 by Jeave Reserva, MD; Monica Janeczek, BS; Cara Joyce, PhD; Amanda Goslawski, MA; Hwala Hong, BA; Feng-Ning Yuan, BS; Neelam Balasubramanian, BA; Laura Winterfield, MD, MPH;
by Jeave Reserva, MD; Monica Janeczek, BS; Cara Joyce, PhD; Amanda Goslawski, MA; Hwala Hong, BA; Feng-Ning Yuan, BS; Neelam Balasubramanian, BA; Laura Winterfield, MD, MPH;
James Swan, MD; and Rebecca Tung, MD
Drs. Reserva, Swan, and Tung are with the Division of Dermatology at Loyola University Chicago in Chicago, Illinois. Dr. Joyce and Ms. Balasubramanian are with the Biostatistics Core – Clinical Research Office at at Loyola University Chicago, Chicago, Illinois. Ms. Janeczek, Ms. Goslawski, Ms. Hong, and Mr. Yuan are with the Stritch School of Medicine in Maywood, Illinois. Dr. Winterfield is with the Department of Dermatology and Dermatologic Surgery, Medical University of South Carolina in Charleston, South Carolina.
Abstract: Background. Melanoma surveillance serves to identify new primary melanomas and curable locoregional or early distant recurrences. Although an optimal melanoma surveillance strategy has not been determined, several clinical guidelines exist.
Objective. The aim of this study was to identify demographic and clinico-pathologic variables associated with poor adherence to National Comprehensive Cancer Network (NCCN) melanoma surveillance guidelines.
Design. We retrospectively reviewed the initial five-year dermatology follow-up visit frequencies of melanoma patients and extracted basic demographic and clinical data from their medical records.
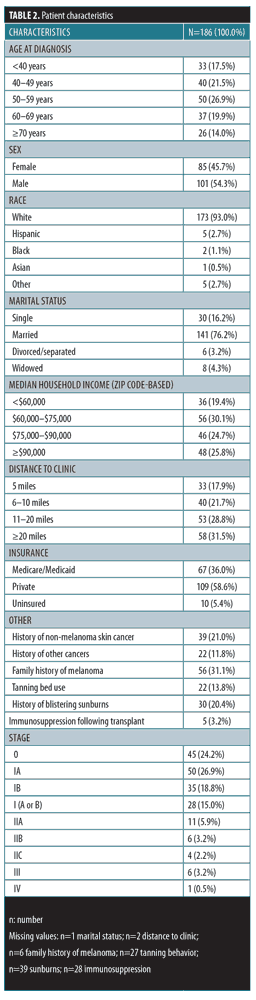
Participants. Of 186 patients included, the mean age was 55 (standard deviation=15); 47.5 percent (n=85) were female, 93.0 percent (n=173) were white, and 76.2 percent (n=141) were married. Sixty percent of patients lived at locations more than 10 miles from the clinic, and 58.6 percent had private insurance.
Measurements. “Aggressive” and “conservative” surveillance schedules were adapted from National Comprehensive Cancer Network visit frequency guidelines.
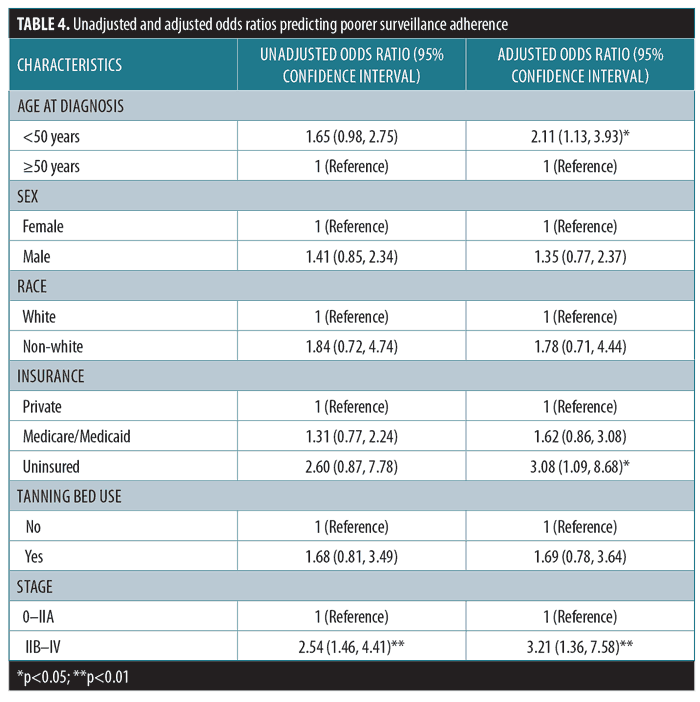
Results. Between 58.4 and 74.5 percent of patients adhered to “aggressive” surveillance, with decreasing rates over the five-year period. Annual rates of poor surveillance adherence (7.3–23.6%) increased over time. Based on adjusted odds ratios, patients younger than 50 years of age (odds ratios 2.11 [95% CI 1.13–3.93], p<0.05), those lacking health insurance (odds ratios 3.08 [95% CI 1.09–8.68], p<0.05), and those with at least Stage IIB disease (odds ratios 3.21 [95% CI 1.36–7.58], p<0.01) are more likely to be poorly adherent to melanoma surveillance.
Conclusion. This study’s findings highlight some variables associated with poor surveillance adherence among melanoma survivors that could help to guide efforts in counseling this at-risk population.
Keywords: Primary cutaneous melanoma, melanoma surveillance, follow-up visit
J Clin Aesthet Dermatol. 2017;10(12):44–48
Introduction
The incidence of a second melanoma is increased in melanoma survivors, with the cumulative risk ranging from 2 to 5 percent at periods from 5 to 20 years after initial diagnosis.1–3 Although an optimal surveillance strategy after treatment of primary cutaneous melanoma has not been determined, several guidelines are available, though they vary slightly between specialty organizing bodies and between countries.4,5 For instance, the National Comprehensive Cancer Network (NCCN) recommends lifelong full-skin examinations (FSE) at least annually for those with Stage 0 disease (melanoma in situ).6 For stage IA disease, NCCN recommends a history and physical exam including FSE with lymph node examination every 3 to 12 months for the first five years and annually thereafter. Similar recommendations are set forth for patients with Stages IB to IV, although with more frequent follow-ups for the first two years (i.e., every 3–6 months).6
Evidence presented in support of the rationale for surveillance visit frequencies has primarily been based on relapse profile over time.7 Second primary melanomas occur at an estimated incidence of 11.4 percent over a five-year period, with one-half to three-fifths of these cases occurring within the first year.8–10 In an extensive review by Francken et al,3 20 to 28 percent developed local or in-transit recurrences, 26 to 60 percent had regional recurrences, and 15 to 50 percent had distant metastases. Nearly two-thirds of relapses occur within the first two years, underscoring the importance of close surveillance for at least two years following initial diagnosis.11
Because patient adherence is central to the effectiveness of any clinical intervention,12,13 clinicians providing care to melanoma patients should be familiar with the complexities of patient adherence as it relates to melanoma surveillance. Much of this responsibility falls in the hands of physicians, mainly dermatologists, but might also be of greater effectiveness if executed in a multidisciplinary fashion.4,7,14 Variations in follow-up visit frequency guidelines, retrospective study design, and small sample size might be at play in the conflicting findings of previous studies investigating factors associated with poor patient adherence to melanoma surveillance.15–17 However, recent evidence suggests that reduced frequency of follow-up visits in early-stage melanoma patients does not negatively affect recurrence, patients’ psychological well-being, or detection of subsequent primary melanoma.18,19 With the ultimate goal of identifying patients who might benefit from additional counseling on the importance of melanoma follow-up, we investigate factors associated with poor melanoma surveillance adherence using two follow-up schedules derived from the NCCN guidelines.
Methods
A retrospective chart review was performed on patient records with diagnosis codes of melanoma of the skin (ICD-9 172.9) and personal history of melanoma (ICD-9 V10.82) between January 2005 and November 2015. Patients without an available pathology report on file to confirm a melanoma diagnosis were excluded. All study procedures were performed after obtaining approval by the institutional review board of Loyola University Chicago’s Health Sciences Division. For patients diagnosed with melanoma within the Loyola University Health System (LUHS), dermatology clinic visits were reviewed for a span of five years starting from the date of initial diagnosis of melanoma. For patients with a known personal history of melanoma diagnosed outside of the LUHS, dermatology clinic visits were reviewed for a span of five years from initial contact with the LUHS. Only dermatology encounters were included in recording follow-up visits. Patients who failed to follow up with dermatology, but were seen in surveillance by oncology or other specialties, were excluded for the purposes of this study.
Basic demographic data , including age, sex, race, marital status, proximity of residence to clinic, median household income (as determined by ZIP code), and health insurance status were obtained. Data on selected risk factors for melanoma were also abstracted from patients’ electronic medical records, when available, including history of non-melanoma skin cancer, tanning bed use, and history of blistering sunburns. Each patient’s clinical stage for melanoma was recorded based on available pathology reports, including data from wide local excision, sentinel lymph node biopsy, and completion lymphadenectomy.
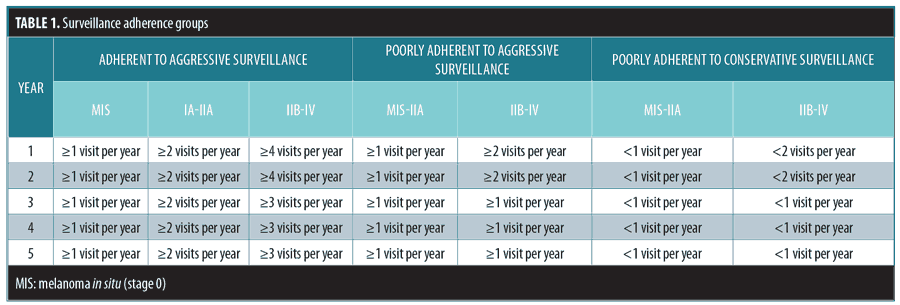
Criteria for aggressive and conservative surveillance adherence. The “aggressive” surveillance schedule was defined as follows: Stage 0 (melanoma in situ): at least one visit each year for five years; Stages IA to IIA: at least two visits each year for five years; Stages IIB to IV: at least four visits for Year 1, at least three visits for Year 2, and at least two visits for Years 3 to 5. The “conservative” surveillance adherence schedule was defined as follows: Stages 0 to IIA: at least one visit each year for five years; Stages IIB to IV: at least two visits in each of the first two years and at least one visit in the next three years. Patient adherence trends were divided into three groups (Table 1) in decreasing order of adherence: “adherent to aggressive surveillance” if they adhered to the “aggressive” surveillance schedule; “poorly adherent to aggressive surveillance” if they adhered to the “conservative” schedule but did not adhere to the “aggressive” adherence schedule; or “poorly adherent to conservative schedule” if they were not adherent to either schedule. Surveillance adherence was not calculated before patients first visited the LUHS, or after they left for another practice.
Statistical analysis. Patient characteristics and annual adherence were presented as counts and percentages. The associations between patient characteristics and adherence were assessed in separate univariable proportional odds mixed models predicting poorer levels of adherence. A multivariable proportional odds regression mixed model included variables with p<0.25 in univariable analysis. The mixed models included random intercepts to account for within-patient correlation over up to five years of follow-up.
Results
Patient characteristics. Of 186 patients included, the average age was 55 (standard deviation [SD]=15) years old, 45.7 percent (n=85) were female, 94.1 percent (n=175) were white, and 75.8 percent (n=141) were married. The majority of patients lived farther than 10 miles from the clinic (111/184, 60.3%) and had private insurance (n=109, 58.6%). Nearly all patients had between Stages 0 to IIA disease (n=169, 90.9%). A minority of patients had a history of tanning bed use (13.8%), blistering sunburns (20.4%), or non-melanoma skin cancer (21.0%). ZIP code-based household income varied, with 19.4 percent of patients living in ZIP codes with a median income of less than $60,000, 30.1 percent with $60,000 to $74,000, 24.7 percent with $75,000 to $90,000, and 25.8 percent with over $90,000 (Table 2).
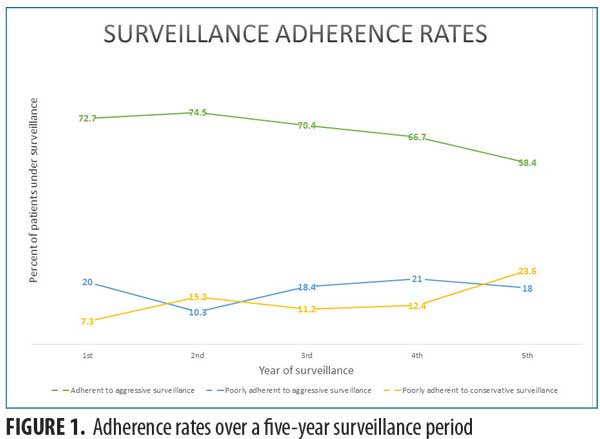
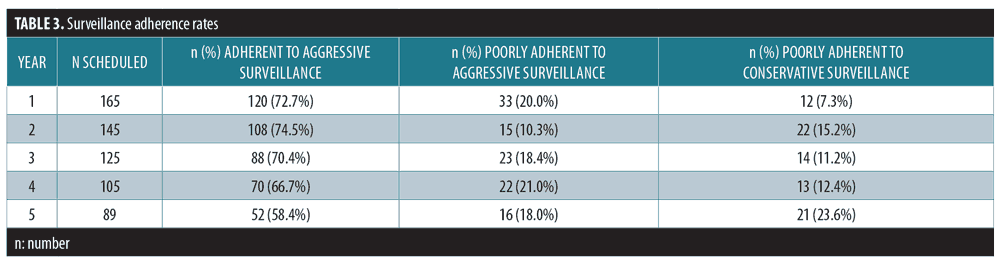
Surveillance adherence rates. Between 58.4 and 74.5 percent of patients were “adherent to aggressive surveillance,” with decreasing rates over the five-year period (Figure 1). The percentage of patients categorized as “poorly adherent to conservative schedule” (7.3–23.6%) increased over time (Table 3).
Demographics and clinicopathologic variables. Based on adjusted odds ratios, patients younger than 50 years of age are twice as likely as those older than 50 years of age to fall under the “poorly adherent to aggressive” category or “poorly adherent to conservative” category. Similarly, the uninsured and those with Stages IIB to IV disease are three times more likely to be poorly adherent to aggressive or conservative surveillance schedules. Table 4 summarizes unadjusted and adjusted ratios for poor adherence to aggressive and conservative surveillance schedules.
discussion
The number of patients requiring melanoma surveillance has grown as a result of its increasing incidence, partly due to earlier detection and improved prognosis.20,21 The present study found surveillance adherence rates of up to 76 percent (including both “aggressive” and “conservative” schedules) at five years after diagnosis. Despite the present study including a “conservative” follow-up schedule, this five-year surveillance adherence rate is much higher than those previously reported by Kalimullah et al15 (22%) and Kitler et al.16(55.3%). Apart from variations in follow-up schedule used, potential causes of such differences in reported adherence rates might, in part, be due to exclusion of melanomas thicker than 1.5mm.16 Interestingly, we found our first-year rate for “poorly adherent to conservative schedule” to be 7.3 percent, a rate similar to the findings (7.8%) reported in a prospective, randomized, controlled trial comparing the effect of a reduced follow-up frequency with a conventional follow-up schedule as recommended by the Dutch melanoma guidelines.18
Previous studies on melanoma surveillance adherence have reported conflicting findings, with one study showing positive correlation between Breslow thickness and surveillance adherence,15 and others showing either no correlation16 or even an inverse association.22 The present study found younger age (i.e., <50 years), higher stage disease (i.e., IIB–IV), and lack of insurance coverage to be the strongest predictors of poor adherence to aggressive or conservative surveillance schedule. We posit that the intensity of visit frequency for higher stages of disease and the financial burdens of surveillance visits prevent these subgroups of melanoma patients from adhering to surveillance guidelines. Similar to our findings, tumor thickness (i.e., >1mm) has previously been associated with longer periods of follow-up, as has patients’ residence proximity to the surveilling clinic location and the absence of co-morbidities.22 Under the assumption that patients older than 50 years of age are more likely to have comorbid conditions, our findings support other authors’ suppositions that patients with comorbidities might be more attuned to adhering to their melanoma surveillance visits as a consequence of their experience in handling multiple doctor visits for their other ailments.22
Follow-up visits for melanoma may be performed by general practitioners or by specialists.4,7,17 However, given dermatologists’ skills and experience in performing full skin examinations, and because “many cancer specialists never ask melanoma patients to disrobe for a proper physical examination,”23 dermatologists should continue to play a central role in melanoma surveillance. However, in an attempt to eliminate potential confounding variables during analysis, the authors only analyzed dermatology follow-up visits and excluded patients also following up with oncology or other specialties. There is, nonetheless, value in a multidisciplinary approach to melanoma surveillance, especially in cases of advanced disease.4,7,14
A brief overview of the literature on surveillance adherence in other malignancies might provide additional insights into the challenges of and potential areas for further investigation in melanoma surveillance adherence. In hepatocellular carcinoma, optimally adherent patients were those with smaller tumor sizes,24 while lower surveillance rates were reported in non-Caucasians and patients of lower socioeconomic status.25 Although the future of the Affordable Care Act (ACA) remains uncertain at this time, similar to the ACA’s effect on screening colonoscopies, policy incentives that mitigate financial barriers to melanoma surveillance experienced by those uninsured or under-insured may improve patient adherence to surveillance recommendations.26 For patients with advanced disease who likely would benefit from a multidisciplinary management approach, a dedicated single-day melanoma surveillance visit might prove beneficial in improving surveillance adherence rates, as it has been reported with colorectal carcinoma.27
Limitations. This study’s single-institution retrospective design, in addition to its relatively small sample size, could limit the ability to generalize of our findings. Furthermore, patients’ reasons for failing to show up for surveillance visits were not directly investigated. It is important to note that system-based patient reminders that might vary between institutions could also play a role in surveillance adherence rates, but have not been directly investigated in the current or previous studies. Prospectively designed, multicentered adherence studies, particularly those that examine patients receiving surveillance from multiple specialties, may address some of these limitations.
Conclusion
The present study highlights factors associated with increased likelihood of poor adherence to aggressive and conservative surveillance schedules in patients diagnosed with melanoma. Because of existing evidence that supports the value of patient-clinician information engagement in improving cancer surveillance adherence,28 it would be reasonable to provide additional surveillance counseling targeted at younger melanoma patients, those with advanced disease, and those without health insurance. Although each patient is unique and a surveillance strategy tailored to an individual patient’s recurrence risk has yet to be fully developed,23 the present study builds onto the current knowledge tackling the complexity of patient adherence, specifically in the realm of cancer surveillance.
Acknowledgments
The project described is supported in part by the Loyola Clinical Research Office (CRO) at Loyola University Chicago, Health Sciences Division, 2160 S. First Avenue, CTRE Bldg. 115, 2nd Floor, Room 253, Maywood, IL 60153. The work is the responsibility of the authors and does not necessarily represent the views of the CRO. This work was presented as an oral and poster presentation on April 28, 2017, at the 76th Annual Meeting of the Society for Investigative Dermatology in Portland, Oregon.
References
- Goggins WB, Tsao H. A population-based analysis of risk factors for a second primary cutaneous melanoma among melanoma survivors. 2003;97(3):639–643.
- DiFronzo LA, Wanek LA, Elashoff R, Morton DL. Increased incidence of second primary melanoma in patients with a previous cutaneous melanoma. Ann Surg Oncol. 1999;6(7):705–711.
- Francken AB, Bastiaannet E, Hoekstra HJ. Follow-up in patients with localised primary cutaneous melanoma. Lancet Oncol. 2005;6(8):608–621.
- Trotter SC, Sroa N, Winkelmann RR, et al. A global review of melanoma follow-up guidelines. J Clin Aesthet Dermatol. 2013;6(9):18–26.
- Cromwell KD, Ross MI, Xing Y, et al. Variability in melanoma post-treatment surveillance practices by country and physician specialty: a systematic review. Melanoma Res. 2012;22(5):376–385.
- National Comprehensive Cancer Network. NCCN Clinical practice guidelines in oncology. Melanoma. Available at: http://www.nccn.org/professionals/physician_gls/pdf/melanoma.pdf. Accessed October 2017.
- Marciano NJ, Merlin TL, Bessen T, Street JM. To what extent are current guidelines for cutaneous melanoma follow up based on scientific evidence?. Int J Clin Pract. 2014;68(6):761–770.
- Slingluff CL,Jr, Vollmer RT, Seigler HF. Multiple primary melanoma: incidence and risk factors in 283 patients. 1993;113(3):330–339.
- Kang S, Barnhill RL, Mihm MC,Jr, et al. Multiple primary cutaneous melanomas. 1992;70(7):1911–1916.
- Ferrone CR, Ben Porat L, Panageas KS, et al. Clinicopathological features of and risk factors for multiple primary melanomas. 2005;294(13):1647–1654.
- Tas F, Erturk K. Recurrence behavior in early-stage cutaneous melanoma: pattern, timing, survival, and influencing factors. Melanoma Res. 2017;27(2):134–139.
- Sohi N, Davis SA. Impact of nonadherence in dermatology. In: Davis SA, ed. Adherence in Dermatology. Switzerland: Springer International Publishing; 2016: 3–6.
- Carlsen KH, Carlsen KM, Serup J. Non-attendance, predictors, and interventions. In: Davis SA, ed. Adherence in Dermatology. Switzerland: Springer International Publishing; 2016: 29–38.
- McKenna DB, Marioni JC, Lee RJ, et al. A comparison of dermatologists’, surgeons’ and general practitioners’ surgical management of cutaneous melanoma. Br J Dermatol. 2004;151(3):636–644.
- Kalimullah FA, Brown CW. Compliance with follow-up among patients with melanoma and non-melanoma skin cancers. Dermatol Online J. 2014;20(2). pii: doj_21537.
- Kittler H, Weitzdorfer R, Pehamberger H, et al. Compliance with follow-up and prognosis among patients with thin melanomas. Eur J Cancer. 2001;37(12):1504–1509.
- Holterhues C, van de Poll-Franse LV, de Vries E, et al. Melanoma patients receive more follow-up care than current guideline recommendations: a study of 546 patients from the general Dutch population. J Eur Acad Dermatol Venereol. 2012;26(11):1389–1395.
- Damude S, Hoekstra-Weebers JE, Francken AB, et al. The MELFO-study: prospective, randomized, clinical trial for the evaluation of a stage-adjusted reduced follow-up schedule in cutaneous melanoma patients-results after 1 year. Ann Surg Oncol. 2016;23(9):2762–2771.
- Scally CP, Wong SL. Intensity of follow-up after melanoma surgery. Ann Surg Oncol. 2014;21(3):752–757.
- National Cancer Institute. Melanoma of the Skin: Cancer Stat Facts. Available at: https://seer.cancer.gov/statfacts/html/melan.html. Accessed October 2017.
- Lin AY, Wang PF, Li H, Kolker JA. Multicohort model for prevalence estimation of advanced malignant melanoma in the USA: an increasing public health concern. Melanoma Res. 2012;22(6):454–459.
- Livingstone E, Krajewski C, Eigentler TK, et al. Prospective evaluation of follow-up in melanoma patients in Germany – results of a multicentre and longitudinal study. Eur J Cancer. 2015;51(5):653–667.
- Bhutiani N, Egger ME, McMasters KM. Optimizing follow-up sssessment of patients with cutaneous melanoma. Ann Surg Oncol. 2017;24(4):861–863.
- Wang C, Chen V, Vu V, et al. Poor adherence and low persistency rates for hepatocellular carcinoma surveillance in patients with chronic hepatitis B. Medicine (Baltimore). 2016;95(35):e4744.
- Singal AG, Yopp A, S Skinner C, et al. Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. J Gen Intern Med. 2012;27(7):861–867.
- Hamman MK, Kapinos KA. Affordable Care Act provision lowered out-of-pocket cost And increased colonoscopy rates among men in Medicare. Health Aff (Millwood). 2015;34(12):2069–2076.
- Cheah LP, Hemingway DM. Improving colorectal cancer follow-up: the dedicated single-visit colorectal cancer follow-up clinic. Ann R Coll Surg Engl. 2002;84(4):260–262.
- Tan AS, Moldovan-Johnson M, Parvanta S, et al. Patient-clinician information engagement improves adherence to colorectal cancer surveillance after curative treatment: results from a longitudinal study. 2012;17(9):1155–1162.

