 by Glynis Ablon, MD, FAAD
by Glynis Ablon, MD, FAAD
Dr. Ablon is with the Ablon Skin Institute and Research Center in Manhattan Beach, California.
Funding: No funding was provided for this article.
Disclosures: The author has no conflicts of interest to relevant to the content of this article.
Abstract: Within the field of dermatology, advances in the use of light emitting diodes (LEDs) have led to their clinical application for a variety of medical and cosmetic uses. Of note, one phototherapy device has demonstrated beneficial effects over a range of clinical applications (Omnilux™; GlobalMed Technologies, Glen Ellen, California). The study included a literature review of published studies. Using LEDs with frequencies of 415nm (blue), 633nm (red), and 830nm (infrared), this device has demonstrated significant results for the treatment of medical conditions, including mild-to-moderate acne vulgaris, wound healing, psoriasis, squamous cell carcinoma in situ (Bowen’s disease), basal cell carcinoma, actinic keratosis, and cosmetic applications. Although photodynamic therapy with the photosensitizer 5-aminolevulinic acid might cause stinging and burning, phototherapy is free of adverse events. We determined that phototherapy using LEDs is beneficial for a range of medical and aesthetic conditions encountered in the dermatology practice. This treatment displays an excellent safety profile.
Keywords: Light-emitting diodes, phototherapy, photodynamic therapy, skin rejuvenation, acne vulgaris, periorbital wrinkles
J Clin Aesthet Dermatol. 2018;11(2):21–27
Introduction
An increasing number of individuals are seeking noninvasive procedures for improving medical and aesthetic dermatologic conditions. Phototherapy refers to the use of nonthermal, noninvasive light to achieve a therapeutic outcome and can apply to a variety of light-emitting devices. Interest in recent advances in the use of light emitting
diodes (LEDs) has led to their clinical application for a variety of medical and cosmetic uses. Depending on the target chromophore, different wavelengths of light are used.1 Three wavelengths of light that have demonstrated several therapeutic applications are blue (415nm), red (633nm), and near-infrared (830nm). Recent publications have reignited an interest in the numerous studies performed or sponsored by a leader in the field of LED phototherapy (Omnilux™; GlobalMed Technologies, Glen Ellen, California) which clearly demonstrate the significant value of phototherapy for a range of clinical applications (Figure 1). In my private practice, LED technology is the most commonly performed procedure and is used each office day to treat a wide variety of medical and aesthetic disorders. This review will describe the broad range of clinical applications and significant results achieved for acne, wound healing, actinic keratosis, precancerous tissue, psoriasis and skin rejuvenation, and post-procedural erythema. Signed photoconsent was provided by the patients pictured herein.
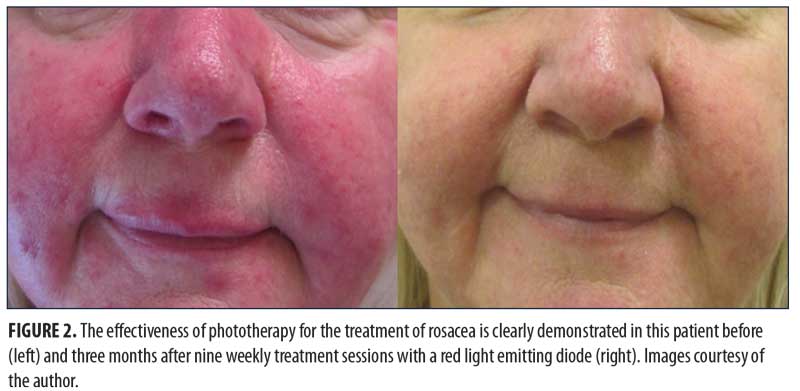
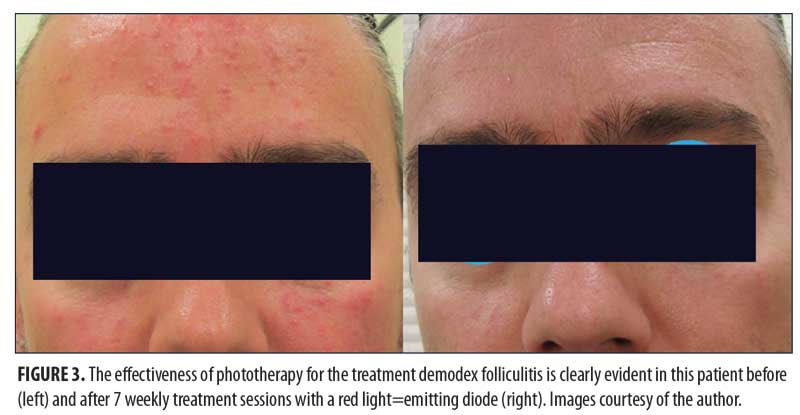
Medical Applications
Mild-to-moderate acne vulgaris. Propionibacterium acnes (P. acnes) is a gram-positive bacterium involved in the pathogenesis of acne vulgaris.2 In-vitro studies have demonstrated that blue light is effective for treating P. acnes because it produces the strongest photoactivation of endogenous porphyrins through a process known as endogenous photodynamic therapy (PDT). The result is free radical formation and destruction of the P. acnes cell membrane.3 An open-label clinical study assessed the safety and efficacy of narrowband blue light on inflammatory and noninflammatory acne lesions in patients with mild-to-moderate facial acne (N=30).4 Subjects had not used topical, oral, or systemic treatments for two weeks and had not received oral retinoids for six months. Baseline lesions were counted and recorded by lesion type. Subjects received eight 10- or 20-minute light treatments using LEDs with peak wavelengths of 409nm to 419nm (40mW/cm2) over a four-week period. Lesion counts were repeated at Weeks 5, 8, and 12. A beneficial effect on inflammatory lesions was observed at Week 5, becoming significant at Weeks 8 and 12. The mean percent reduction in lesion counts at each time point was 25 percent, 53 percent (p<0.001), and 60 percent (p<0.001), respectively; however, there was little effect on noninflammatory lesions. Adverse events included mild and transient erythema, skin dryness, and pruritis.
A second open-label study assessed the effects of phototherapy using LEDs emitting blue 415nm light at 48J/cm2 to treat subjects with mild-to-moderate acne (N=45).5 Subjects received two 20-minute treatments weekly for 4 to 8 weeks, and clinical assessments were made at baseline and two, four, and eight weeks post-treatment. Therapeutic response was measured using a global improvement scoring system: 0 (no improvement), 1 (0–25% improvement), 2 (25–50% improvement), 3 (51–75% improvement), and 4 (76–100% improvement). Among the evaluable subjects (n=43), the mean improvement score was 3.14 at four weeks and 2.90 at eight weeks. Nine patients experienced complete clearing at eight weeks, and 50 percent of subjects were highly satisfied with the treatment. There were no adverse events.
Since it had been demonstrated that phototherapy with combined blue and red light could achieve even greater efficacy in the treatment of acne,6 an open-label study was designed to assess the efficacy of combining 415nm blue light and 633nm red light for treating subjects with mild-to-moderate facial acne.7 Enrolled subjects (N=24) with Fitzpatrick Skin Types II to V had not received treatment with oral or topical acne agents during the six weeks preceding the trial or oral retinoid use in the previous nine months. Subjects with a history of photosensitivity disorder were excluded. Each subject received two treatments per week, three days apart, alternating between 415nm blue light (20 minutes/session, 48J/cm2) and 633nm red light (20 minutes/session, 96J/cm2) for four weeks using an LED-based therapy system. Patients received a mild microdermabrasion prior to each treatment session. The purpose of microdermabrasion is to provide a nonchemical superficial removal of the stratum corneum. This allows products or other procedures to pass more readily through the protective barrier of the epidermis. While recent studies have reported some histologic changes in the dermis on collagen density with microdermabrasion, published data demonstrate improvement of acne when microdermabrasion is used in combination therapy.8
Acne severity was assessed at baseline and at Weeks 2, 4, 8, and 12. Among the evaluable subjects (n=22), a mean reduction in lesion count was observed at each follow-up evaluation. At the four-week follow-up, the mean lesion count was reduced by 46 percent (p=0.001) and by 81 percent at the 12-week follow-up (p=0.001). Severe acne showed a marginally better response than mild acne, although comedones did not respond as well as inflammatory lesions. This is a common finding in light therapy studies because noninflammatory acne lesions have fewer chromophores (coproporphyrin III and protoporphyrin IX).9 Adverse events were mild and transient.
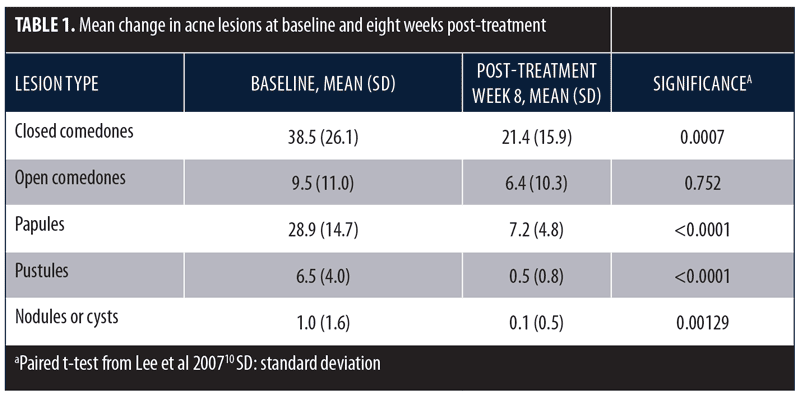
A similar study assessed the same treatment in subjects with Fitzpatrick Skin Type IV and mild-to-moderately severe facial acne (N=24).10 Subjects had not used topical acne treatment or systemic antibiotics within the two weeks of the trial or systemic retinoids within three months. Subjects with a history of photosensitivity or recent use of photosensitizing drugs were excluded. Treatment was performed twice weekly for four weeks with alternating quasi-monochromatic blue (415nm) and red (633nm) light. Clinical assessments were conducted at baseline and following Treatments 2, 4, and 6, as well as two, four, and eight weeks after the final treatment. Evaluations included lesion counts and an acne grading scale. The final mean percentage improvements in noninflammatory and inflammatory lesions were 34.2 percent and 77.9 percent, respectively. Changes in acne lesion type are summarized in Table 1.
Phototherapy and wound healing. Pre-clinical in-vitro studies and early clinical studies demonstrated LEDs developed by the National Aeronautics and Space Administration (NASA) had beneficial effects on wound healing.11,12 Based on these reports, the following controlled pilot study was performed to determine whether LED phototherapy might enhance wound healing following surgical aesthetic and resurfacing procedures.13 Male (n=2) and female (n=8) subjects with a mean age of 52.3 years (range 44–59 years) underwent combined blepharoplasty and Er:YAG/CO2 laser ablative resurfacing. Subsequently, one-half of each subject’s face was randomly selected and treated with a 633nm (96J/cm2) red LED for 20 minutes immediately after surgery, 48 hours post-surgery, and twice more the following week. Subjects were assessed 24 and 48 hours after surgery, at seven and 10 days, and at two, three, and six weeks. Resolution of erythema, edema, bruising, and days to healing was assessed using digital photography and was reviewed by a blinded and independent plastic surgeon. The LED-treated side healed after a mean (SD) of 13.5 (0.34) days versus 26.8 (0.49) days for the untreated side (p<0.0001; Table 2).
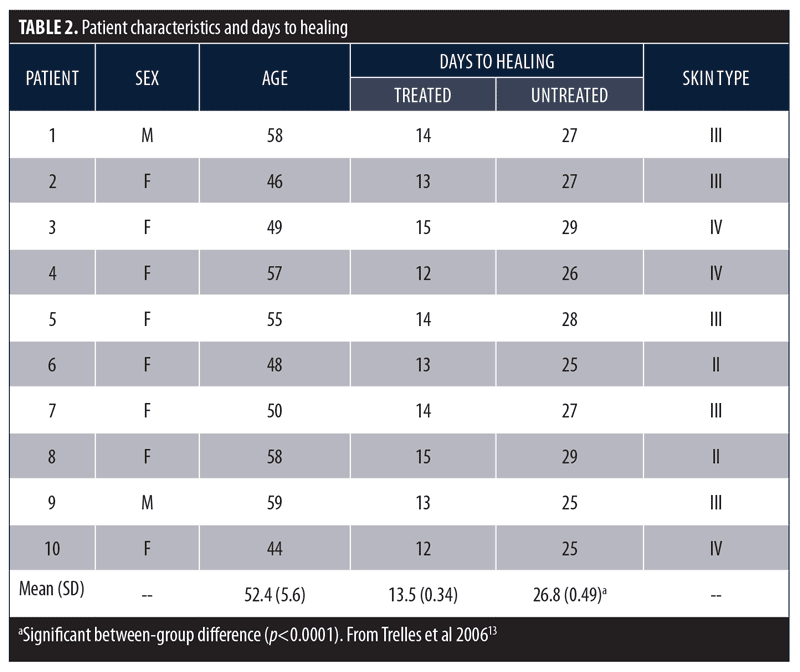
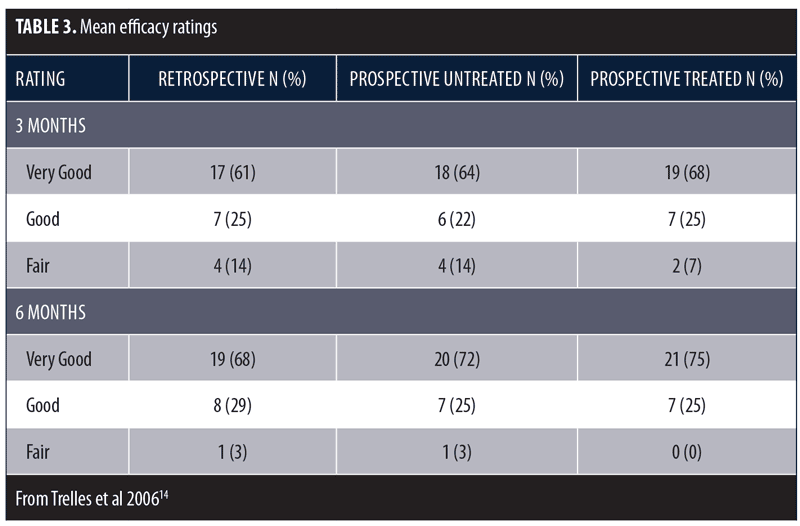
These same investigators conducted a larger study to further assess the beneficial effects of LED phototherapy on wound healing.14 The study involved a prospective treatment arm and a retrospective analysis of a matched cohort. The prospective study population included female patients (N=28) who underwent ablative Er:YAG/CO2 laser resurfacing (4 full-face, 8 periocular, 16 perioral). This was immediately followed by exposure to LEDs emitting infrared 830nm light (55 J/cm2) for 20 minutes and red 633nm light (98 J/cm2) for 20 minutes, which was repeated after 72 hours. Two additional treatments were performed three days apart during the following week and a final session during the third week. Digital images were obtained after 24 hours, three days, one, two, four, and six weeks, and three and six months post-resurfacing. The retrospective group included the same number of age- and treatment-matched subjects who did not receive LED therapy and were evaluated three and six months post-resurfacing. Healing response was rated as Very Good (85–100% improvement), Good (65–84% improvement), Fair (45–64% improvement), Poor (<45% improvement), or Bad (little improvement or worsened). Overall, mean efficacy results were similar across treatment groups (Table 3). No subjects were rated as Poor or Bad. At the three-month assessment, the overall efficacy of the prospective-treated group (Very Good + Good) was significantly better than both the prospective-control and retrospective groups (p<0.01). At the six-month assessment, the prospective-treated group showed slightly better improvement than the other two groups, but the difference was not significant. The symptoms of exudation, crusting, pain, and edema resolved approximately 50 percent faster in the prospective-treated group (p<0.001) as did erythema (p<0.0001). At six months, there was no significant difference in wrinkle improvement between the prospective-treated and untreated side, but the skin appeared younger-looking on the LED-treated side. These results have been confirmed in several subsequent studies.13,15–17
Psoriasis. In the Unites States, the incidence of psoriasis in adults has been increasing from 50.8/100,000 during the 1970s to 100.5/100,000 during the 1990s.18 As increasing attention was being paid to visible red (633nm) and near infrared (830nm) light-emitting diodes for treating various dermatological conditions, the following pilot study was designed to assess the efficacy of combining 830nm and 633nm LED phototherapy for treating recalcitrant psoriasis.19 Informed and consenting subjects with psoriasis (N=9) with a mean age of 34.3 years and Fitzpatrick Skin Types I to IV were enrolled. All had chronic plaque psoriasis (n=8) and guttate psoriasis (n=1) of up to 35 years duration and affecting 15 to 80 percent of their body surface area. Most had become resistant to conventional treatments.
Each subject was treated sequentially with LED arrays delivering continuous-wave 830nm, 60J/cm2 and 633nm, 126J/cm2 during two weekly 20-minute sessions for 4 to 5 weeks with two days between sessions. Specified psoriatic plaques or guttate papules were chosen to be treated. All subjects completed their LED regimens with five subjects requiring a second treatment regimen. Among evaluable subjects (n=7) at three- to four-month follow-up evaluations, clearance rates ranged from 60 to 100 percent of specified treatment sites. Overall patient satisfaction was very high. Protoporphyrin present in psoriatic skin apparently acts as a photosensitizer. A subsequent study further demonstrated the beneficial effects of phototherapy for treating psoriasis.20
Photodynamic therapy (PDT): phototherapy combined with 5-aminolevulinic acid. PDT involves administration of a light-sensitive substance, or photosensitizer, followed by exposure to the wavelength of light that corresponds to the absorbance band of the sensitizer. Both red and blue LED, as well as intense pulsed light technology, have been used to activate the photosensitizer. Cytotoxic free radicals form in the presence of oxygen which causes cell death.21 The most common photosensitizer is 5-aminolevulinic acid (ALA). Photodynamic therapy with ALA has been used to treat a range of conditions, including from pre-cancerous and cancerous lesions22,23 and photoaged skin.24,25
Squamous cell carcinoma in situ (Bowen’s disease). The safety and efficacy of PDT was compared with cryotherapy for treating Bowen’s disease.26 Lesions were randomized to undergo cryotherapy with liquid nitrogen (n=20) or PDT (n=20) using ALA as the photosensitizer. ALA was applied topically four hours prior to irradiation (125J/cm2; 70 mW/cm2). Subjects were evaluated every two months and retreated as needed. Cryotherapy produced clearance in 10 lesions after one treatment, with the remaining 10 lesions requiring two or three additional treatments. PDT resulted in clearance of 15 lesions after one treatment and the remaining five lesions after a second treatment. The probability that a lesion cleared after one treatment was significantly greater with PDT (p<0.01). Cryotherapy was associated with ulceration (n=5), infection (n=2) and recurrent disease (n=20). No adverse events were associated with the use of PDT.
Another controlled study assessed the relative efficacy of red and green light for the treatment of Bowen’s disease.27 Four hours following the application of 20% ALA, lesions were exposed to red 630±15nm (125J/cm2) (n=32) or green 540±15nm (62.5J/cm2) (n=29) light. The initial clearance rate for lesions treated by red light was 94 percent versus 72 percent (21 of 29) for green light (p=0.002). Over the following 12 months, there were two recurrences in the red light group and seven in the green light group, reducing the overall clearance rates to 88 percent and 48 percent, respectively.
Based on these results, an open-label study assessed the efficacy of PDT using 630±15nm (100J/cm2) to treat Bowen’s disease consisting of 40 large lesions greater than 20mm in diameter and 45 multiple lesions.28 ALA was used as the photosensitizer. Among the large lesions, 35 (88%) cleared after 1 to 3 treatments. Five lesions failed to clear but all showed partial response. Among the multiple lesions, 44 (98%) cleared following one or two treatments; however, four lesions cleared after 12 months, reducing the clearance rate to 89%.
Basal cell carcinoma. Despite the success of PDT using ALA for Bowen’s disease, superficial basal cell carcinoma of the skin (BCC) often respond poorly. The results of several studies report relapse rates of 50 percent or greater.29–31 The objective of this open-label study was to determine whether a second PDT treatment after seven days could improve outcomes. One hour following the application of 20% ALA, six subjects with 26 BCC lesions were treated with 630±15nm red light (120–134J/cm2; 50±100mW/cm2). The treatment was repeated after seven days. A complete response rate of 100 percent was observed one month after treatment. During a median follow-up of 27 months (range, 15–45 months), relapse of one lesion occurred after 16 months. Cosmetic results were excellent.
Actinic keratosis (AK). AK refers to rough, scaly lesions that can occur following long-term sun exposure in fair-skinned individuals. Consequently, they most often occur on the face, forearms, and back of the hands.32 AKs are precancerous and might eventually progress to squamous cell carcinoma if left untreated.21 PDT provides good cure rates and excellent cosmetic outcomes when used for the treatment of AK.21,33 Our review found that when PDT was used with 20% ALA cream or solution, long-term cure rates were reported to be 78 to 89 percent using blue light and 85 to 89 percent using red light.21 The following trial described the efficacy of PDT for the treatment of AK.
Initially, human epidermal keratinocytes were incubated for 24 hours with ALA ranging from 100 to 500µmol/L and irradiated using 633nm light (3 to 24J/cm2).34 Cell viability was significantly reduced. Maximal cytotoxic effects were achieved using a light dose of 24J/cm2. Subsequently, a clinical ALA-PDT study was performed on 40 subjects with 294 AK lesions.34 Subjects were included only if they showed a lesion distribution suitable for a two-sided comparison. Most lesions (81%) were located on the face or scalp, 15 percent were located on the hands, and four percent were located on the limbs. The treatment groups were very similar with respect to number of lesions and lesion grades. ALA 20% in a cream base was applied to each lesion and 5mm of surrounding normal tissue. After a four-hour incubation period, subjects were treated with 633nm light (40J/cm2). Immediately following treatment, subjects scored pain severity using an 11-point (0–10) scale. Subjects were evaluated after six, 12, and 24 weeks.
The overall six- and 24-week complete response rates were 84.3 percent and 38.8 percent, respectively.34 All treated lesions developed erythema and crusting 2 to 4 days after treatment, which lasted approximately 10 to 14 days. After that time, all subjects showed excellent cosmetic results. The most common adverse event (100%) was stinging and burning pain during and after irradiation with a mean (SD) pain score of 6.95 (1.73). No patient discontinued treatment due to discomfort.
Aesthetic Applications
Skin rejuvenation. LEDs have also been used to improve the appearance of photoaged skin. In contrast with thermal-based skin-tightening devices, such as radiofrequency and focused ultrasound,35 LEDs do not produce thermal injury. The skin-rejuvenating effects of LED systems are produced by a mechanism known as photobiomodulation.36,37 This nonthermal process involves exciting endogenous chromophores to elicit photophysical and photochemical events. Photobiomodulation stimulates fibroblast proliferation, collagen synthesis, growth factors, and extracellular matrix production by activating cellular mitochondrial respiratory pathways. The result is lifting and tightening lax skin and the reduction of rhytids.
One small, controlled study (N=23) demonstrated the effectiveness of a 633nm (96 J/cm2) LED light when administered as 20-minute treatments three times weekly for three weeks.38 Subjects had not received any aesthetic treatments within the previous six months before initiation of the study treatment. Improvements included reduction in fine lines and wrinkles and softer, smoother skin. No adverse events were reported. Another small controlled study (N=31) demonstrated the effectiveness of combining a 633nm (126J/cm2) LED and a 830nm (66J/cm2) for treating periorbital wrinkles.39 Exclusion criteria included laser treatment or other ablative or nonablative cosmetic intervention within the previous six months, including injectables or fillers, a history of laser treatment or trauma to the test site, and Fitzpatrick Skin Type VI.
Subjects were irradiated with the 830nm LED for 20 minutes on Days 1, 3, 5, 15, 22, and 29 and with the 633nm LED for 20 minutes on Days 8, 10, and 12. Most subjects achieved softening of periorbital wrinkles, improvement in photoaging scores, and improvements in softness, smoothness, and firmness of their skin. Mild erythema was reported by eight subjects.
Based on these promising results, the following randomized, double-blind, controlled study was designed to assess the efficacy of 830nm and 633nm LEDs, alone or together, for facial rejuvenation.40 Exclusion criteria included a history of photosensitivity or recent use of photosensitizing drugs including systemic retinoids or recent use of topical retinoic acid; recent skin disease, operation, trauma, or systemic disease that could affect the skin status; or aesthetic procedures, such as botulinum toxin, dermal filler, laser resurfacing, chemical peels, dermabrasion, or nonablative rejuvenation treatments within the three years on enrollment previous to the trial.
Subjects with facial wrinkles (N=76) were randomized to receive treatment with a quasi-monochromatic 830nm (126J/cm2) LED alone (Group 1, n=28), 633nm (66J/cm2) LED alone (Group 2, n=28), 830nm, 633nm LEDs sequentially (Group 3, n=28) on one side of the face, or sham treatment (Group 4, n=28) on the other side of the face. Treatments were performed twice weekly for four weeks with a 3- to 4-day interval between sessions. Digital images of both periorbital areas of each subject were obtained at baseline, Week 3 during treatment, and two, four, and eight, and 12 weeks post-treatment. Profilometric evaluation using silicon imprints was performed on the outer canthus of both periorbital areas at baseline and at two, four, eight, and 12 weeks post-treatment. Objective measurements of the melanin level were performed before and after each treatment session, and measurements of the skin elasticity were performed at baseline, Weeks 2, 3, and 4 during treatment, and at Weeks 2, 4, 8, and 12 post-treatment. There were 4 to 6 subjects in each group who volunteered for punch biopsies for tissue assays. Assessment scales were used to assess clinical improvement and subject satisfaction.
There was a significant decrease in mean wrinkle severity among subjects in Groups 1, 2, and 3 and a significant decrease in melanin in Group 2. At the final assessment, the proportion of improved subjects in Group 1 (95.2%), Group 2 (72.3%), and Group 3 (95.5%) were much greater than sham-treated subjects (13.3%). Investigator assessments in overall wrinkle severity are summarized in Table 4. The highest mean scores were in Groups 1 and 3. Group 4 results demonstrate sham treatment produced no effect.
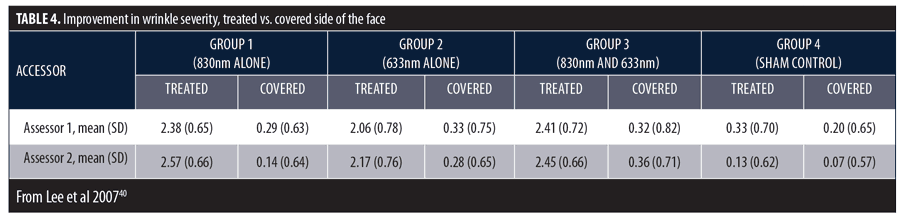
Tissue assay results showed increased collagen throughout the entire dermis in Groups 1, 2, and 3 and particularly in the perifollicular areas and the papillary and upper reticular dermis. Collagen bundles were more packed and well-organized and the thickness of each bundle appeared greater than before treatment. Microscopic examination revealed the presence of highly activated fibroblasts in the active treatment groups and an increase in the size and number of collagen and elastic fibers. Fibroblasts appeared enlarged with numerous dilated endoplasmic reticula, surrounded by abundant thick collagen bundles and normal-structured elastic fibers.
Photodynamic rejuvenation with 5-aminolevulinic acid (5-ALA). The objective of this two-part pilot study was to 1) evaluate the optimum dose and tolerance of 5-ALA when used with a 633nm LED light in healthy volunteers compared to a placebo cream and 2) determine whether ALA-PDT treatments improve the signs of aging skin.41 Subjects with normal forearm skin and Glogau Scale II photodamage were enrolled (N=6). Subjects had no inflammatory skin conditions in the areas to be treated, history of photosensitivity and were not taking photosensitizing medications.
For the dose ranging study, 5%, 10%, and 20% ALA or placebo cream were applied to the forearm and an occlusive dressing applied. After 30 to 120 minutes, the dressing and excess cream were removed, and the test areas were exposed to 633nm at 126 J/cm2 light for 20 minutes. The treated areas were examined for evidence of edema, erythema, and changes in pigmentation immediately post-exposure and one, two, three, and seven days post-exposure. Subjects were queried about potential adverse events during each assessment.
Minimum erythema with no significant change in pigmentation was achieved with 5% ALA and a 30-minute occlusion time. The 10% ALA with a 30-minute occlusion resulted in more consistent erythema and mild pigmentation. The 10% ALA with a 60-minute occlusion time increased pigmentation in several sites with adverse textural changes to the skin. The 20% ALA caused severe erythema, some ulceration and pigmentation, and adverse textural skin changes at all occlusion times. For this reason, the combination of 5% 5-ALA incubated for 30 minutes was chosen as the treatment parameter that gave the desired amount of erythema with no side effects.
For the second part of the study, 5% ALA was applied to the skin immediately around the periorbital region and an occlusive dressing was applied for 30 minutes. After that time, the dressing and excess cream were removed and the area was exposed to 633nm light for 20 minutes. Digital photography was used to record baseline appearance of the lateral canthal areas and changes after one, two, three, and seven days. Subjects were queried about potential adverse events during each assessment.
The use of 5% ALA and a 30-minute occlusion time resulted in mild, self-limiting erythema at Day 1 and mild skin peeling at Day 3, which resolved by Day 7. Clinical assessment of the periorbital region showed a significant treatment response in four subjects (67%) with a reduction in fine lines. Skin softness was improved in all subjects at the test area. The treatment was well tolerated with no reported adverse events.
Discussion
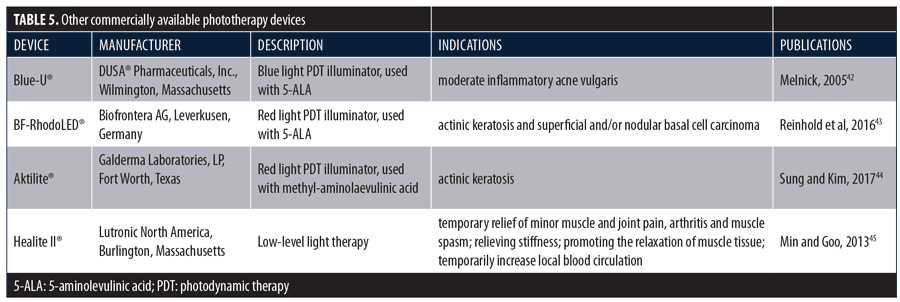
LED phototherapy is a valuable tool in the dermatologist’s armamentarium. It can be used alone or in combination with other therapies to treat a wide variety of dermatological conditions, many of which have been described in this paper. Moreover, the technology has numerous aesthetic applications, both as a stand-alone treatment and as an adjunctive to other aesthetic procedures, including such commonly performed procedures as injections, laser resurfacing, peels, intense pulsed light, and microneedling. There is no other technology available to the practicing dermatologist that has such broad utility in such a wide variety of medical and aesthetic applications. The effectiveness of other devices used for phototherapy, primarily PDL, has also been clinically demonstrated.42–45 Devices with published efficacy data are shown in Table 5.
One of the most important aspects of LED phototherapy devices is their safety. LEDs are nonablative and nonthermal, and when used alone (i.e., without topical photosensitizers in PDT applications) do not cause damage to the epidermis or dermal tissue. There are no adverse events associated with the use of these devices and little to no downtime for the patient. When LED phototherapy is used alone, patients do not experience redness, peeling, blistering, swelling, or pain. In fact, patients can have a treatment during their lunch hour and return to work immediately afterwards.
While home use devices have been available for several years, there are many differences between those devices and those specifically designed for use by physicians. The home use devices necessarily deliver significantly less power and typically do not have light panel arrays large enough to treat the entire face at once, for example. As they often are hand-held, it might be cumbersome, time-consuming, and impractical to treat the entire face in a single session. In contrast with the medical LED units and their protocols, home use devices have not been validated by controlled clinical studies published in peer-reviewed journals. In some cases, home units may be used adjunctively with dermatologist-provided treatment to address specific areas of concern, but they are dissimilar enough from the medical-grade units to not be considered an alternative to these tested technologies.
Conclusion
Phototherapy using LEDs is beneficial for a broad range of medical and aesthetic conditions encountered in the dermatology practice. The treatment modality displays an excellent safety profile and can be effectively used for the treatment of acne vulgaris, wound healing applications following surgical aesthetic and resurfacing procedures, actinic keratosis, squamous cell carcinoma in situ, and basal cell carcinoma. LED phototherapy is also an effective means for rejuvenating aged skin when used alone or together with a photosensitizer, such as 5-ALA, as well as post-procedural erythema. Having implemented this technology in my practice since 2009, I have also had success treating chemotherapy-induced rashes and many other difficult to treat skin conditions. More research should be performed, as this type of phototherapy shows great promise treating even more diagnostic conditions than are currently published.
Acknowledgments
The author acknowledges the editorial assistance of Dr. Carl S. Hornfeldt, Apothekon, Inc., with funding provided by GlobalMed Technologies, Glen Ellen, CA.
References
- Calderhead RG. The photobiological basics behind light-emitting diode (LED) phototherapy. Laser Ther 2007;16:97–108.
- Zaenglein AL, Thiboutot DM. Acne Vulgaris. In: Bolognia JL, Jorizzo JL, Schaffer JV, editors. Dermatology. Atlanta: Elsevier Inc; 2016.
- Ashkenazi H, Malik Z, Harth Y, et al. Eradication of Propionibacterium acnes by its endogenic porphyrins after illumination with high intensity blue light. FEMS Immunol Med Microbiol. 2003;35:17–24.
- Morton CA, Scholefield RD, Whitehurst C, et al. An open study to determine the efficacy of blue light in the treatment of mild to moderate acne. J Dermatolog Treat. 2005;16:219–23.
- Tremblay JF, Sire DJ, Lowe NJ, et al. Light-emitting diode 415nm in the treatment of inflammatory acne: an open-label, multicentric, pilot investigation. J Cosmet Laser Ther. 2006;8:31–3.
- Papageorgiou P, Katsambas A, Chu A. Phototherapy with blue (415nm) and red (660nm) light in the treatment of acne vulgaris. Br J Dermatol. 2000;142:973–8.
- Goldberg DJ, Russell BA. Combination blue (415nm) and red (633nm) LED phototherapy in the treatment of mild to severe acne vulgaris. J Cosmet Laser Ther. 2006;8:71–5.
- El-Domyati M, Hosam W, Abdel-Azim E, et al. Microdermabrasion: a clinical, histometric, and histopathologic study. J Cosmet Dermatol. 2016;15:503–13.
- Lee SYC. Shedding Light on Acne: From Myth to Science (Laser and Light Therapy for Acne). In: Roth DE, editor. Dermatology Research Focus on Acne. Hauppauge, NY: Nova Science Publishers, Inc; 2009.
- Lee SY, You CE, Park MY. Blue and red light combination LED phototherapy for acne vulgaris in patients with skin phototype IV. Lasers Surg Med. 2007;39:180–8.
- Whelan HT, Smits RL Jr, Buchman EV, et al. Effect of NASA light-emitting diode irradiation on wound healing. J Clin Laser Med Surg. 2001;19:305–14.
- Whelan HT, Buchmann EV, Dhokalia A, et al. Effect of NASA light-emitting diode irradiation on molecular changes for wound healing in diabetic mice. J Clin Laser Med Surg. 2003;21:67–74.
- Trelles MA, Allones I. Red light-emitting diode (LED) therapy accelerates wound healing post-blepharoplasty and periocular laser ablative resurfacing. J Cosmet Laser Ther. 2006;8:39–42.
- Trelles MA, Allones I, Mayo E. Combined visible light and infrared light-emitting diode (LED) therapy enhances wound healing after laser ablative resurfacing of photodamaged facial skin. Med Laser Appl. 2006;21:165–75.
- Kim JW, Lee JO. Low level laser therapy and phototherapy-assisted hydrogel dressing in burn wound healing: light-guided epithelial stem cell biomodulation. In: Innovations in Plastic and Aesthetic Surgery. New York: Springer; 2008. p. 36–41.
- Kim JW, Lee JO, Calderhead RG. The improvement of hypertrophic scar and keloidal scar by combining drilling tiny pinholes with carbon dioxide laser and 830 nm Omnilux PDT LED. J Korean Soc Laser Med Surg. 2005;9:1–6.
- Kim JW, Lee JO, Calderhead RG, et al. Synergic effect of ablating erbium Yag laser skin resurfacing combined with non-ablating quasilaser light 415nm, 633nm, 830nm LED array in Asian patients. J Korean Soc Laser Med Surg. 2005;9:15–23.
- Griffiths CE, van de Kerkhof P, Czarnecka-Operacz M. Psoriasis and atopic dermatitis. Dermatol Ther (Heidelb). 2017;7:31–41.
- Ablon G. Combination 830nm and 633nm light-emitting diode phototherapy shows promise in the treatment of recalcitrant psoriasis: preliminary findings. Photomed Laser Surg. 2010;28:141–6.
- Kleinpenning MM, Otero ME, van Erp PE, et al. Efficacy of blue light vs. red light in the treatment of psoriasis: a double-blind, randomized comparative study. J Eur Acad Dermatol Venereol. 2012;26:219–25.
- Ericson MB, Wennberg AM, Larkö O. Review of photodynamic therapy in actinic keratosis and basal cell carcinoma. Ther Clin Risk Manag. 2008;4:1–9.
- Yang X, Palasuberniam P, Kraus D, et al. Aminolevulinic acid-based tumor detection and therapy: molecular mechanisms and strategies for enhancement. Int J Mol Sci. 2015;16:25865–80.
- Saini R, Lee NV, Liu KY, et al. Prospects in the application of photodynamic therapy in oral cancer and premalignant lesions. Cancers (Basel). 2016;8:E83.
- Yang G, Xiang LF, Gold MH. 5-Aminolevulinic acid-based photodynamic intense pulsed light therapy shows better effects in the treatment of skin photoaging in Asian skin: a prospective, single-blinded, controlled trial. J Clin Aesthet Dermatol. 2010;3:40–3.
- Le Pillouer-Prost A, Cartier H. Photodynamic photorejuvenation: a review. Dermatol Surg. 2016;42:21–30.
- Morton CA, Whitehurst C, Moseley H, et al. Comparison of photodynamic therapy with cryotherapy in the treatment of Bowen’s disease. Br J Dermatol. 1996;135:766–71.
- Morton CA, Whitehurst C, Moore JV, et al. Comparison of red and green light in the treatment of Bowen’s disease by photodynamic therapy. Br J Dermatol. 2000;143:767–72.
- Morton CA, Whitehurst C, McColl JH, et al. Photodynamic therapy for large or multiple patches of Bowen disease and basal cell carcinoma. Arch Dermatol. 2001;137:319–24.
- Cairnduff F, Stringer MR, Hudson EJ, et al. Superficial photodynamic therapy with topical 5-aminolaevulinic acid for superficial primary and secondary skin cancer. Br J Cancer. 1994;69:605–8.
- Fink-Puches R, Soyer HP, Hofer A, et al. Long-term follow-up and histological changes of superficial nonmelanoma skin cancers treated with topical delta-aminolevulinic acid photodynamic therapy. Arch Dermatol. 1998;134:821–6.
- Lui H, Salasche S, Kollias N, et al. Photodynamic therapy of nonmelanoma skin cancer with topical aminolevulinic acid: a clinical and histologic study. Arch Dermatol. 1995;131:737–8.
- Pariser DM, Houlihan A, Ferdon MB, et al. Randomized vehicle-controlled study of short drug incubation aminolevulinic acid photodynamic therapy for actinic keratoses of the face or scalp. Dermatol Surg. 2016;42:296–304.
- Berman B, Ablon GR, Bhatia ND, et al. Expert consensus on cosmetic outcomes after treatment of actinic keratosis. J Drugs Dermatol. 2017;16:260–64.
- Babilas P, Kohl E, Maisch T, et al. In vitro and in vivo comparison of two different light sources for topical photodynamic therapy. Br J Dermatol. 2006;154:712–8.
- Britt CJ, Marcus B. Energy-based facial rejuvenation: advances in diagnosis and treatment. JAMA Facial Plast Surg. 2017;19:64–71.
- Kim JW. Clinical trial of nonthermal 633nm Omnilux LED array for renewal of photoaging: clinical surface profilometric results. J Korean Soc Laser Med Surg. 2005;9:69–76.
- Nestor M, Andriessen A, Berman B, et al. Photobiomodulation with non-thermal lasers: mechanisms of action and therapeutic uses in dermatology and aesthetic medicine. J Cosmet Laser Ther. 2017;Feb 17:1–9.
- Bhat J, Birch J, Whitehurst C, et al. A single-blinded randomised controlled study to determine the efficacy of Omnilux Revive facial treatment in skin rejuvenation. Lasers Med Sci. 2005;20:6–10.
- Russell BA, Kellett N, Reilly LR. A study to determine the efficacy of combination LED light therapy (633nm and 830nm) in facial skin rejuvenation. J Cosmet Laser Ther. 2005;7:196–200.
- Lee SY, Park KH, Choi JW, et al. A prospective, randomized, placebo-controlled, double-blinded, and split-face clinical study on LED phototherapy for skin rejuvenation: clinical, profilometric, histologic, ultrastructural, and biochemical evaluations and comparison of three different treatment settings. J Photochem Photobiol B. 2007;88:51–67.
- Lowe NJ, Lowe P. Pilot study to determine the efficacy of ALA-PDT photo-rejuvenation for the treatment of facial ageing. J Cosmet Laser Ther. 2005;7:159–62.
- Melnick S. Cystic acne improved by photodynamic therapy with short-contact 5-aminolevulinic acid and sequential combination of intense pulsed light and blue light activation. J Drugs Dermatol. 2005;4:742–5.
- Reinhold U, Dirschka T, Ostendorf R, et al. A randomized, double-blind, phase III, multicentre study to evaluate the safety and efficacy of BF-200 ALA (Ameluz®) vs. placebo in the field-directed treatment of mild-to-moderate actinic keratosis with photodynamic therapy (PDT) when using the BF-RhodoLED® lamp. Br J Dermatol. 2016;175:696–705.
- Sung JM, Kim YC. Photodynamic therapy with epidermal ablation using fractional carbon-dioxide laser in the treatment of Bowen’s disease: a case series. Photodiagnosis Photodyn Ther. 2017;19:84–85.
- Min PK, Goo BL. 830nm light-emitting diode low level light therapy (LED-LLLT) enhances wound healing: a preliminary study. Laser Ther. 2013;22:43–9.

