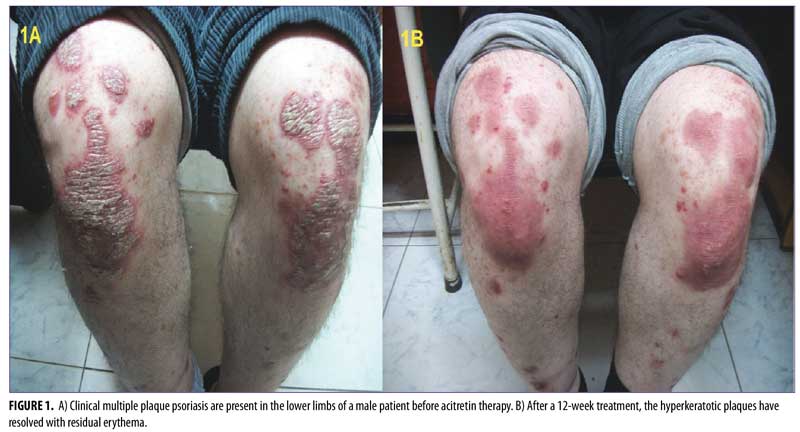
 by Mohamed H. Khater, MD; Mohamed Soliman, MD; Amin Amer, MD;
by Mohamed H. Khater, MD; Mohamed Soliman, MD; Amin Amer, MD;
Fatehy Khater, MD; and Manal R. Abd elhaleem, mD
Dr. M. Khater is Assistant Professor of Dermatology and Venereology, Dr. Amer is Assistant Professor of Dermatology and Venereology, Dr. Soliman is a Lecturer of Dermatology and Venereology, Dr. F. Khater is Professor of Parasitiology, and Dr. Elhaleem is Assistant Professor of Histology—all with Zagazig University in Zagazig, Egypt.
Funding: No funding was received.
Disclosures: The authors have no financial conflicts relevant to the content of this article.
Abstract: Psoriasis is one of the most common chronic inflammatory skin diseases, characterized by erythema and the formation of plaques. The diagnosis of psoriasis is based on clinical examination, and its severity is assessed by the Psoriasis Area and Severity Index. Histologic examination is still the standard method for the final diagnosis. Sonography has proved to be a suitable noninvasive imaging method for studying soft tissue in dermatologic diseases such as psoriasis. This study evaluated the effect of Doppler sonography in the assessment of psoriasis in comparison with histopathology. Clinical, multifrequency sonography, and histological examinations were completed in 30 patients with chronic plaque psoriasis before and after acitertin treatment. After a 12-week treatment period, there was a notable decrease of psoriatic plaques in 28 patients with Psoriasis Area and Severity Index-1 severity and in two patients with Psoriasis Area and Severity Index-2 severity. Multifrequency sonography results after treatment of the same plaques showed normal finding in five patients, mild sonographic changes in 24 patients, and moderate sonographic changes in one patient. Histopathology findings after treatment were normal epidermis and dermis in six patients, mild histopathological changes in 22 patients, and moderate changes in two patients. In conclusion, there were significant correlations between sonography and histopathology in the diagnosis and evaluation of a psoriatic skin treatment regimen.
Keywords: Multifrequency Doppler, psoriasis, psoriatic histopathology
J Clin Aesthet Dermatol. 2017;10(11):33–38
Introduction
Psoriasis is one of the most common immune-mediated chronic inflammatory skin diseases.1 The diagnosis of psoriasis is based on history and clinical examination, and its severity is assessed by the Psoriasis Area and Severity Index (PASI), a specific validated method for evaluating the extension of psoriatic lesions.1 However, the histopathology examination is still helpful in detecting the severity of psoriasis and for follow-up purposes during treatment.2,3
Sonography has proved to be a noninvasive imaging technique for studying affected soft tissue in patients with dermatologic diseases.4 The availability of sonographic equipment with high-resolution multifrequency sonography (i.e., higher than 10MHz) enables the detection of the character of the skin layers and allows accurate high-resolution imaging of the epidermis, dermis, and hypodermis. Furthermore, it is very sensitive for the visualization of the blood flow up to the dermal level.5–7 So, multifrequency sonography is an effective way to measure and monitor skin changes in patients affected by psoriasis.8,9
Acitretin is a systemic therapy for cases of severe psoriasis resistant to other systemic drugs or topical medication.10 Its mechanism of action is to regulate epidermal cell proliferation, differentiation, and cornification. It also stimulates normal differentiation of the epidermis. Acitretin is not a cytotoxic or immunosuppressive drug.11,12 Acitretin is safer than methotrexate in treating psoriasis, is easy to monitor, and is associated with good results.13,14 Acitretin is also cheaper than biological therapies for treating psoriasis in Egypt.
In this study, we examine the use of Doppler sonography as a noninvasive tool for assessing and monitoring treatment effects in psoriasis, compared histopathological method, among patients treated with acitretin.
Methods
Patients. Thirty patients (21 men and 9 women) were selected from the dermatology outpatient clinic at Zagazig University Hospital in Zagazig, Egypt. Their age varied between 25 and 50 years (mean: 37.5 years) and all had a clinical diagnosis of chronic plaque psoriasis. The disease duration ranged from 5 to 10 years. The study duration was from September, 2013 to April, 2014.
The inclusion criteria for this study were 1) age more than 20 years, 2) the presence of moderate-to-severe plaque psoriasis, and 3) a negative pregnancy test (for women). The exclusion criteria were 1) use of systemic methotrexate, 2) use of psoralen-ultraviolet A therapy, and 3) the presence of an active inflammatory skin disease or recent skin infection.
One month before the beginning of the study, any topical therapy was discontinued. Liver enzymes and fasting serum cholesterol and triglycerides were assessed in all subjects every two weeks during the two-month study period, and at the conclusion of the study. Blood sugar levels were also monitored at similar intervals in those subjects with diabetes who were on insulin or oral antiglycemic drugs before therapy.10
Patients. The relevant institutional ethics committee approved the study, and informed consent was obtained from all patients prior to performing skin biopsy and/or multifrequency sonography examinations.
The clinical, multifrequency sonography, and histological examinations were carried out at the Dermatology, Radiology and Histology Departments of Zagazig University, respectively. Patients who had multiple instances of plaque psoriasis were prescribed acitretin 25mg per day for 12 weeks.8 Typical plaques with scaling, erythema, and infiltration were chosen for photographs, multifrequency sonography, and histopathology. The same plaque from each patient was examined at the end of therapy to ensure adequate comparison within the study.
The PASI score, sonography, and histological examination findings were assessed and recorded for all patients at baseline and at 12 weeks after acitretin treatment.
Clinical assessment. All patients underwent a clinical examination and were scored using the PASI score. Each patient was given a PASI score using a semiquantitative 4-point scoring method (0=normal, 1=mild, 2=moderate, 3=marked); scores of 0 or 1 indicated psoriatic lesions covering less than 10 percent of the body surface area (BSA), a score of 2 indicated a BSA of 10 to 20 percent, and a score of 3 indicated a BSA of more than 20 percent.8
 Multifrequency sonography assessment. All patients rested for at least 30 minutes prior to superficial linear probe examination in order to stabilize the blood flow. Multifrequency sonography examinations were performed by an experienced sonographer, blinded to patient PASI score, using a superficial linear probe with a Doppler examination ultrasound system. The multifrequency sonography system (MyLab 70 XVG; Esaote Biomedica, Genoa, Italy) was equipped with a broadband frequency transducer ranging from 6MHz to 18MHz and a Doppler frequency ranging from 7MHz to 14MHz. At the focal area, the probe had an axial resolution of 30µm and a lateral resolution of 60µm. The fields of view in depth and laterally were 50mm and 60mm, respectively.4
Multifrequency sonography assessment. All patients rested for at least 30 minutes prior to superficial linear probe examination in order to stabilize the blood flow. Multifrequency sonography examinations were performed by an experienced sonographer, blinded to patient PASI score, using a superficial linear probe with a Doppler examination ultrasound system. The multifrequency sonography system (MyLab 70 XVG; Esaote Biomedica, Genoa, Italy) was equipped with a broadband frequency transducer ranging from 6MHz to 18MHz and a Doppler frequency ranging from 7MHz to 14MHz. At the focal area, the probe had an axial resolution of 30µm and a lateral resolution of 60µm. The fields of view in depth and laterally were 50mm and 60mm, respectively.4
The multifrequency sonography settings were standardized as follows:
High-resolution multifrequency 6MHz to 18MHz linear superficial array transducer grayscale slices and Doppler examination were performed. Psoriatic lesions were assessed by sonography as follows: the transducer was placed over the examined area directly perpendicular to the surface and a relevant quantity of gel was applied over the skin in order to provide a correct acoustic interface. No standoff pad was used.5,7
Sonography results were evaluated and graded on the basis of the 0 to 3 semiquantitative scoring already described. The dermis area with the highest expression of Doppler signal was used to score the psoriatic plaque.8
Histological assessment. In all patients, biopsies were performed using the same plaque examined by multifrequency sonography. In order to have more detailed information about epidermal and dermal changes, staining results were analyzed4; specifically the skin tissue sections were embedded in paraffin and stained with hematoxylin and eosin stain and examined by a histopathologist for epidermal and dermal changes. The results were rated using the 0 to 3 scoring by an experienced pathologist who was blinded to the clinical and sonography data.8
Statistical analysis. Statistical analysis was performed using MedCalc version 9.5.1 for Windows XP (Mariakerke, Belgium). Standard descriptive results were expressed as medians and 95-percent confidence intervals (CIs) for the median. Wilcoxon signed-rank test was used for the comparison of the findings at baseline and follow-up. Correlations between clinical, histological, and multifrequency sonography scores were analyzed by Spearman’s rank correlation test. P less than 0.05 was considered significant.8
Results
Thirty patients with psoriasis were involved this study and were treated with acitretin for 12 weeks. Histological and multifrequency sonography examinations were completed using the same psoriatic plaque at baseline and at the end of the 12-weeks study period for each patient.
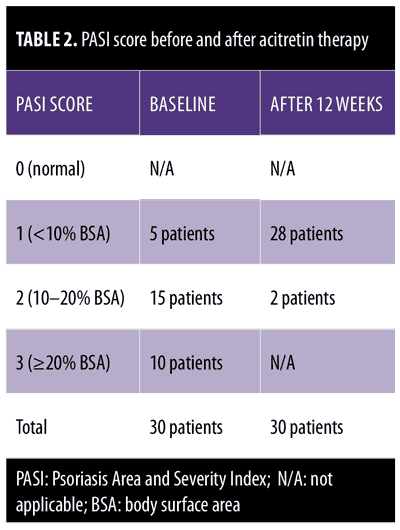
Clinical examination revealed five patients with a PASI score of 1, 15 patients with a PASI score of 2, and 10 patients with a PASI score of 3.
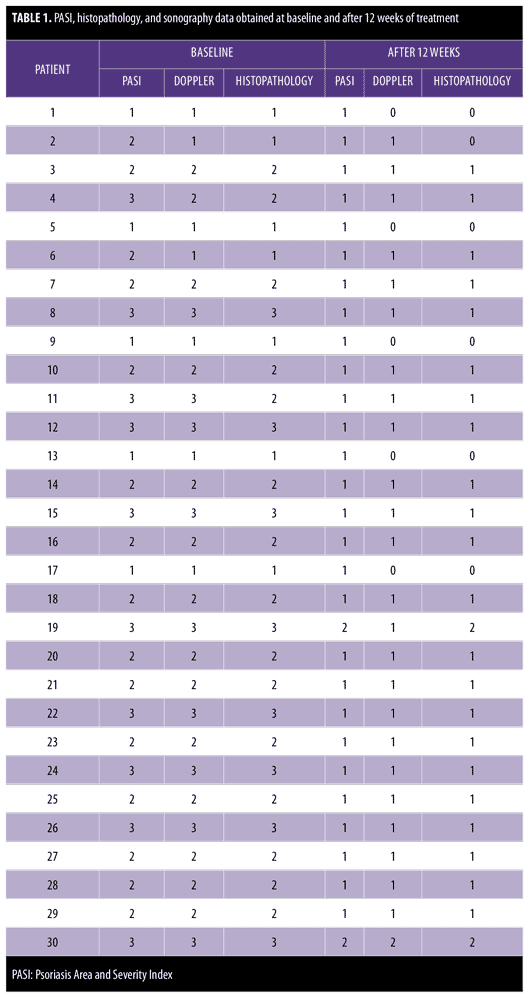
Table 1 shows the clinical (PASI), histopathology, and sonography data, obtained at baseline and after 12 weeks of treatment.
After 12 weeks of therapy, there was significant improvement in psoriatic plaques according to PASI score. The psoriatic plaques decreased in 28 patients, with a reduction to
PASI-2 (10–20% BSA) in two patients, a reduction to PASI-1 (<10% BSA) in 23 patients, and a reduction to PASI-0 (0% BSA) in five patients.
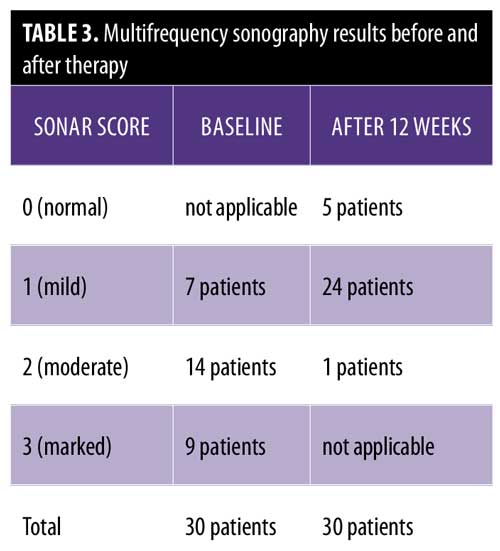
Multifrequency sonography examination before treatment revealed epidermal thickening and multiple dermal vessels. These Doppler findings were marked in nine patients, moderate in 14 patients, and mild in seven patients.
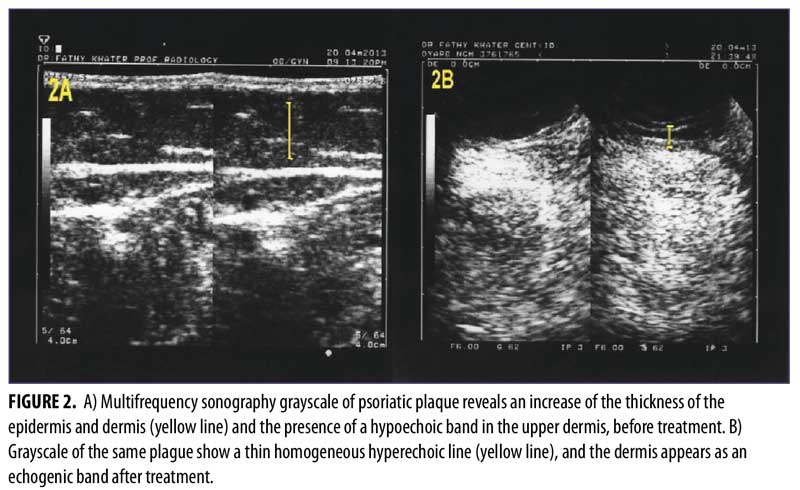
The multifrequency sonography results after 12 weeks of treatment of the same psoriatic plaques revealed significant improvement, with a decrease epidermal thickening and a decrease in dermal vasculature. Five patients showed normal sonographic epidermal and dermal findings, 24 patients showed mild sonographic improvement findings, and only one patient showed moderate sonographic improvement findings (Figure 2 and Table 3).
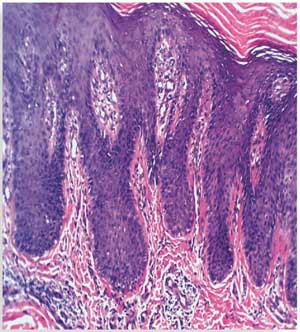
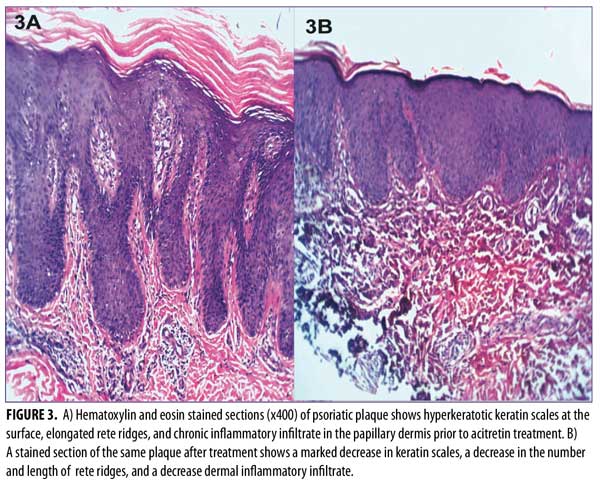
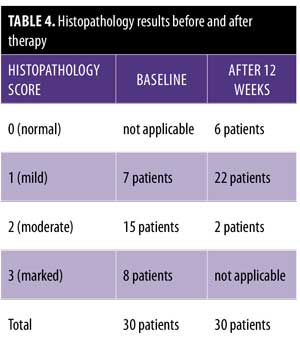
Histopathological results before treatment showed acanthosis, hyperkeratosis, and elongated rete ridges of the epidermis. The dermis showed inflammatory cells and microabscesses. These histopathological changes in the epidermis and dermis (marked hyperkeratosis dermal microabscesses) were marked in eight patients; moderate (slight epidermal hyperkeratosis and dermal inflammatory infiltrate) in 15 patients; and mild (epidermis nonspecific, dermal mild edema with inflammatory infiltrate) in seven patients.
After 12 weeks of therapy, there was significant improvement in the histopathological results. Six patients showed normal epidermis and dermis, two patients showed moderate histopathological improvement, and 22 patients showed mild histopathological improvement (Figure 3 and Table 4). Side effects that were noticed with treatment included dry lips in 15 patients, pruritus in five patients, epistaxis in one patient, and conjunctivitis in two patients. All of these side effects were reversible following drug discontinuation.
Discussion
The use of multifrequency Doppler with main grayscale and Doppler sonographic findings provides advanced evaluation of psoriatic plaque. The grayscale imaging aids in identifying epidermal and dermal thickening that results from high keratinocyte proliferation. Another common sonographic feature is a hypoechoic band in the upper dermis, which represents inflammatory edema and vasodilatation within the papillary dermis; this hypoechoic band corresponds with the clinically diagnosed palpable psoriatic plaque.15-17
The reduction of both the epidermal and dermal thicknesses and the disappearance of the hypoechoic band at the superficial dermis level were described as the grayscale sonographic indicators of effective therapy.4 However, the hypoechoic band is not definitively indicative of psoriasis, because it can also present in other inflammatory conditions, such as contact dermatitis and atopic eczema.8 In multifrequency Doppler examination, the findings of the grayscale sonography imaging of the epidermis and dermis are more important than the hypoechoic band in the upper dermis (because the hypoechoic band is not specific for psoriasis).8,18,19
In contact dermatitis, Doppler examination might show thickened dermis with homogeneous echotexture and foci of hypoechoic edema in comparison with normal skin.20 Doppler examination of cutaneous lymphoma in the diffuse form typically shows a hyperechoic area with poorly defined margins and an increase of the thickness of the dermis and the subcutaneous layers. In cases of nodular lymphoma, Doppler examination shows a hypoechoic coalescing solid nodule with ill-defined margins.21
Other imaging modalities such as computed tomography or magnetic resonance imaging require the use of an intravenous contrast medium, are limited to skin lesions measuring larger than 5mm, and are more expensive. Variable-frequency ultrasound scan, on the other hand, is capable of demonstrating lesions in the depth of 60mm with a single probe and up to 200mm with a combination of probes.7 Wortsman and Wortsman22 examined the diagnostic accuracy of ultrasound examination in various skin lesions as compared with clinical diagnosis. They found that skin ultrasound is sufficiently sensitive and specific.22 In our study, we chose to use multifrequency ultrasonography with a superficial probe as a diagnostic method for psoriasis.
In a study by Gutierrez et al,4 30 patients with a clinical diagnosis of psoriasis were assessed using a sonography system equipped with a variable-frequency transducer ranging from 6MHz to 18MHz. Gutierrez et al4 showed sonography to be effective in the evaluation and follow-up of psoriasis treatment, but the researchers did not use the PASI score in the evaluation of psoriasis. Also, they did not compare the sonographic results with the histopathology results of the same lesion, as was done in our study.
The clinical standard evaluation of psoriasis treatment is the PASI score, but histopathological assessment has both invasive and practical constraints,8 so multifrequency sonography represents an easy additional method for clinical evaluation and monitoring of disease activity and therapeutic effect. In our study, multifrequency sonography showed a significant correlation with histopathology and PASI score in the evaluation of psoriatic plaques before and after treatment. Our results indicate that multifrequency sonography is an effective method of monitoring and assessing treatment effect in psoriatic skin disease. Because it can be performed noninvasively, multifrequency sonography might be a more ideal method of monitoring psoriasis treatment for many patients compared to histopathology.
Limitation. This study did not have a placebo control, which limits the results.
Conclusion
In conclusion, multifrequency sonography provides a useful and easy method for monitoring disease activity in patients with psoriatic plaques. The highly significant correlation between the sonography findings and those obtained by both clinical and histological examinations supports the value of this inexpensive, noninvasive imaging technique. However, additional placebo-controlled investigations studying larger groups of patients are needed to further support these observations.
References
- Schon MP, Boehncke WH. Psoriasis. N Engl J Med. 2005; 352(18):1899–1912.
- Sabat R, Philipp S, Höflich C, et al. Immunopathogenesis of psoriasis. Exp Dermatol. 2007;16(1): 779–798.
- Zaba LC, Cardinale I, Gilleaudeau P, et al. Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J Exp Med. 2007;204(13):3183–3194.
- Gutierrez M, Wortsman X, Filippucci E, et al. High frequency sonography in the evaluation of psoriasis: nail and skin involvement. Ultrasound Med. 2009;28(11):1569–1574.
- Joshua F, de Carle R, Rayment M, et al. Power Doppler ‘blanching’ after the application of transducer pressure. Australas Radiol. 2005;49(3):218–221.
- Feldman SR, Krueger GG. Psoriasis assessment tools in clinical trials. Ann Rheum Dis. 2005;64 Suppl 2:ii65–68; discussion ii69–73.
- Goldberg I, Sprecher E, Schwartz ME, et al. Comparative study of high-resolution multifrequency ultrasound of the plantar skin in patients with various types of hereditary palmoplantar keratoderma. 2013; 226(4):365–370.
- Gutierrez M, De Angelis R, Bernardini ML, et al. Clinical power Doppler sonography and histological assessment of the psoriatic plaque: short-term monitoring in patients treated with etanercept. Br J Dermatol. 2011;164(1):33–37.
- Wortsman X, Holm EA, Wulf HC, et al. Real-time spatial compound ultrasound imaging of skin. Skin Res Technol. 2004;10(1):23–31.
- Ormerod AD, Campalani E, Goodfield MJ; BAD Clinical Standards Unit. British Association of Dermatologists guidelines on the efficacy and use of acitretin in dermatology. Br J Dermatol. 2010;162(15):952–963.
- Larsen FG, Steinkjer B, Jakobsen P, et al. Acitretin is converted to etretinate only during concomitant alcohol intake. Br J Dermatol. 2000;143(6):1164–1169.
- Pang ML, Murase JE, Koo J. An updated review of acitretin—a systemic retinoid for the treatment of psoriasis. Expert Opin Drug Metab Toxicol. 2008; 4(7):953–964.
- El-Darouti M, Abdel Hay R. Psoriasis: highlights on pathogenesis, adjuvant therapy and treatment of resistant and problematic cases (part I). J Egypt Women Dermatol Soc. 2010;7(2):64–70.
- Lynde CW, Kraft JN, Lynde CB. Acitretin revisited. Skin Therapy Lett.2011;16(3):1–4.
- Gupta AK, Turnbull DH, Harasiewicz KA, et al. The use of high-frequency ultrasound as a method of assessing the severity of a plaque of psoriasis. Arch Dermatol. 1996;132(6):658–662.
- El Gammal S, Auer T, Popp C, et al. Psoriasis vulgaris in 50 MHz B-scan ultrasound: characteristic features of stratum corneum, epidermis and dermis. Acta Derm Venereol Suppl (Stockh). 1994;186:173–176.
- Guastalla P, Guerci VI, Fabretto A, et al. Detection of epidermal thickening in GJB2 carriers with epidermal US. 2009;251(1):280–286.
- Wortsman X, Jemec GBE. Dermatologic Ultrasound with Clinical and Histologic Correlations. 1st ed. New York, NY: Springer; 2013: 623.
- Filippucci E, Farina A, Carotti M, et al. Grey scale and power Doppler sonographic changes induced by intra-articular steroid injection treatment. Ann Rheum Dis. 2004;63(6):740–743.
- Schmid-Wendtner MH, Burgdorf W. Ultrasound scanning in dermatology. Arch Dermatol. 2005;141(2):217–224.
- Giovagnorio F. Sonography of cutaneous non-Hodgkin’s lymphomas. Clin Radiol. 1997;52(4):301–303.
- Wortsman X, Wortsman J. Clinical usefulness of variable-frequency ultrasound in localized lesions of the skin. J Am Acad Dermatol. 2010;62(2):247–256.

