF), representing the American Academy of Allergy, Asthma, and Immunology (AAAAI); the American College of Allergy, Asthma & Immunology; and the Joint Council of Allergy, Asthma, and Immunology8
The guidelines were reviewed, noting their similarities, differences, and updates since the last updates in 2014 for the AAD9–12 and 2012 for the JTF.13–15 Additionally, both organizations utilized GRADE (Grading of Recommendations, Assessment, Development, and Evaluations) to systematically review the guidelines and management. Each organization had distinct approaches to developing their respective guidelines.
The AAD partnered with the Southern California Evidence Review Center (SCERC) at the University of Southern California to conduct components of the systematic review process, including literature searches, study selection, risk of bias assessment, data extraction, and analysis. The Southern California Evidence Review Center searched the literature for all Patient/Problem, Intervention, Comparison, and Outcome (PICO) questions using MEDLINE (via PubMed), EMBASE, and clinicaltrials.gov to identify reports of randomized controlled trials (RCTs). In addition, MEDLINE, the Cochrane Database of Systematic Reviews, and PROSPERO were queried to identify systematic reviews for reference mining. They specifically restricted their search to publications from November 1, 2012, through May 21, 2020 to identify RCTs published since completion of the search that informed the topical therapy recommendations in the AAD’s 2014 guidelines of care for the management of AD. Their searches identified 2161 citations.
The JTF guidelines differ from their previous guidelines as the 2023 guidelines focused on five main questions addressing therapy. Multiple new therapies have emerged in the last ten years, including biologics, small molecules, and a topical PDE4 inhibitor. To create recommendations, the panel relied on evidence synthesized in systematic reviews and network meta-analyses (NMA) led by the Evidence in Allergy Group. These included the following:
- Systematic review and meta-analysis of bleach baths versus usual baths for atopic dermatitis16
- Systematic review and meta-analysis of dietary elimination versus usual diet for atopic dermatitis17
- Systematic review and meta-analysis of allergen immunotherapy (AIT) versus no AIT for atopic dermatitis18
- Systematic review and meta-analysis of cancer risk with topical topical calcineurin inhibitors (TCIs), pimecrolimus and tacrolimus, for atopic dermatitis19
- Systematic review and NMA of topical treatments for atopic dermatitis—referred to here as the topicals NMA20
- Systematic review and NMA of systemic treatments (monoclonal antibodies, small molecules [e.g. JAK inhibitors, cyclosporine, methotrexate], ultraviolet [UV] light therapy [phototherapy]) for atopic dermatitis—referred to here as the systemics NMA21
- Systematic review of values and preferences of patients and caregivers regarding treatment of atopic dermatitis22
Results
While both groups differed in their selection of studies and criteria for inclusion, both achieved similar conclusions and efficacy scales for each recommendation. (Table 1) They both utilized high-quality clinical studies or reviews that included high-quality studies in their analysis. However, there were some notable areas of disagreement.
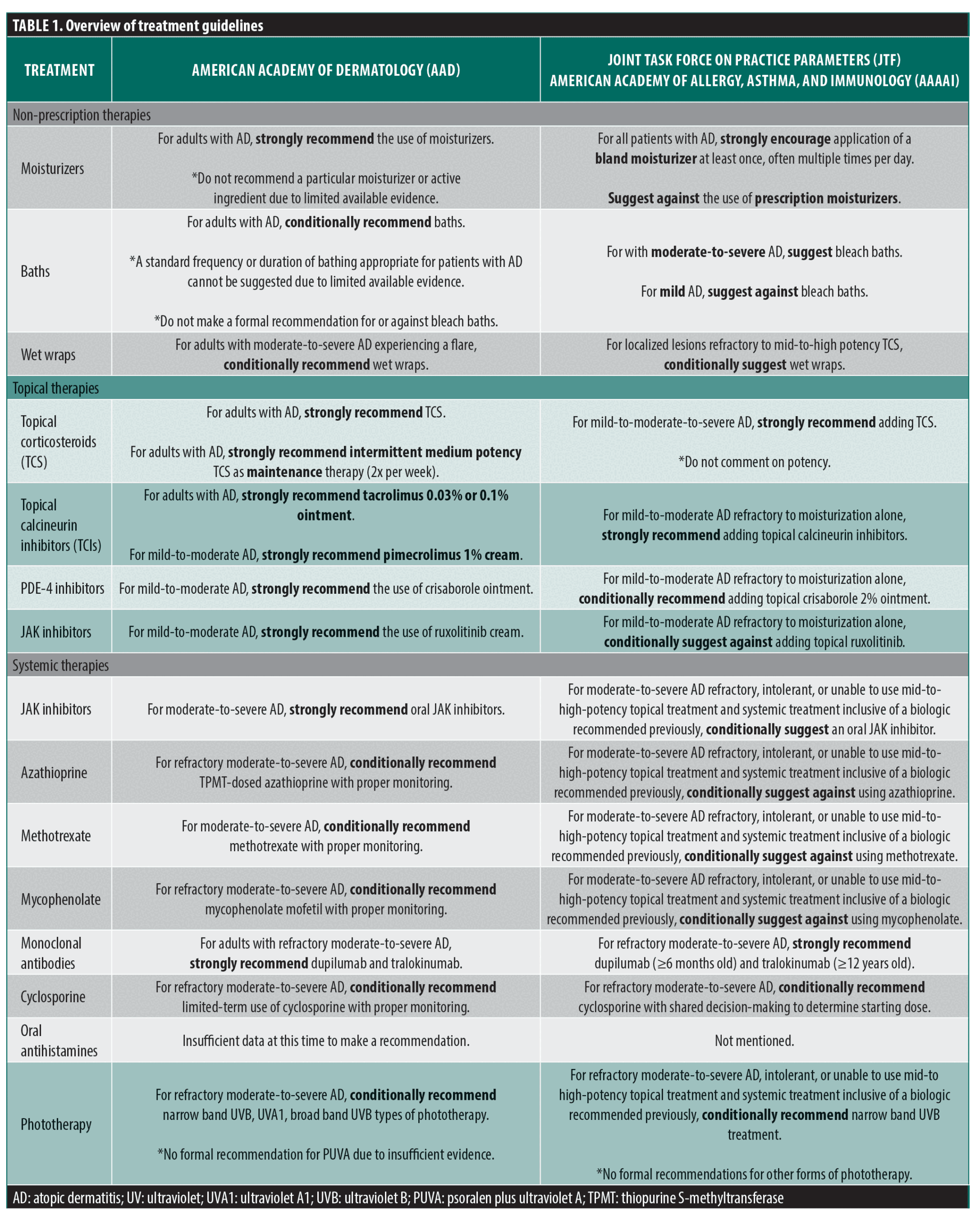
Topical therapy. Moisturizers and non-prescription therapies. Both guidelines recommend topical therapies as first-line treatment for milder cases of AD. The AAD recommends moisturizers as the first line in reducing symptoms and inflammation, often in conjunction with other therapies. Their analysis of five moisturizer studies showed a slight reduction in AD severity.6,23–26 The AAD makes note that due to the various types of moisturizers—including emollients, occlusive agents, and humectants—that are commercially available, studies examining moisturizer use in AD vary by type of moisturizer, study design, and outcomes. They concluded that no single moisturizer or ingredient can be recommended due to the limited evidence.6 The JTF guidelines also emphasize moisturizers as critical for AD care but discourage prescription moisturizers,20 citing similar reasoning for the close balance between over-the-counter moisturizers and possible economic burdens on patients.8 Neither study takes into account the placebo effect in their analysis. Both studies recommend moisturizers for patients with AD with a “strong” level of evidence.
Another non-prescription therapy that both guidelines mention is bathing. While the AAD guidelines mention bathing in general and with additives such as magnesium chloride, the JTF guidelines mainly only discuss dilute bleach baths. The level of evidence of bleach baths is minimal in adults especially. The AAD concludes that given the low certainty of the evidence, bathing can be conditionally recommended. However: “a standard for the frequency or duration of bathing, the temperature of the water, type of soap, and use of water softeners, and other bathing accessories, including bleach, for those with noninfected AD cannot be suggested based on the limited available evidence.”6 They specifically mention the use of bleach baths only with clinical signs of a secondary bacterial infection. However, the evidence certainty was graded as “very low.”6 The JTF guidelines, in contrast, suggest bleach baths as conditionally having more benefit than baths without diluted bleach. Their recommendations suggest a similar finding: “In patients with moderate-severe atopic dermatitis, the JTF panel suggests, in addition to topical therapy, dilute bleach baths over usual baths (conditional recommendation, low-certainty evidence).”8 This is in contrast with the JTF’s previous 2012 guidelines, which strongly recommended dilute bleach baths twice weekly.13 The JTF panel was, overall, more supportive of bleach baths and concluded that patients would find dilute bleach baths worthwhile given the minimal downsides. However, they similarly only ranked this recommendation as conditional, given the level of evidence in adults. Of note, the JTF recommends against bleach baths in patients with mild atopic dermatitis, reserving their recommendation only for moderate-to-severe cases.8
The use of wet wrap therapy in conjunction with topical corticosteroids, topical calcineurin inhibitors, or other treatments is mentioned in both documents. The AAD gives a conditional recommendation due to the scarcity of data and variability in both topical agent and wrap material to recommend it for a flare of moderate-to-severe AD. The JTF similarly gives wet wrap therapy a conditional recommendation with a very low-certainty evidence rating.27–31
Topical corticosteroids. Previous guidelines from JTF and AAD both emphasized the importance of addressing patients’ fears associated with topical corticosteroid use. However, over the past decade, topical corticosteroids have become a mainstay for therapy in AD, specifically in patients who have AD refractory to moisturizer alone. These are now widely known and accepted AD therapy.
Overall, both groups strongly recommend the use of topical corticosteroids for the management of acute flares and maintenance therapy in AD.6,8 The AAD and JTF guidelines differentiate topical steroids into seven classes of potency and describe how some dermatologists prefer high and very high potency steroids (at least initially) to control active disease, others use the lowest potency agent needed for the situation and increase potency if needed.6 Both documents reference the adverse effects of higher potency topical corticosteroids and the caution advised when treating thinner and intertriginous skin, older patient age, and long-term continuous use. The AAD guideline then dives into comparisons between each corticosteroid potency and the data behind them.6 Additionally, both mention frequency of application, but only the JTF guidelines make a formal recommendation to suggest once a day over twice a day for mid-to-high-potency topical medications, supported by a meta-analyses of nine randomized controlled trials which found little to no difference in outcomes, including AD severity, flares, and adverse effects.20
A slight difference between the two guidelines is the discussion of combination therapy with calcineurin inhibitors. The AAD and JTF outline the main evidence in support of and against combination therapy, demonstrating the mixed evidence for its support. The JTF individually recommends topical corticosteroids and topical calcineurin inhibitors, and they state there is low certainty for modest added benefits compared to using either agent alone.20 The AAD mentions studies on both sides of the debate but does not make a formal recommendation on the use of combination therapy.6,32
Although neither group recommends using systemic corticosteroids, this, colloquially, may still be common practice amongst community allergists and dermatologists. While these guidelines provide important evidence-based recommendations, raising awareness is key to ensuring they are implemented.
Topical calcineurin inhibitors. Both guidelines recommend topical calcineurin inhibitors, specifically tacrolimus 0.03% or 0.1% and pimecrolimus 1%, with a strong rating and a high level of certainty.6,8 Both acknowledge the boxed warning on TCIs and the low clinical concern for lymphoma regarding these labels. Of note, the AAD guidelines briefly mention comparative studies between topical calcineurin inhibitors and topical corticosteroids and state that high and very high potency steroids are more effective than pimecrolimus 1% cream.33 However, based on this limited data, they have yet to recommend it formally.
Topical antimicrobials/antiseptics and antihistamines. The JTF and AAD guidelines both strongly recommend against the usage of topical antimicrobials in the treatment of uninfected AD lesions. This recommendation was conditional based on the current evidence, given that the patient has no active bacterial skin infection.6 The JTF guidelines identified some special considerations, including immunocompromised or immunosuppressed individuals who may benefit from adding antimicrobials.8 The AAD guidelines specifically reference the addition of topical sodium hypochlorite, which may be suggested to reduce disease severity. Notably, the JTF guidelines point out that while the initial hypothesis for the mechanism of action of dilute bleach baths in AD was the presumed antibacterial activity via sodium hypochlorite, subsequent investigations have revealed that they are not actually antimicrobial at the concentrations used clinically.
The AAD also mentions a study that utilized topical doxepin, a topical antihistamine, for the treatment of AD and ultimately recommends against the usage of topical antihistamines as well.6
It should be noted that the AAD determined there was insufficient data to make a formal recommendation for or against oral antihistamines, while the JTF makes no mention of this cornerstone therapy used for many years to provide sedation and pruritus relief for patients with AD.8,11,34
Topical PDE4 inhibitors. PDE4 inhibitors are a new therapy added to the market since the previous guidelines from the JTF or AAD. Regarding the PDE4 inhibitor, 2% crisaborole ointment, the AAD strongly recommends it for treating mild-to-moderate AD with a high level of evidence.6 The JTF, in comparison, also recommends crisaborole. However, they conditionally recommend it instead of strongly recommending it. Additionally, they specify that the benefits are more likely to be seen in patients with milder AD.8
Topical JAK inhibitors. Topical JAK inhibitors are a new therapy added to the market since the previous guidelines from JTF or AAD. An area of disagreement between the two guidelines is the usage of topical JAK Inhibitors, specifically ruxolitinib cream. The AAD cites moderate certainty of evidence and strongly recommends the usage of topical JAK inhibitors.35–37 In contrast, the JTF suggests against topical ruxolitinib over continued usual care alone. They cite the safety profile and uncertain small increase in potential harm (including an increase in death, cancer, thrombosis, and serious infectious).8 This is one of the first major points of divergence between the two guidelines. Specifically, the JTF authors note: “The panel inferred that most patients with mild-moderate AD would prefer to avoid the uncertain increase in death, cancer, thrombosis, and serious infections, particularly when there are multiple safer treatment options with larger certain benefits and higher certainty for safety.”8
Allergy testing and elimination diets. Previously, the JTF recommended avoidance of many triggering factors, including aeroallergens, and went as far as to recommend humidity and temperature control of the home. Within the new guidelines, neither the AAD nor JTF address patch testing, and only the JTF addresses elimination diets.
Neither the AAD nor JTF addresses patch testing, and only the JTF addresses elimination diets. The JTF suggests against the use of elimination diets, citing that avoidance of food allergens is strongly associated with promoting the development of IgE-mediated food allergy.8 Furthermore, pursuing elimination diets is burdensome to manage and will increase the risk of malnutrition complications, making this practice unfavorable.8 The JTF recognized that the decision to pursue an elimination diet is often informed by patch testing or oral challenge results. However, they caution that these tests have high rates of false positives.8 These are important and common elements in the care and management of AD that the AAD guidelines have failed to acknowledge, which poses a danger that dermatologists may not comprehensively address all factors of care for AD.
Systemic therapy and new biologics. These were all investigative therapies when the last set of guidelines were published. Dupilumab, now the most commonly used biologic, has been widely used for more than five years.
Monoclonal antibodies — dupilumab and tralokinumab. Both AAD and JTF guidelines recommend only using systemic or biologic therapies for moderate-to-severe AD recalcitrant to topical treatments. The current United States Food and Drug Administration (FDA)-approved biologics are dupilumab for six months and older and tralokinumab for 12 years and older. Both guidelines agree that adding dupilumab or tralokinumab is recommended for moderate-to-severe AD. Both also mention the caveat that tralokinumab is somewhat less effective than dupilumab; however, this may not be a clinically appreciable difference. The AAD guidelines warn that no studies directly compare the efficacy of tralokinumab against any other systemic therapies.7 The JTF warns that there has been no evidence thus far that addresses using both of these agents simultaneously, and as such, recommends using either agent independently.8
Conjunctivitis is the most common adverse effect noted for these drugs by both groups. They recommend considering referrals to ophthalmology if the conjunctivitis is severe, refractory to conservative measures such as artificial tears or lubricant drops and if the patient is experiencing severe ocular symptoms such as blurred vision, changes in acuity, purulent discharge, photophobia, or eye pain. The JTF recommends topical tacrolimus or pimecrolimus to reduce eczema around the eyes and subsequently reduce ocular itching or rubbing.8 The AAD guidelines indicate that the evidence for monoclonal antibodies’ adverse events has statistically inconsistent analyses, indicating that further research is needed to determine which adverse events are most prevalent and impactful.7
Compared to the AAD guidelines, the JTF guidelines consider additional practical factors associated with monoclonal antibody treatment, which may indicate that allergists may be more attentive to patient desired outcomes and to minimizing financial harm. They account for patient preferences for convenient and nonvisible treatments and patient willingness to undergo recurrent injections or to self-inject. They acknowledge the additional coordination of insurance paperwork, difficulty obtaining the medications, keeping the drug temperature controlled, and, in case injections need to be administered by a healthcare professional, the extra time and cost of travel and appointments for injections. These are significant inconveniences and costs for patients; however, the potential relief and normalization of daily activities make the process worthwhile for patients.8
JAK Inhibitors — abrocitinib, baricitinib, upadacitinib. Both AAD and JTF guidelines moderately/conditionally recommend oral JAK inhibitors (abrocitinib and upadacitinib) for moderate-to-severe AD that is refractory to other treatments, including biologics, or for which other therapies are inadvisable. The conflict between high efficacy and the need for more conclusive, long-term evidence for potential adverse events makes it difficult to recommend JAK inhibitors firmly. The AAD moderately recommends abrocitinib and upadacitinib because they demonstrated high efficacy with rapid onset of action in Phase III trials, but the statistical evidence regarding associated adverse events was inconsistent.7 JTF guidelines only conditionally recommend JAK inhibitors because, for most patients, the benefits of JAK inhibitors outweigh the risks, but all patients may not share the same values or preferences.8
Neither the AAD nor the JTF guidelines advocate for the use of baricitinib. The AAD mentions that although baricitinib has yet to be studied head-to-head against abrocitinib or upadacitinib, network meta-analyses suggest that baricitinib is less efficacious than the others.7 The current FDA-approved JAK inhibitors are abrocitinib and upadacitinib (both approved for ages 12 and older). Baricitinib is not yet FDA-approved for atopic dermatitis in the United States, though it is available in other markets globally.
Compared to the AAD guidelines, the JTF guidelines consider additional practical factors associated with JAK inhibitor treatment, which may indicate that allergists are more attentive to patient preferences and convenience. The JTF guidelines mention that patients with multiple comorbid autoimmune conditions may prefer JAK inhibitors over AD-specific immunotherapy, as JAK inhibitors may simultaneously treat multiple conditions.8 They acknowledge special circumstances during which clinicians or patients may prefer JAK inhibitor therapy, even if conventional indications are not met. Furthermore, JTF guidelines discuss patient preferences that would sway patients’ preferences toward or against JAK inhibitors. For example, until randomized trials robustly address the uncertainty associated with adverse event outcomes, patients who place a very high value on reducing symptoms and improving current quality of life and a lower value on the uncertain serious harms that some of these agents may cause are likely to choose the most effective interventions (e.g. the included oral JAK inhibitors). Patients may also prefer JAK inhibitors due to their oral route of administration. Contrastingly, patients who find it inconvenient to go through the screening and monitoring associated with JAK inhibitors may be more opposed to JAK inhibitor treatment.8 These guidelines also dive into the access and availability of JAK inhibitors depending on cost and insurance plans, which could further modify patient preferences for JAK inhibitors.8
Comorbidities. A prominent difference between the AAD and JTF guidelines is that the AAD categorizes evidence and comments on the certainty of association for each group of comorbidities, while the JTF makes general statements regarding comorbidities. The JTF’s stated aim for the 2023 guidelines update was to address intervention recommendations, not comorbidities. The AAD is highly certain that AD is associated with food allergies, and simply mentions that AD is associated with several atopic conditions (food allergy, asthma, allergic rhinitis).5,8 Additionally, the AAD states with high certainty that AD is not associated with autism spectrum disorders.5 The JTF highlights that ophthalmic and ocular diseases, some potentially sight-threatening, occur as comorbidities or complications of AD treatment, such as conjunctivitis from monoclonal antibody therapies.8 The AAD does not mention ophthalmic and ocular comorbidities. Both groups agree that AD is associated with osteoporosis, although the mechanism for this is unclear.5,8 There is uncertainty among both groups as to whether metabolic disorders are associated with AD.
Discussion
Despite the different methods both guidelines utilized to determine their recommendations, both groups ultimately have overwhelmingly similar themes for clinicians. Specifically, regarding topical treatments, both guidelines support using moisturizers, topical calcineurin inhibitors, and topical corticosteroids. However, the AAD recommended topical PDE4 inhibitors, while the JTF was only conditionally in favor. JAK inhibitors were an important point of disagreement in topical therapy, with the AAD recommending their usage while the JTF was conditionally against, apparently largely due to patient input.
Systemic therapies also had points of disagreement. While both guidelines recommend dupilumab and tralokinumab, the JTF only conditionally supported abrocitinib, baricitinib, and upadacitinib. The AAD and JTF conditionally recommend UVB phototherapy and cyclosporine but had some small differences in the rest of their systemic recommendations. The AAD conditionally recommends using azathioprine, methotrexate, and mycophenolate, while the JTF is conditionally against these. The final recommendation from both groups was against systemic corticosteroids, with the JTF conditionally recommending against them. The recommendation against these legacy immunosuppressive medications is striking, as their relatively low cost and long history of use have allowed them to remain on most guideline documents, including more recent guidelines from Europe and Asia.38–40 The JTF notes for methotrexate (and with similar language for mycophenolate and azathioprine): “The panel inferred that most well-informed patients would prefer to avoid the modest benefits (with slow onset) and more certain harms and burdens associated with methotrexate use compared with continued standard care, or alternative, more effective options.”8
A glaring oversight amongst these guidelines is the lack of discussion about allergy testing, which should be an important tenant of care for patients with AD who have a propensity for allergies. While the JTF makes some mention of allergy testing for food allergens and the high rates of false positives, the AAD never mentions these components.
We can also see small stylistic recommendations from both organizations, including the frequency of application and ancillary management. These differences occur most often in areas with less supporting evidence. These guidelines also underline the importance of further study, especially in areas of disagreement due to lack of evidence. Ultimately, seeing the difference in perspectives, especially from clinicians from distinct disciplines, and gaining a broader perspective on AD management strategies is extremely valuable. Despite these guidelines being reinforced with evidence, the decision on each treatment should be tailor-made depending on the patient and clinician. Certain circumstances may require deviations from these recommendations. From these guidelines, clinicians can better understand the current literature supporting therapeutic options for each patient and ultimately make personalized choices for the best possible outcomes.
References
- Ramírez-Marín HA, Silverberg JI. Differences between pediatric and adult atopic dermatitis. Pediatr Dermatol. 2022;39(3):345–353.
- Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015;66 Suppl 1:8–16.
- Laughter MR, Maymone MBC, Mashayekhi S, et al. The global burden of atopic dermatitis: lessons from the Global Burden of Disease Study 1990-2017. Br J Dermatol. 2021;184(2):304–309.
- Saavedra JM, Boguniewicz M, Chamlin S, et al. Patterns of clinical management of atopic dermatitis in infants and toddlers: a survey of three physician specialties in the United States. J Pediatr. 2013;163(6):1747–1753.
- Davis DMR, Drucker AM, Alikhan A, et al. American Academy of Dermatology Guidelines: Awareness of comorbidities associated with atopic dermatitis in adults. J Am Acad Dermatol. 2022;86(6):1335-1336.e18.
- Sidbury R, Alikhan A, Bercovitch L, et al. Guidelines of care for the management of atopic dermatitis in adults with topical therapies. J Am Acad Dermatol. 2023;89(1):e1–e20.
- Davis DMR, Drucker AM, Alikhan A, et al. Guidelines of care for the management of atopic dermatitis in adults with phototherapy and systemic therapies. J Am Acad Dermatol. 2024;90(2):e43–e56.
- Chu DK, Schneider L, Asiniwasis RN, et al. Atopic dermatitis (eczema) guidelines: 2023 American Academy of Allergy, Asthma and Immunology/American College of Allergy, Asthma and Immunology Joint Task Force on Practice Parameters GRADE– and Institute of Medicine–based recommendations. Ann Allergy Asthma Immunol. 2024;132(3):274–312.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70(2):338–351.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71(1):116–132.
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71(2):327–349.
- Sidbury R, Tom WL, Bergman JN, et al. Guidelines of care for the management of atopic dermatitis: Section 4. Prevention of disease flares and use of adjunctive therapies and approaches. J Am Acad Dermatol. 2014;71(6):1218–1233.
- Schneider L, Tilles S, Lio P, et al. Atopic dermatitis: a practice parameter update 2012. J Allergy Clin Immunol. 2013;131(2):295-299.e1–e27.
- Lio PA, Lee M, LeBovidge J, Timmons KG, Schneider L. Clinical management of atopic dermatitis: practical highlights and updates from the atopic dermatitis practice parameter 2012. J Allergy Clin Immunol Pract. 2014;2(4):361–369.
- Boguniewicz M. Atopic dermatitis: the updated practice parameter and beyond. Allergy Asthma Proc. 2014;35(6):429–434.
- Bakaa L, Pernica JM, Couban RJ, et al. Bleach baths for atopic dermatitis: A systematic review and meta-analysis including unpublished data, Bayesian interpretation, and GRADE. Ann Allergy Asthma Immunol. 2022;128(6):660–668.
- Oykhman P, Dookie J, Al-Rammahy H, et al. Dietary Elimination for the Treatment of Atopic Dermatitis: A Systematic Review and Meta-Analysis. J Allergy Clin Immunol Pract. 2022;10(10):2657–2666.e8.
- Yepes-Nuñez JJ, Guyatt GH, Gómez-Escobar LG, et al. Allergen immunotherapy for atopic dermatitis: Systematic review and meta-analysis of benefits and harms. J Allergy Clin Immunol. 2023;151(1):147–158.
- Devasenapathy N, Chu A, Wong M, et al. Cancer risk with topical calcineurin inhibitors, pimecrolimus and tacrolimus, for atopic dermatitis: a systematic review and meta-analysis. Lancet Child Adolesc Health. 2023;7(1):13–25.
- Chu DK, Chu AWL, Rayner DG, et al. Topical treatments for atopic dermatitis (eczema): Systematic review and network meta-analysis of randomized trials. J Allergy Clin Immunol. 2023;152(6):1493–1519.
- Silverberg JI. Clinical Management of Atopic Dermatitis. Professional Communications, Inc; 2023.
- National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division, Food and Nutrition Board, Committee on Food Allergies: Global Burden, Causes, Treatment, Prevention, and Public Policy. Finding a Path to Safety in Food Allergy: Assessment of the Global Burden, Causes, Prevention, Management, and Public Policy. National Academies Press; 2017.
- Belloni G, Pinelli S, Veraldi S. A randomised, double-blind, vehicle-controlled study to evaluate the efficacy and safety of MAS063D (Atopiclair) in the treatment of mild to moderate atopic dermatitis. Eur J Dermatol. 2005;15(1):31–36.
- Breternitz M, Kowatzki D, Langenauer M, Elsner P, Fluhr JW. Placebo-controlled, double-blind, randomized, prospective study of a glycerol-based emollient on eczematous skin in atopic dermatitis: biophysical and clinical evaluation. Skin Pharmacol Physiol. 2008;21(1):39–45.
- Tan WP, Suresh S, Tey HL, Chiam LY, Goon AT. A randomized double-blind controlled trial to compare a triclosan-containing emollient with vehicle for the treatment of atopic dermatitis. Clin Exp Dermatol. 2010;35(4):e109–e112.
- Abramovits W, Fleischer AB Jr, Jaracz E, Breneman D. Adult patients with moderate atopic dermatitis: tacrolimus ointment versus pimecrolimus cream. J Drugs Dermatol. 2008;7(12):1153–1158.
- Beattie PE, Lewis-Jones MS. A pilot study on the use of wet wraps in infants with moderate atopic eczema. Clin Exp Dermatol. 2004;29(4):348–353.
- Foelster-Holst R, Nagel F, Zoellner P, Spaeth D. Efficacy of crisis intervention treatment with topical corticosteroid prednicarbat with and without partial wet-wrap dressing in atopic dermatitis. Dermatology. 2006;212(1):66–69.
- Hindley D, Galloway G, Murray J, Gardener L. A randomised study of “wet wraps” versus conventional treatment for atopic eczema. Arch Dis Child. 2006;91(2):164–168.
- Janmohamed SR, Oranje AP, Devillers AC, et al. The proactive wet-wrap method with diluted corticosteroids versus emollients in children with atopic dermatitis: a prospective, randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2014;70(6):1076–1082.
- Schnopp C, Holtmann C, Stock S, et al. Topical steroids under wet-wrap dressings in atopic dermatitis–a vehicle-controlled trial. Dermatology. 2002;204(1):56–59.
- Gong JQ, Lin L, Lin T, et al. Skin colonization by Staphylococcus aureus in patients with eczema and atopic dermatitis and relevant combined topical therapy: a double-blind multicentre randomized controlled trial. Br J Dermatol. 2006;155(4):680–687.
- Guttman-Yassky E, Ungar B, Malik K, et al. Molecular signatures order the potency of topically applied anti-inflammatory drugs in patients with atopic dermatitis. J Allergy Clin Immunol. 2017;140(4):1032–1042.e13.
- Henderson RL, Fleischer AB Jr, Feldman SR. Dermatologists and allergists have far more experience and use more complex treatment regimens in the treatment of atopic dermatitis than other physicians. J Cutan Med Surg. 2001;5(3):211–216.
- Kim BS, Sun K, Papp K, Venturanza M, Nasir A, Kuligowski ME. Effects of ruxolitinib cream on pruritus and quality of life in atopic dermatitis: Results from a phase 2, randomized, dose-ranging, vehicle- and active-controlled study. J Am Acad Dermatol. 2020;82(6):1305–1313.
- Kim BS, Howell MD, Sun K, et al. Treatment of atopic dermatitis with ruxolitinib cream (JAK1/JAK2 inhibitor) or triamcinolone cream. J Allergy Clin Immunol. 2020;145(2):572–582.
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: Results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85(4):863–872.
- Wollenberg A, Kinberger M, Arents B, et al. European guideline (EuroGuiDerm) on atopic eczema: part I – systemic therapy. J Eur Acad Dermatol Venereol. 2022;36(9):1409–1431.
- Wollenberg A, Kinberger M, Arents B, et al. European guideline (EuroGuiDerm) on atopic eczema – part II: non-systemic treatments and treatment recommendations for special AE patient populations. J Eur Acad Dermatol Venereol. 2022;36(11):1904–1926.
- Kulthanan K, Tuchinda P, Nitiyarom R, et al. Clinical practice guidelines for the diagnosis and management of atopic dermatitis. Asian Pac J Allergy Immunol. 2021;39(3):145–155.
