by Roberta Del Campo, MD; Yu Zhang, PhD; and Charles Wakeford, PhD
Dr. Del Campo is a physician at South Beach Dermatology in Miami Beach, Florida. Dr. Zhang is associated with the Department of Food Science and Human Nutrition at the University of Florida in Gainesville, Florida. Dr. Wakeford is a managing member of statistics at Triangle Biostatistics in Raleigh, North Carolina.
Funding: A research grant for this study was received from the Miracle Fruit Oil Company, manufacturers of the study product.
Disclosures: Compensation for statistical analysis related with this study was to Dr. Wakeford. Drs. Del Campo and Zhang have no financial disclosure relevant to the content of this article.
Abstract: Background. Hair breakage is a common unrecognized form of hair loss in women most often the result of hair weathering and traumatic grooming practices. Lipids are major determinants of the physical properties of the hair. Synsepalum dulcificum seed oil (MFSO®; Miracle Fruit Oil Co., Miami Beach, Florida), is an exotic fruit oil with physicochemical properties suited to providing a superior ability to reduce hair breakage.
Objective. To assess the safety and efficacy of a hair oil containing MFSO and its effects on hair breakage rates.
Methods. Healthy, long-haired women (age range: 19–63 years, mean age: 36.7 years, standard deviation: 10.77 years) with excessive hair breakage were randomized in this double-blind, placebo-controlled study to receive MFSO (n=24), vehicle (n=17), or argan oil (n=16). Measurements of hair length, hair diameter, and Hair Mass Index were performed at baseline, Month 4, and Month 8. Hair Breakage Index and the Healthy Hair Index values were calculated from the trichometer measurements, and subject self-assessment questionnaires were conducted. The primary efficacy endpoints were the percent change in Healthy Hair Index 75 and Healthy Hair Index 50 measurements from baseline to the eighth month.
Results. The Healthy Hair Index calculations, expressed as percent change from baseline to Month 4 and from baseline to Month 8, revealed that the MFSO® treatment group improved by 103.6 percent and 215.7 percent for the Healthy Hair Index 75 and 133.7 and 188.3 percent for the Healthy Hair Index 50 values, respectively. When compared with the vehicle and the argan oil brand groups, the Healthy Hair Index levels were significantly higher (p < 0.001) for the MFSO® treatment group, indicating a much greater ability to increase the levels of unbroken hairs by reducing hair breakage. With respect to the mean percent improvements from baseline to Month 4 and Month 8, the MFSO® hair oil treatment group was better than each of the other two treatment groups by at least 117.6 percent and 234.9 percent for the Healthy Hair Index 75 and 316.5 percent and 312 percent for the Healthy Hair Index 50 values, respectively, thereby achieving the primary efficacy objective. Subjects favored the MFSO® hair oil treatment, rating it as safe, effective, and aesthetically pleasing.
Conclusions. The MFSO hair oil product is a safe and effective option for the treatment of women suffering from hair breakage and damaged hair.
Keywords: miracle fruit seed oil, Synsepalum dulcificum, hair breakage, Healthy Hair Index, Hair Breakage Index, trichometer, randomized controlled study
J Clin Aesthet Dermatol. 2017;10(11):39–48
Introduction
The aesthetic appearance of hair can play an important role in people’s overall physical appearance, self-perception, and self-confidence during social interactions. Hair breakage is a common but often unrecognized form of hair loss in women with long hair.1, 2 It is frequently misdiagnosed as thinning due to female pattern hair loss or shedding due to telogen effluvium in women who present to the physician with complaints of hair loss. Although certain nutritional and medical disorders can result in hair breakage, it is most often the result of hair weathering and grooming practices.3–7 Hair shaft fragility results from natural aging and wear and tear due to exposure to a variety of environmental stresses, such as ultraviolet radiation, salt water, and pollution.8–11 There are many common hair grooming practices that can cause structural damage to the hair fiber, causing the hair to break by producing mechano-physical and chemical insults. These include shampooing, combing, brushing, blow-drying, braiding, weaving, adding hair extensions, straightening, waving, perming, bleaching, dyeing, and the use of hot irons. These traumatic hair care practices strip hair oils and cause the cuticle to become raised and porous, exposing the cortex to further damage, which can ultimately lead to reduced hair fiber strength and elasticity followed by fracture and hair loss.12
Research studies designed to measure hair breakage have traditionally been conducted in laboratories using instrumentation that measures a single fiber’s tensile strength after fatigue or on tresses of cut normal human hair in humidity-controlled environments.13–15 After a single application of test product, tresses are washed with water and exposed to bleach followed by repeated mechanical grooming. The detection of broken hair shafts is performed using a weight measuring method or by counting the number of individual broken hairs. However, the results from these biophysical in-vitro models might not be relevant to real-life situations in which many distinct consumer grooming habits and behavioral practices adversely affect the integrity of the hair. The trichometer offers clinicians an opportunity to quantitatively measure the severity of hair damage due to breakage and the response of the hair to various products in vivo.16–20
Lipids are considered to be major determinants of the physical properties of hair, with the total amount in scalp hair being generally around 10 percent of the weight of the hair.21 Lipids contribute to the conditioning, defense, integrity, anti-aging, and maintenance of healthy hair. The normal function of scalp sebaceous glands is to produce and secrete sebum, a group of complex oils consisting mostly of triglycerides, fatty acids, wax esters composed mainly of palmitic acid, and squalene.22–25 In humans, wax esters and squalene are emollients that provide lubrication and are uniquely sebaceous. Squalene also acts as a potent antioxidant.26, 27 The progressive loss of lipids, which tends to occur from the root to the distal end, due to normal weathering and grooming practices, contribute to a significant decrease in tensile properties of hair.28 Hair tends to break more easily at its distal end with longitudinal splitting, which leads to the common phenomenon of “split ends.” Sebum protects hairs from weathering and could also assist in repairing damaged hair by smoothing flaws, such as fragmented cracks and split ends.
Application of naturally occurring oils from plants and fruit seeds has been known for centuries to help smooth, soften, shine, and otherwise condition human hair, leading to a healthy attractive appearance. The bioactive substances in oils that confer beneficial functional properties, such as moisturizing, conditioning, anti-inflammatory, and antioxidant activity, are largely contained within the non-saponifiable (non-soap forming) lipid (NSL) fraction of the oil.29 Although most oils on the market have undergone chemical refinement methods that significantly reduce their levels of natural bioactive components, hair care oils from plants and fruits are purported to be effective and are still in wide use today in a large number of cosmetic hair care products.
Laboratory research studies have shown that due to their physical and chemical composition, certain oils exhibit substantially stronger affinities for hair and better capacities to penetrate human hair.30-34 In contrast with large polyunsaturated fatty acids that do not penetrate well but are able to coat the hair providing slip, small saturated fatty acids are more capable of penetrating and strengthening the hair shaft. This unique property, primarily seen with highly saturated oils, is very desirable since it reduces the swelling of the hair fibers, and thus provides an optimum beneficial effect on the hair. Therefore, certain oils should perform better than others in their ability to lubricate, waterproof, and strengthen hair, thereby minimizing breakage while providing protection from self-inflicted damage and environmental assault. In view of these findings, there exists a need to test the clinical efficacy of hair oils and compare their effects on preventing hair damage due to breakage.
Synsepalum dulcificum seed oil, commonly known as Miracle Fruit Seed Oil® (MFSO®) (Miracle Fruit Oil Company, Miami Beach, Florida), is a rare and exotic fruit oil derived from the seed of the miracle fruit (MF) berry.35 The MF seed constitutes the greatest portion of the berry by weight, and its lipids are mainly composed of palmitic acid, the major fatty acid found in sebum, with a balanced saturated/unsaturated fatty acid ratio of nearly 1:1. Due to its physicochemical properties, MFSO® is ideally suited to coat and penetrate the hair, thereby providing a superior ability to prevent hair damage and breakage.
Although many available hair oil treatment products claim to provide patients with healthier looking, stronger hair that resists breakage, few well-controlled clinical studies have been conducted that demonstrate the significant benefits of the specific active oil. The objective of this preliminary clinical study was to assess the safety and efficacy of a hair oil containing MFSO® as compared with its vehicle formulation and a leading commercially available argan oil brand on its ability to measurably reduce hair breakage in a population of healthy long-haired women with excessively damaged hair.
Methods
Study design. An eight-month, double-blind, placebo-controlled, randomized clinical study was performed to assess the efficacy of a hair oil formulation containing MFSO in comparison with a placebo in terms of the ability to influence the percentage of broken and unbroken hairs in adult long-haired women with excessively damaged hair. At each of the three study visits, hair breakage evaluations were performed on study participants with the use of a quantitative trichometer measuring device.16 In addition, the perception of the efficacy and degree of satisfaction with the use of the study product were assessed by users through self-assessment questionnaires.
Ethical conduct and consent. All clinical study procedures were approved by the relevant institutional review board for the protection of human subjects, and the study protocol was conducted in accordance with the Declaration of Helsinki. Before any study procedures were performed, the experimental procedures and risks/benefits were discussed with the subjects, who provided written informed consent.
Subjects. The study recruited and enrolled long-haired (defined as length of 12 inches or longer) women with hair damage aged 18 to 65 years old. At screening, subjects completed questionnaires that would provide investigators with information on their medical and hair health history and demographics. Each subject’s hair length, styled layer length, average hair diameters, Hair Mass Index (HMI), Hair Breakage Index (HBI), and Healthy Hair Index (HHI) readings were measured, calculated, and recorded.
To be eligible for enrollment, all female subjects had to meet certain inclusion/exclusion criteria at the time of screening. An attempt was made to disqualify subjects who might have partial hair regrowth following an episode of telogen effluvium due to the potential for a false positive (excessive breakage) trichometer reading.16 Exclusion comprised the following:
- Pregnancy or willingness to become pregnant during the study
- Having an episode of hair shedding in the past six months
- Being in the 12-month period following childbirth
- Being in the 12-month period following the discontinuation of a contraceptive drug
- Having a history of shampooing hair less than three times a week as part of their current hair care regimen
- Taking a prescription or over-the-counter (OTC) medicine that could significantly affect the study outcome within the past month
- Willing to take any new prescription drugs or OTC products that could significantly affect the study outcome;
- Willing to have their hair cut more than three inches in length;
- Willing to discontinue shampooing hair on different days at least three times a week
- Willing to continue the use of their current hair oil, if any
- Willing to substantially change their diet or daily routines
- An illness with fever in the past month
- A history of a scalp or hair loss disorder, such as alopecia, in the past 12 months
- A history of greater than 20 percent weight loss in the past 12 months
- A history of nutritional deficiency or being on a new diet in the past six months
- A history of a thyroid problem
- A history of medical or surgical event in the past year that may significantly affect the study outcome, including cardiovascular disease, metabolic, renal, hepatic, or musculoskeletal disorders
- A history of depression or severe anxiety in the past six months
- Participating in another clinical trial or taking an investigational product in the past three months.
Inclusion criteria comprised the following:
- A hair length measurement of at least 12 inches
- A hair length measurement of at least 10 inches of the shortest layer if hair styled in a layered cut
- Evidence of significant hair breakage as defined by a HBI75 calculation of greater than 30 percent (subjects with negligible hair breakage generally have values less than 15%) as assessed with trichometer taken from a hair bundle located at a hair length of 75 percent from the scalp to the distal tips of hair.
Product. The miracle fruit seeds were secured from local growers in Africa and the MFSO was extracted using standard supercritical CO2 fluid extraction methods.36 The unfractionated and unrefined virgin MFSO obtained directly from the extraction methods used, considered the highest quality grade of oil without undergoing any physical or chemical modification, was the type of oil used in the clinical study. The hair oil treatment product containing Synsepalum dulcificum seed oil (MFSO) and the vehicle control containing no MFSO were formulated in a liquid silicone base and produced in the United States for the Miracle Fruit Oil Company (Miami Beach, Florida). A commercially available leading brand of hair oil treatment containing Argania spinosa kernel oil (argan oil) in a liquid silicone base was also utilized as a control. All hair oil treatment samples were received blinded at the study site, bottled (4oz) under code, stored at ambient humidity and temperature, and dispensed to study subjects using a random allocation sequence.
As part of the study, the MFSO was separated into fractions and the NSL fraction underwent laboratory investigations to identify the presence of certain classes of bioactive components that could be beneficial for hair care. Reference standards and other reagents for these studies were purchased from Sigma-Aldrich (St. Louis, Missouri) and Thermo Fisher Scientific (Waltham, Massachusetts). The extraction of the NSL fraction of the MFSO and the thin layer chromatography (TLC) separation were performed as previously described with slight modification.37 Briefly, the extracted NSL fraction of MFSO was separated using TLC on 20cm x 20cm plates coated with 0.3mm silica. After development, the spots were visualized with iodine-vapor staining for 20 minutes at 50°C.
Treatments. The clinical study to determine the effects of the investigational hair oil treatment containing MFSO on its ability to increase the amount of unbroken hairs in long-haired women was designed as a single-center, randomized, double-blind, placebo-controlled, parallel design, institutional review board-approved study. Participants were seen and evaluated by staff on three visits during the study: at an initial screening/baseline pre-treatment visit (Visit 1), after four months of treatment (Visit 2), and after eight months of treatment at the end of the study (Visit 3). The study participants were randomly assigned a treatment using a web-based research randomizer (http://randomizer.org).
At baseline, subjects that met the inclusion/exclusion criteria were enrolled into the clinical study and were randomly assigned to treatment arms in a 1:1:1 manner, respectively, to the following three hair oil treatment groups: Group 1 (hair oil treatment containing MFSO); Group 2 (identical vehicle to that of Group 1 but with no MFSO); and Group 3 (commercially available argan oil brand).
The subjects that enrolled and received study product were instructed to 1) continue their current hair care regimen, 2) discontinue their use of any hair oils, 3) wash their hair at least three times on different days each week, 4) apply the hair oil study treatment each time after washing their hair to damp hair without rinsing it off, 5) immediately report the occurrence of any adverse event to the staff and temporarily discontinue the use of the hair oil treatment product until re-evaluated, 6) complete a diary log form to document adherence with the weekly use of the hair oil study treatment product and to record all adverse events, and 7) immediately report the occurrence of a pregnancy to the staff and temporarily discontinue the use of the hair oil treatment product.
All subjects who received the study product were provided with written and oral instructions and with a demonstration on the correct method of application of the hair oil treatment study product. Briefly, based on the hair length, subjects were told to pump a coin-sized amount of study product on their palm, rub their palms together, and thoroughly stroke the hair oil treatment product throughout their entire hair from the hair root just above the scalp to the ends of the hair. The first week of product use required a trial-and-error period to identify the correct amount needed to obtain the perceived beneficial behavior of their hair. Once the satisfactory amount of product to use was established, subjects were told to continue to apply the same amount each time and enter the amounts of pumps per application into their diary log. In addition to the weekly reporting of adherence and adverse events with the use of the study treatment product in the diary log form, subjects were also asked to note the frequency of study product use, the use of physical and chemical agents, and the methods of drying their hair. Subjects were encouraged to maintain their normal daily hair care grooming routines during the study, excluding the use of hair oils.
During the two treatment visits, the subject diary log and questionnaire forms were collected and reviewed for documenting adherence with the use of the study product, the reporting of any adverse events, the development of any medical events, the description of their hair care grooming routine, and other comments. Then, safety evaluations were conducted and the subjects were asked to report any adverse events. Additionally, any changes in the use of the concomitant medications related with the treatment conditions were documented. Finally, the bottles of hair oil treatment product were inspected for signs of use.
Efficacy assessments. During all study visits, the participants were subjected to hair tests that included measurements of hair length, shortest styled layer length, hair diameter, and HMI with the use of the trichometer.
Hair diameter was measured using a standard digital micrometer (0.001mm; Mitutoyo Corp., Kawasaki, Japan). Ten hairs from a distant site on the occipital scalp region were cut (or combed/brushed off by the subject) and their diameters were measured. The highest and lowest readings among the 10 hairs were discarded and the remaining eight hair readings were used to calculate the average diameter.
Serial hair breakage assessments were performed on subjects at the same mid-scalp site using the trichometer as previously described.16 At each study visit, subjects had three separate HMI measurements performed on the same bundle of hair at three different locations moving from proximal to distal along its length. The first HMI measurement, designated the proximal, was obtained at two inches from the scalp, the second and third HMI measurements were taken distally from the scalp at a distance of 50 percent and 75 percent, respectively, along the total length of the hair bundle. For example, if the hair length was 16 inches, then the proximal measurement was completed at two inches, the 50-percent measurement was completed at eight inches, and the 75-percent measurement was completed at 12 inches from the scalp. At each visit, each participant’s HBI was calculated to determine the percentage of broken hairs at 50- and 75-percent hair length distances, respectively, according to the following formula: HBI=([HMI proximal – HMI distal]/HMI proximal) x 100%.
The HHI was defined as the inverse of the HBI (i.e., 1/HBI) and provides a measure of the amount of healthy unbroken hairs. Given this reciprocal relationship, as the HBI decreases, the HHI increases (i.e., a favorable outcome) and as the HBI increases, the HHI decreases (i.e., an unfavorable outcome). The HHI values usually range from 1 to 20 (HBI range from 99% to 5%) with higher values denoting greater amounts of healthy unbroken hairs. Typical HHI values are generally greater than 6.7 (HBI at 15%), with values less than 3.3 (HBI at 30%) considered low and below 2.5 (HBI at 40%) as very low. To determine the quantitative effects of the hair oil treatment on its ability to protect hairs from breaking and to improve the overall health of the hair, the percent improvement in the HHI was calculated by comparing the HHIs over time among the three study visits (baseline to Month 8, baseline to Month 4, and Month 4 to Month 8). For example, the percent improvement in the HHI75 between baseline and Month 8 (the end of the study) was calculated as: ([HHI75 at Month 8 – HHI75 at Baseline]/HHI75 at baseline) x 100%.
At entry, the subjects completed a self-assessment hair health questionnaire reporting their medical history, current hair care grooming techniques, and product use. Subjects were also asked to complete a hair satisfaction questionnaire containing 13 questions and to provide a rating for their level of satisfaction with their hair characteristics (e.g., degree of damage, breakage, hair loss, ease of combing, presence of fly-away, tangles). At Month 4 and Month 8, in addition to noting any changes that occurred in the medical history or in grooming habits and product use since the beginning of the study, subjects were asked to recomplete the hair satisfaction questionnaire and rate their present level of satisfaction with their hair characteristics. Results were interpreted by comparing the change from the baseline score, with a higher score indicating a more favorable response. At Month 8, subjects completed an additional questionnaire rating the performance of the hair oil treatment product among three categories that included increases in unbroken hairs (e.g., reduction in hair breakage/split ends), hair cosmetic properties/qualities (e.g., shine, softness, body, density, manageability, ease of combing), and overall product performance.
Study endpoints. The primary efficacy endpoints were the percent change in HHI75 and HHI50 values from baseline to Month 8 (the end of the study). The secondary efficacy endpoints comprised the percent change in HHI75 and HHI50 values from baseline to Month 4 and from Month 4 to Month 8, and the overall clinical response and product satisfaction as assessed by the subject’s questionnaires.
Statistical analysis. All data were collected in a blinded fashion by the clinical investigator and given to the nonclinical investigator for transmission to an independent statistician for unblinding and data analysis. The primary analysis was conducted on the percent improvement in HHI50 and HHI75 values from baseline to Month 8, where improvement was defined as a favorable change from baseline. A negative improvement implied a worsening from baseline. The same analysis was performed on hair diameter, HBI50, and HBI75 values. Secondary analyses included percent improvement from baseline to Month 4 and percent improvement from Month 4 to Month 8.
For these variables, the MFSO treatment group was compared in a pair-wise manner to each of the two control groups using an analysis of variance-based t-test. To account for multiple comparisons, a step-down Bonferroni adjustment was used to control the type I error (alpha). For the two pair-wise tests, the largest difference in means was tested first at the 0.05/2 (=0.025) level. If the first test was significant, then the second largest difference in means was tested at the 0.05 level.
Subject and product satisfaction questionnaire data were summarized by treatment group by presenting the mean score (i.e., the higher, the more favorable) across all questions, or for a given category of hair characteristics. No statistical testing was performed on questionnaire data. Study data were analyzed using SAS® software version 9.4 (SAS Institute, Cary, North Carolina).
Results
Subject disposition, demographics, and hair characteristics. Sixty-nine healthy long-haired women who met the entrance criteria were enrolled and randomized into the study. Twelve subjects voluntarily withdrew from the study prior to their first treatment visit (Month 4). A total of 57 women (age range: 19–63 years; mean age: 36.7 years; SD: 10.77 years) with perceptible hair damage completed at least one treatment visit (MFSO, n=24; vehicle, n=17; argan oil, n=16). From this group, one subject failed to return for their final treatment visit, resulting in a total of 56 participants who completed the study. Reasons for early study termination included loss to follow-up and nonadherence.
Subject demographics, hair anatomy, and hair care characteristics for each group at baseline showed a homogeneous and representative distribution of all the variables among the three treatment groups. The ethnic groups were white non-Hispanic (n=24), Hispanic (n=19), Black (n=8), and Asian (n=6). Subjects had an average hair length of 18.6 inches. The hair calibers observed were coarse (n=23), average (n=17), and fine (n=17), and the curvature patterns seen were wavy (n=22), straight (n=20), curly (n=11), and kinky (n=4). All subjects complained of excessive hair damage associated with broken hairs, and a majority (80.7%) claimed to have noticeable hair loss. Twenty-four subjects (42.1%) reported the use of a commercial hair oil product within the previous six months as part of their hair care regimen. All subjects confirmed that they participated in hair care practices that are known to damage hair. Eighty-five percent of subjects reported using at least one of five chemical (bleach, coloring agent, perm, straightener, and fixative) and one of three physical (rubbing hair to dry, using a hairdryer, and using a hot iron) grooming treatments. Four or more of these eight grooming practices were routinely used by 49.1 percent of the subjects. Subjects with a baseline HHI75 value of less than 2.5 used an average of 3.6 grooming treatments versus an average of 2.9 in subjects with a HHI75 value greater than 2.5.
Self-reported adherence to the study product (percentage of diary-logged weeks on which the study hair oil treatment was used) and the continued use of hair grooming modalities that damage hair were uniformly high among all subjects across all three of the treatment groups (>90%). No subject reported any adverse reaction with the use of any of the hair oil treatments that were provided for the study.
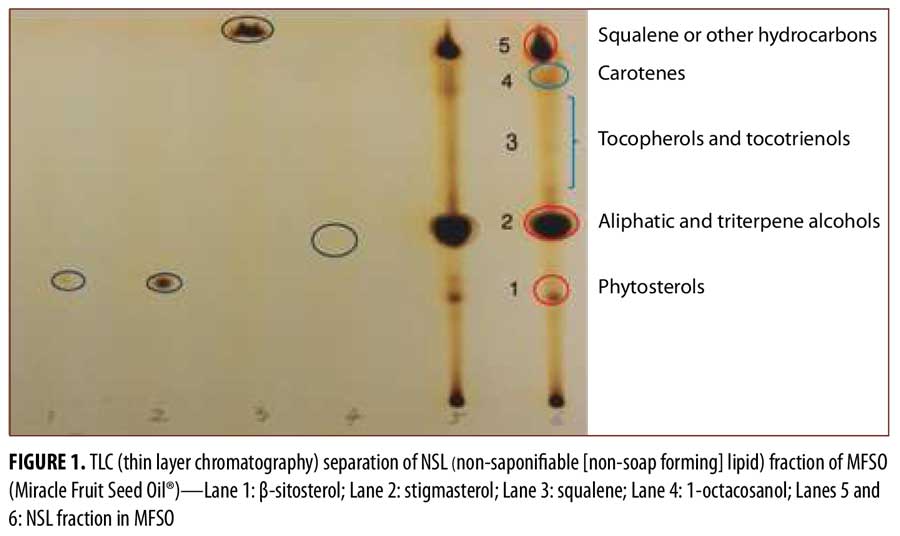
MFSO components. The content of the NSL fraction within the MFSO was 20.5±1.3g/100g (mean±SD, n=3). Figure 1 shows the TLC analysis from the NSL fraction of the MFSO. Five spots were identified with retardation factor (Rf) values that matched the locations for 1) phytosterols, 2) aliphatic and triterpene alcohols, 3) tocopherols/tocotrienols, 4) carotenes, and 5) squalene and other hydrocarbons. The size of the spots suggested that the three major classes of components were aliphatic/triterpene alcohols, squalene/hydrocarbons, and phytosterols.
Instrumental hair measurements. The instrumental hair measurements with calculations for each treatment group at baseline are shown in Table 1. There was a homogeneous and representative distribution of all variables among the three treatment groups. There were no clinically remarkable changes in hair diameter measurements among all subjects during treatment. Thirteen subjects (22.8%) with very fine hair (defined as a hair diameter <50µm) had a 10.8-percent higher level of hair breakage (HBI75) on average in comparison with subjects with hair diameters greater than 50µm. These subjects were homogeneously distributed among the groups, and no association was observed with response to treatment.
At baseline, all participants had very high amounts of hair breakage and low levels of healthy unbroken hairs as demonstrated by the HBI75/HHI75 and HBI50/HHI50 calculations from the HMI measurements obtained with the trichometer. The baseline HBI75/HHI75 and HBI50/HHI50 values ranged from 31.0 to 68.5 percent/3.2 to 1.5 (mean: 47.9%/2.2 and SD: 10.48%/0.52; normal: <15%/> 6.6) and from 13.1 to 45.3 percent/7.6 to 2.2 (mean: 26.2%/4.13 with SD: 7.39%/1.25; normal <10%/> 10), respectively. Treatment groups were balanced with respect to baseline hair breakage measurements, ethnicity, hair caliber, hair curvature, hair length, and prior use of hair oils.
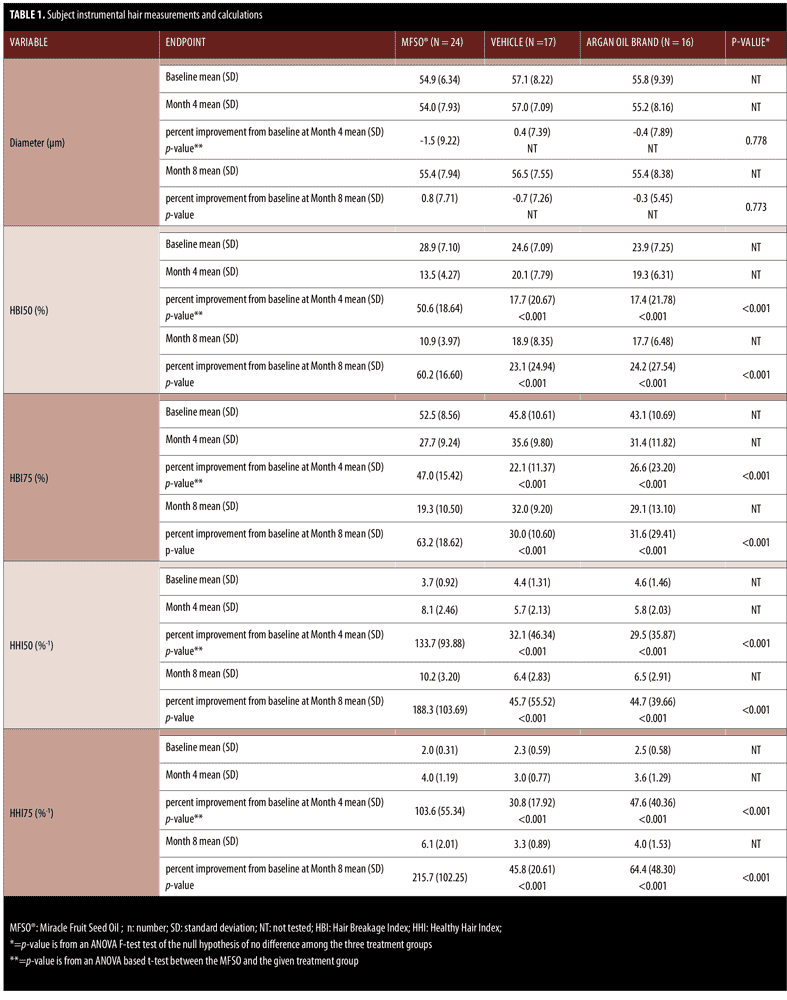
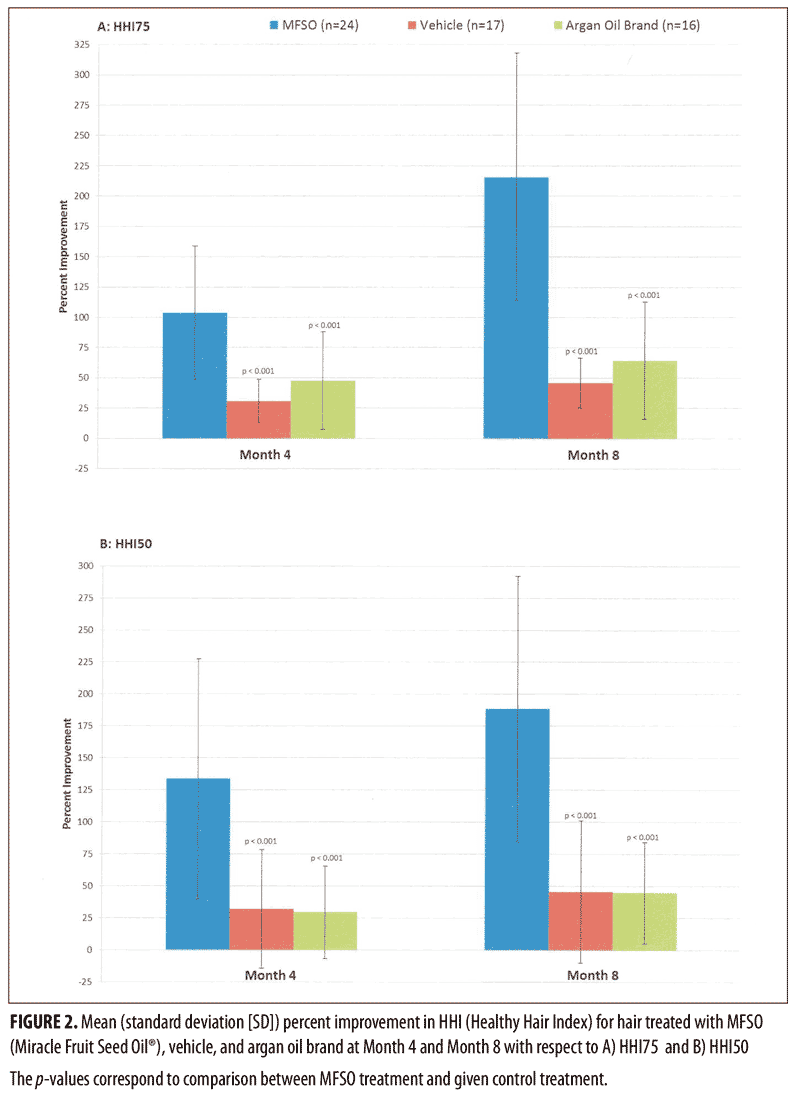
Figure 2 shows the mean (SD) percent improvement in the 1) HHI75 and 2) HHI50 values derived from the measurements obtained with a trichometer among the three treatment groups from baseline to Month 4 and from baseline to Month 8 (the primary efficacy endpoint at the end of the study). With respect to the mean percent improvement from baseline at Month 4 and from baseline at Month 8, the MFSO treatment group improved by 103.6 percent and 215.7 percent for the HHI75 and 133.7 percent and 188.3 percent for the HHI50 values, respectively. At both treatment visits, the HHI calculations, expressed as percent change from baseline, were significantly higher (p<0.001) for the MFSO hair oil treatment group as compared with the non-MFSO vehicle and the argan oil brand, thus indicating a much greater ability to increase the levels of unbroken hairs by reducing hair breakage. With respect to the mean percent improvement from baseline at Month 4, from Month 4 to Month 8, and from baseline to Month 8, the MFSO treatment group showed better results than each of the other two treatment groups by at least 117.6 percent (56 percentage points [pp]), 409% (47.4pp), and 234.9 percent (151.3pp) for the HHI75 and 316.5 percent (101.6pp), 165 percent (20.3pp), and 312 percent (142.6 pp) for the HHI50 values, respectively.
After four and eight months of treatment, all subjects treated with the MFSO® exhibited an increase in their levels of unbroken hairs from baseline as shown with the calculated HHI values. In contrast, one subject in the vehicle group and two subjects in the argan oil brand treatment group had a reduction in the amount of unbroken hairs, indicating a worsening effect during treatment. A large segment of the MFSO-treated subjects had a return to “normal” levels of unbroken hairs at the completion of the study; specifically, 11 subjects (46%) had a HHI75 greater than 6.7 and 12 subjects (50%) had a HHI50 greater than 10. In comparison, in the vehicle-treated group, no subject returned to normal HHI75 levels and two subjects (12%) returned to normal HHI50 levels. In the argan oil brand group, one subject (6%) returned to normal HHI75 levels and one subject (6%) returned to normal HHI50 levels. Furthermore, at the completion of the study, 20 subjects (83%) treated with the MFSO® had an HBI75 below 30 percent, and thus would no longer have met the inclusion criteria for enrollment into this study. In contrast, eight subjects (47%) in the vehicle-treated group and 10 subjects (62%) in the argan oil brand group would have been ineligible based on this criterion.
Apart from this randomized study, six additional subjects who did not participate in the study, but who had similar entry criteria for the study, were simultaneously followed and assessed with no treatment according to the study timelines. This exploratory analysis was conducted to observe the typical variability in hair health over time. In these six subjects, the changes in hair health indicators and breakage were unremarkable after four and eight months.
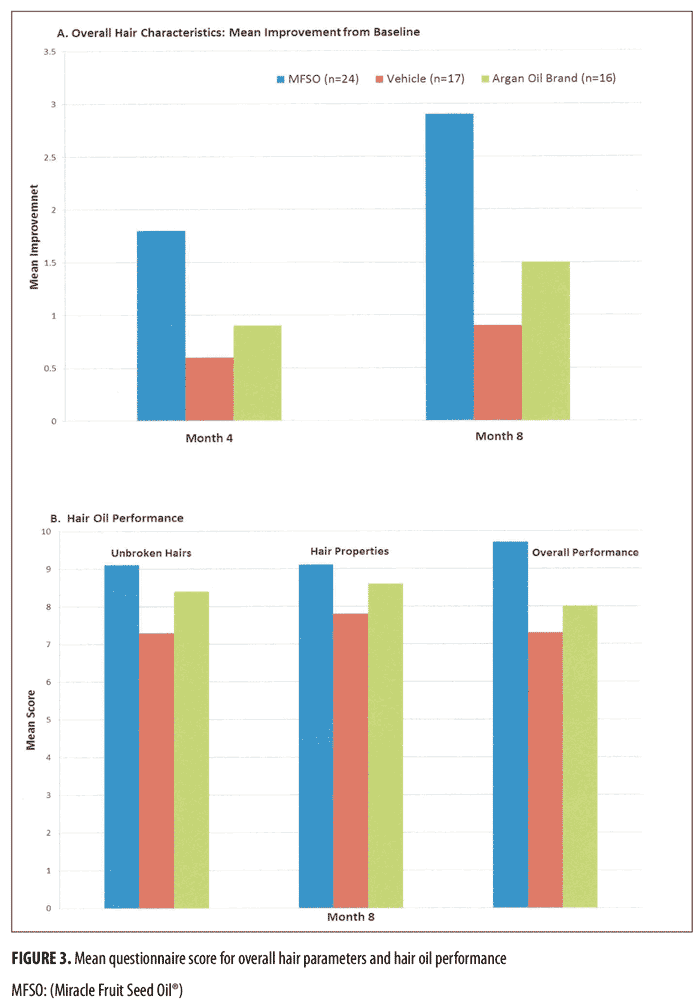
Self-assessment. Figure 3 shows the mean rating scale scores from the subject and product satisfaction questionnaires among the three treatment groups during treatment. The MFSO hair oil treatment group had a more favorable response than either of the other treatment groups with respect to the overall improvement in their hair characteristics at Month 4 and Month 8, respectively. A total of 15 subjects (26.3%) reported no visible hair loss due to breakage at the completion of the study; this number can be stratified as 10 subjects (41.7%) in the MFSO group, two subjects (11.8%) in the vehicle group and three subjects (18.8%) in the argan oil brand group. At Month 8, the MFSO hair oil treatment group also reported a greater level of satisfaction with the performance of their hair product than did individuals in either of the other treatment groups across all hair categories in the questionnaire.
Discussion
This is the first clinical trial to demonstrate the safety and efficacy of a hair oil containing MFSO for the treatment of broken hairs in long-haired women with severely damaged hair. This was also the first randomized, placebo-controlled, double-blind clinical study that compared the efficacy of a hair oil to its vehicle and a leading brand of commercially available hair oil. In this preliminary study, the group of women that used the MFSO hair oil product at least three times a week on different days for a period of eight months experienced a greater improvement by reducing breakage and increasing their amount of unbroken healthy hairs (HHI as measured by the trichometer) from baseline during treatment and significantly (p<0.001) outperformed subjects using only its vehicle and the argan oil brand. This beneficial result occurred as early as Month 4 and was long-lasting, with continued improvements observed at Month 8, thus achieving the primary and key secondary efficacy endpoints. Furthermore, the self-assessments revealed that a much greater percentage of subjects in the MFSO group reported no visible hair loss due to breakage at the completion of the study as compared with subjects in the vehicle and argan oil brand groups. Subjects also favored the MFSO hair oil treatment on almost all tested attributes, rating it as effective and aesthetically pleasing, with the majority noting that they would purchase the product. As expected, subjects who used the vehicle containing silicones or argan oil brand also showed improvements in their HHI50 and HHI75 values from baseline to Month 8. However, their levels of improvement were statistically inferior compared with those of the MFSO hair oil product group. At Month 8, the average improvements in HHI75 of the MFSO-treated group were nearly fivefold and threefold higher than in subjects using the vehicle or argan oil brand, respectively.
Hair loss due to hair breakage is a very common complaint among the general population and is seldom addressed by the dermatologist. Although mainly a cosmetic concern, patients might seek treatment based on dissatisfaction with their appearance. Treatments that are clinically proven effective would be expected to have a positive impact on the self-image of the individual. Many hair care products have been manufactured and cosmetic claims made in an attempt to help consumers reduce their levels of hair breakage. However, there exists a scarcity of clinical data from well-designed studies demonstrating that these products have any significant benefits. It is important for the practicing dermatologist to have clear evidence-based results that the hair product has the actual effect promised. These results are valuable for the dermatologist to make informed product recommendations to their patients.
MFSO has similarities in chemical composition to the oil made by the sebaceous glands in our scalps and contains lipids that naturally waterproof, lubricate, moisturize, strengthen, protect, and restore hair from damage.35,38,39 This naturally derived fruit seed oil has only just begun to be clinically tested.40 Its mechanism of action is likely due to the balanced ratio of saturated/unsaturated fatty acids that imparts a double functionality to protect hair from damage by strengthening hair as a result of its penetration into the shaft and provision of external lubrication to reduce friction and water pickup. MFSO might act to restore hairs from damage by sealing and smoothing the cuticle cracks and other flaws that occur along the damaged hair shaft. Furthermore, MFSO also contains a number of bioactive substances that might play a role in limiting the development and progression of hair damage.35,41 For example, the triterpene alcohols have anti-inflammatory activity, and squalene acts as an emollient with a potent antioxidant activity capable of protecting hair from environmental insults such as ultraviolet radiation.26,27,42,43 By preventing refinement methods like bleaching and deodorization, these valuable bioactive components are fully preserved. At greater than 20 percent of its weight, the bioactive-rich NSL content of the unrefined MFSO is the highest recorded for a fruit seed (higher than unrefined shea butter at 6–17%). In addition, MFSO also contains among the highest content of the combination of palmitic acid (42%) and squalene (1.5%), two characteristic components found in high amounts in human sebum. Argan oil was chosen as the comparative hair oil treatment due to its popularity in the commercial hair care market. When comparing the amounts of components that might play a role in impacting hair breakage, it was not surprising that MFSO performed better than argan oil since it has a more beneficial profile with a 1) a saturated/unsaturated free fatty acid ratio of 1:1 versus 1:4, 2) a palmitic acid concentration of 42 versus 12 percent, and 3) a squalene concentration of 1.5 versus 0.3 percent.44
The current laboratory methods used for the evaluation of products that affect hair breakage have limitations and might not be a reasonable representation of real-life conditions, given the many variables associated with intrinsic hair aging and the multiple environmental insults that affect hair (e.g., grooming practices, exposure to ultraviolet rays, pollution).
The ability of a hair oil product to beneficially affect hair in vivo might be associated with factors that influence its coating or penetration into the hair shaft, such as the product application, shampooing frequency, and degree of hair damage. For example, the application of oil without shampooing provides more time for the deposition of oil to persist on hair, enhancing its protective coating ability. In addition, it might be easier for hair oils to penetrate and seal chronically damaged broken hairs due to the pre-existing lifts or cracks in the cuticle. Of note, the majority of the published in-vitro studies performed their assessments using normal healthy hairs and not damaged hair, which might more closely mimic real-life conditions.
There are no well designed clinical studies addressing the anti-breakage efficacy of hair oils and comparing them with their vehicle or other hair care product brands. Therefore, the anti-breakage claims among hair oil products in the market have not been clinically substantiated. The trichometer is a convenient and useful tool to clinically assess changes in hair mass caused by hairs breaking, making it possible to objectively evaluate the efficacy of a hair oil product over time.18 Subjective measures such as investigator assessments or the comparison of “before and after” global photographs were not utilized due to their limited value in the evaluation of hair breakage. Subjects were treated for an extended period of eight months to document evidence of sufficient long-term therapeutic improvement. The results of this study showed that the benefits of using the hair oil treatment containing MFSO were long-lasting and incremental. The improvement in hair breakage as evidenced by the beneficial increase in the levels of unbroken hairs (HHI) was seen over 4 to 8 months of treatment in subjects exhibiting high levels of hair breakage who continued using grooming practices that are known to damage hair. In addition, as a group, subjects with very fine hairs with an increased propensity for hair breakage also benefited. These findings are encouraging and indicate that subjects have the potential to reverse hair breakage and normalize their levels of unbroken healthy hairs despite their level of severity, small hair diameter, and the continuation of damaging hair care habits. The degree and speed of HHI normalization could possibly be accelerated if the use of the MFSO is combined with a change in behavior associated with the implementation of healthier grooming practices.
Additional studies examining the use of MFSO in women and men with hair damage, the distinct benefits of other hair oils, and the effects of specific grooming practices on hair breakage should be areas of focused research studies in the future.
Conclusion
MFSO is safe and effective for the treatment of women suffering from hair breakage and damaged hair. MFSO is significantly more effective in reducing hair breakage and in increasing the amounts of unbroken hairs when compared with the use of its vehicle and a leading commercial argan oil brand.
Acknowledgments
The authors thank Bernard Cohen, MD, for editorial assistance and Liwei Gu, PhD, for technical assistance in the analysis of the lipids.
References
- Cohen B. Hair breakage: an underappreciated cause of hair loss in women. Hair Transpl Forum Int. 2008;18:95–96.
- Sinclair RD. Healthy hair: What is it?. J Investig Dermatol Symp Proc. 2007;12(2):2–5.
- Rook A. The clinical importance of ‘weathering’ in human hair. Br J Dermatol. 1976;95(1):111–112.
- Tolgyesi E. Weathering of hair. Cosmet Toilet. 1983;98:29–33.
- Dawber R. Cosmetic and medical causes of hair weathering. J Cosmet Dermatol. 2002;1(4):196–201.
- McMichael AJ. Hair breakage in normal and weathered hair: focus on the Black patient. J Investig Dermatol Symp Proc. 2007;12(2):6–9.
- Kamath YK, Robbins C. Hair breakage by combing and brushing—a comment on: T. A. Evans and K. Park, A statistical analysis of hair breakage. II. Repeated grooming experiments, J Cosmet. Sci., 41, 439-456 (2010). J Cosmet Sci. 2011;62(6):579–585.
- Lee WS, Oh TH, Jeon SY. Hair shaft aging. 2005;211:27.
- Trüeb RM. Aging of hair. J Cosmet Dermatol. 2005;4(2):60–72.
- Braida D, Dubief C, Lang G. Photoaging of hair fiber and photoprotection. Skin Pharmacol. 1994;7(1-2):73–77.
- Kim KS, Shin MK, Park HK. Effects of ultraviolet B radiation on physicochemical properties of human hair shaft. Microsc Res Tech. 2012;75(7):949–954.
- Gavazzoni Dias MF. Hair cosmetics: an overview. Int J Trichology. 2015;7(1):2–15.
- Robbins C. Hair breakage during combing. I. Pathways of breakage. J Cosmet Sci. 2006;57(3):233–243.
- Evans TA, Robbins C. Fatigue testing of hair-a statistical approach to hair breakage. J Cosmet Sci. 2009;60(6):599–616.
- Evans TA, Park K. A statistical analysis of hair breakage. II. Repeated grooming experiments. J Cosmet Sci.2010;61(6):439–455.
- Cohen B. The cross-section trichometer: a new device for measuring hair quantity, hair loss, and hair growth. Dermatol Surg.2008;34(7):900–910.
- Stough D. Commentary on the cross section trichometer: a new device for measuring hair quantity, hair loss, and hair growth. Derm Surg. 2008;34(7):910–911.
- Mhaskar S, Kalghatgi B, Chavan M, et al. Hair breakage index: an alternative tool for damage assessment of human hair. J Cosmet Sci.2011;62(2):203–207.
- Hendriks MA, Geerts PA, Dercksen MW, et al. Evaluation of Cohen’s cross-section trichometer for measuring hair quantity. Dermatol Surg.2012;38(4):631–634.
- Wikramanayake TC, Mauro LM, Tabas IA, et al. Cross-section trichometry: A clinical tool for assessing the progression and treatment response of alopecia. Int J Trichology. 2012;4(4):259–264.
- Robbins CR. Chemical and Physical Behavior of Human Hair. 4th Ed. New York, NY: Springer; 2013.
- Downing DT, Stewart ME, Wertz PW, et al. Skin lipids: an update. J Invest Dermatol. 1987;88 (3 Suppl):2s–6s.
- Thody AJ, Shuster S. Control and function of sebaceous glands. Physiol Rev. 1989;69:383–416.
- Ramasastry P, Downing DT, Pochi PE, Strauss JS. Chemical composition of human skin surface lipids from birth to puberty. J Invest Dermatol. 1970;54(2):139–144.
- Nikkari T. Comparative chemistry of sebum. J Invest Dermatol. 1974;62(3):257–267.
- Ohsawa K, Watanabe T, Matsukawa R, et al. The possible role of squalene and its peroxide of the sebum in the occurrence of sunburn and protection from the damage caused by U.V. irradiation. J Toxicol Sci. 1984;9(2):151–159.
- Lens M, Podesta Marty MH. Assessment of the kinetics of the antioxidative capacity of topical antioxidants. J Drugs Dermatol. 2011;10(3):262–267.
- Duvel L, Chun H, Deppa D, et al. Analysis of hair lipids and tensile properties as a function of distance from scalp. Int J Cosmet Sci.2005;27(4):193–197.
- Dabas D, Shegog RM, Ziegler GR, Lambert JD. Avocado (Persea americana) seed as a source of bioactive phytochemicals. Curr Pharm Des. 2013;19(34):6133-6140.
- Rele AS, Mohile RB. Effect of mineral oil, sunflower oil, and coconut oil on prevention of hair damage. J Cosmet Sci. 2003;54(2):175–192.
- Keis K, Persaud D, Kamath YK, et al. Investigation of penetration abilities of various oils into human hair fibers. J of Cosmet. Sci. 2005;56(5):283–295.
- Keis K, Huemmer CL, Kamath YK. Effect of oil films on moisture vapor absorption on human hair. J Cosmet Sci. 2007;58(2):135–145.
- Fregonesi A, Scanavez C, Santos L, et al. Brazilian oils and butters: the effect of different fatty acid chain composition on human hair physiochemical properties. J Cosmet Sci. 2009;60(2):273–280.
- Gode V, Bhalla N, Shirhatti V, et al. Quantitative measurement of the penetration of coconut oil into human hair using radiolabeled coconut oil. J Cosmet Sci. 2012;63(1):27–31.
- Guney, S, Nawar, WW. Seed lipids of the miracle fruit (Synsepalum dulcificum). J Food Biochem. 1977;1(2):173–184.
- Del Valle JM, de la Fuente JC. Supercritical CO2extraction of oilseeds: review of kinetic and equilibrium models. Crit Rev Food Sci Nutr. 2006;46(2):131–160.
- Lercker G, Rodriguez-Estrada MT. Chromatographic analysis of unsaponifiable compounds of olive oils and fat-containing foods. J Chromatogr A. 2000;881(1-2):105–129.
- Stewart ME, Downing DT. Chemistry and function of mammalian sebaceous lipids. Adv Lipid Res.1991;24:263–301.
- Nicolaides N. Skin lipids: their biochemical uniqueness. 1974;186(4158):19–26.
- Gorin S, Wakeford C, Zhang G, et al. Beneficial effects of an investigational wristband containing Synsepalum dulcificum (miracle fruit) seed oil on the performance of hand and finger motor skills in healthy subjects: A randomized controlled preliminary study. Phytotherapy Research. 2018; in press.
- Inglett GE, Chen D. Contents of phenolics and flavonoids and antioxidant activities in skin, pulp, and seeds of miracle fruit. J Food Sci. 2011;76(3):C479–C482.
- Akihisa T, Yasukawa K, Oinuma H, et al. Triterpene alcohols from the flowers of compositae and their anti-inflammatory effects. 1996;43(6):1255–1260.
- Okoye NN, Ajaghaku DL, Okeke HN, et al. Beta-Amyrin and alpha-amyrin acetate isolated from the stem bark of Alstonia boonei display profound anti-inflammatory activity. Pharm Biol. 2014;52(11):1478–1486.
- Khallouki F, Younos C, Soulimani R, et al. Consumption of argan oil (Morocco) with its unique profile of fatty acids, tocopherols, squalene, sterols and phenolic compounds should confer valuable cancer chemopreventive effects. Eur J Cancer Prev. 2003;12(1):67–75.

