J Clin Aesthet Dermatol. 2019;12(2):27–30
 by James Q. Del Rosso, DO, and Leon Kircik, MD
by James Q. Del Rosso, DO, and Leon Kircik, MD
Dr. Del Rosso is Research Director of JDR Dermatology Research in Las Vegas, Nevada; is with Thomas Dermatology in Las Vegas, Nevada; and is Adjunct Clinical Professor (Dermatology) with Touro University Nevada in Henderson, Nevada. Dr. Kircik is Clinical Associate Professor with Icahn School of Medicine at Mount Sinai in New York, New York; Clinical Associate Professor with Indiana Medical Center in Indianapolis, Indiana; and Medical Director of Physicians Skin Care, PLLC and DermResearch, PLLC in Louisville, Kentucky.
FUNDING: No funding was received for the preparation of this article.
DISCLOSURES: Dr. Del Rosso and Dr. Kircik both serve as consultants to Verrica Pharmaceuticals (West Chester, Pennsylvania), manufacturer of the formulation of cantharidin 0.7% solution discussed in this article.
Abstract: Topical application of cantharidin, a vesicant that is naturally derived from the blister beetle, has a long track record of being used to treat primarily cutaneous molluscum contagiosum and verrucae. Although not approved by the United States Food and Drug Administration, cantharidin has been available through a variety of compounding sources without standardization of manufacturing, formulation, or method of application. Randomized, controlled studies assessing safety and efficacy of topical cantharidin are limited, to date, with the majority of published evidence gleaned from collective clinical experience. A recent Phase II pilot study evaluating a specific formulation of cantharidin 0.7% solution [w/v] (VP-102) has demonstrated promising efficacy and safety results, with additional studies forthcoming, including the designated use of a patented application device that contains the solution.
Cantharidin is a naturally occurring terpenoid compound derived from the alimentary tract of the male blister beetle (family Meloidae) that has been manufactured since the 1950s. The vesicant activity of cantharidin occurs secondary to targeting of the desmosomal dense plaque, which produces intraepidermal acantholytic detachment of desmosomes from tonofilaments that result in separation of keratinocytes.1 As the vesiculation-induced changes do not typically extend beyond the epidermis, with the basal layer remaining intact, scar formation is not a common sequelae after proper application of cantharidin, although residual dyschromia might occur.1 Cantharidin induces localized vesiculation within a few hours to two days following application to human skin, which has led to its use primarily for treatment of cutaneous verrucae and molluscum contagiosum (MC).1–3 In a survey of pediatric dermatologists (N=95), cantharidin was reported to be one of the most common treatments for MC.4
Despite widespread use in dermatology for over six decades, cantharidin has lacked consistent commercial availability because it has not yet received United States Food and Drug Administration (FDA) approval; lack of FDA approval has also resulted in an absence of standardized, optimized formulations, as well as a lack of uniform manufacturing processes in accordance with Good Manufacturing Practices (GMP).1,2,4,5 This article provides an overview of topical cantharidin use, discusses management of MC, and reviews early clinical trial data on a specific, ether-free, standardized, pharmaceutical-grade formulation of topical cantharidin 0.7% [w/v] (VP-102) for use in the treatment of MC. In addition, a patented delivery device designed to facilitate ease of use and improve the consistency of cantharidin application to affected lesions is also discussed.
Etiology and Clinical Manifestations of Molluscum Contagiosum
Caused by a superficial cutaneous and/or mucosal infection with a pox virus, MC presents as multiple or solitary skin colored–to-tan, domed-shaped, discrete, small papules.6 With the exception of small micropapular MC lesions, most of the MC papules exhibit central umbilication containing caseous material that, if manually expressed, demonstrates the pox virus on microscopy. The causative agent of MC is a brick-shaped deoxyribonucleic acid (DNA) pox-family virus that exhibits a usual incubation period ranging from 14 days to six months in humans.6 Usually, MC diagnosis is clinically straightforward. However, when clinical examination alone is not deemed sufficiently definitive, diagnosis can be confirmed by histopathologic evaluation of an intact MC lesion obtained for biopsy by careful curettage or by examining, via a glass slide smear, the expressed contents, stained with methylene blue, from the central umbilication of a lesion for the presence of molluscum bodies.3
Patients with atopic skin/atopic dermatitis (AD) or with cell-mediated immunosuppression due to an underlying disease or certain medications appear to be more prone to the development of MC, typically with more numerous lesions and a greater body surface area affected.7,8 In some patients with AD, multiple MC lesions develop within a field of eczematous dermatitis, referred to as molluscum dermatitis, possibly reflecting an id-like immunologic response to the MC lesions that the host attempts to clear innately.7
Increased severity of MC in patients with AD has been associated with epidermal barrier dysfunction, with filaggrin gene mutations correlated directly with early onset of AD, more severe clinical course of AD, and increased risk of persistent MC lesions.9 Interestingly, in individuals who are infected with human immunodeficiency virus (HIV) and in whom MC lesions are often numerous and diffuse, recovery of immune function with highly active antiretroviral therapy (HAART) might also result in clearance of MC lesions.3 In addition to the roles played by both epidermal barrier impairment and the abnormalities inherent in atopic skin that predispose it to emergence of MC, this latter observation noted in individuals with HIV underscores the importance of host immune function in preventing the emergence of MC lesions and promoting their resolution.
Current Treatment Options for Molluscum Contagiosum
A thorough review of publications on therapies used to treat MC reveals a long list of suggested topical treatments and physical modalities encompassing a variety of ablative, immunologic, and antiviral approaches.3 Treatment options vary in the number of publications and levels of supporting evidence, with the review3 serving as a central listing to which clinicians may refer when considering management of MC. The listed topical options include salicylic acid (various concentrations and vehicles), silver nitrate 40% paste, cantharidin, podophyllotoxin 0.5%, potassium hydroxide 10% solution, retinoids (tretinoin, adapalene), imiquimod 5% cream, cidofovir (3%), diphencyprone, Australian lemon myrtle 10% solution, and Malaleuca alternifolia oil and iodine.3 Physical modalities and surgical approaches for MC include cryotherapy, curettage, duct tape application, pulsed dye therapy, and photodynamic therapy.3 Other options include oral cimetidine, intralesional injection of Candida antigen, and subcutaneous injection of interferon-alpha.3 Some treatment options, such as cidofovir and interferon, are mainly used in children who have significant underlying immunodeficiency due to primary disease or HIV infection.
The myriad of topical therapies, physical modalities, and surgical approaches that exist for the treatment of MC suggests that no single intervention or therapeutic approach has been shown to be consistently effective.3.10,11 Clinicians tend to favor using the therapeutic approaches they learned during their training, from other clinicians/colleagues, at educational conferences, and/or from publications, as well as approaches they have developed through experience in clinical practice. In the United States, the lack of a dedicated development program and absence of any FDA-approved topical therapies for MC limit the availability of large-scale randomized, controlled trials (RCTs), which often provide strong evidence that assists clinicians in therapeutic decision-making.3,11 There is a definite need for RCTs evaluating MC therapy in children, adolescents, and adults to provide more high-quality evidence to help clinicians advise their patients on optimal treatment choices, including benefits and risks. It is also important to recognize the role of long-term management of MC, as the lesions can persist or recur and new lesions can develop from re-exposure to the MC virus.12
Importantly, because MC lesions might spontaneously resolve, some clinicians, especially pediatricians, suggest allowing the lesions to resolve on their own, without the help of topical or surgical treatment.3,8 This non-treatment approach to MC might help patients avoid time-consuming, costly therapies that potentially could be painful, result in skin discoloration and/or scarring, and have variable efficacy. There are risks, however, associated with allowing MC lesions to resolve on their own, in particular the possibility of lesions persisting for several months to years, which subsequently increases the likelihood of transmitting the virus to others in the patient’s household and community, repeated auto-inoculation in the patient, and social stigma due to physical appearance of the lesions and public fear of transmission at schools and daycare facilities.8,10,12
In a study completed in the United Kingdom among 306 children (age range 4–15 years) with MC,12 subjects were prospectively evaluated monthly to assess any natural recovery from MC lesions and any transmission to other children in their household, until the enrolled subject no longer demonstrated any visible MC lesions. The average time to natural resolution of MC was 13.3 months, with 80 of 269 followed subjects (30%) failing to achieve complete lesion resolution by 18 months and 36 of 269 subjects (13%) failing to achieve complete lesion resolution by 24 months.12 Transmission of MC to other children in the household was recorded in 102 of 250 cases (41%).
The adverse impact that MC lesions can have on quality of life, based on assessment of measured quality of life indices, correlated directly with a greater number of MC lesions.12 These data on the natural history of untreated MC support the importance of fully educating patients and parents/caretakers on the risks associated with allowing MC lesions to resolve on their own.
Pediatricians from the Society of Pediatric Dermatology (N=95) responded to a survey that queried their approaches to management of MC using cantharidin and other therapies, their impressions of efficacy, and observed cutaneous reactions/side effects to various therapies.4 The most common approaches to MC treatment reported by the survey respondents were cantharidin, imiquimod, benign neglect, curettage, cryotherapy, and topical retinoids. Ultimately, despite numerous publications on MC treatment using a wide variety of therapies, several authors stressed the need for more studies, especially large-scale RCTs, to provide evidence on the optimal treatment of MC beyond anecdotal impressions and case report analyses.3,10,13
Topical Cantharidin for Treatment of Molluscum Contagiosum
Therapeutic benefits and adverse effects. Review of available literature suggests there is an overall perception by clinicians that topical cantharidin is an effective treatment option for MC in both pediatric and adult patients; however, topical cantharidin appears to be used more often in pediatric patients, likely because children typically present with multiple lesions.3–6,10,13–15
Although cantharidin and cryotherapy are both locally ablative methods of therapy for MC, a major advantage of cantharidin over cryotherapy, especially in children, is that the associated discomfort with cantharidin application is delayed in onset and variable in intensity. Cryotherapy, on the other hand, typically induces pain immediately upon application and intensifies as the freezing dissipates (thawing effect).
A comprehensive literature review was conducted by Torbeck et al13 on the use of topical cantharidin as a treatment modality for a variety of skin conditions; 37 articles met inclusion criteria for the review. The authors reported that topical cantharidin appeared to be effective and safe, overall, for treatment of verrucae and MC. Closer monitoring for local adverse effects (AEs) was recommended when used in children. The most commonly recognized AEs associated with topical cantharidin were blistering (10–100%), discomfort/pain (7–86%), and dyschromia (2–54%), which are directly related to its vesicant mode of action.13 No systemic AEs were noted.3,4,6,7,11,13–15 Importantly, the authors stated there is a paucity of high-powered clinical studies involving the use of cantharidin.13
In a systematic review, Vakharia et al14 evaluated 20 studies, completed between the years 1958 and 2018, that included a total of 1,752 adult and pediatric patients who were treated with topical cantharidin for either verrucae (12 studies) or MC (8 studies).14 In this review, the authors reported clearance rates for MC ranging from 15.4 to 100 percent, noting that the cantharidin formulations and methodologies utilized in the evaluated studies were not uniformly standardized. Combinations of cantharidin with other agents (e.g., podophyllotoxin, salicylic acid) were used primarily to treat verrucae.14 Reported AEs were similar to those reported by Torbeck et al.13
In another review, Silverberg et al15 examined the charts of 537 children who presented with MC, 300 of whom were treated with cantharidin. The researchers also interviewed the parents of the children treated with cantharidin. The chart review revealed that cantharidin therapy cleared MC in 90 percent of the patients and required an average of 2.1 visits to the doctor’s office for treatment.15 Blistering was reportedly observed in 92 percent of the cases; temporary burning, pain, erythema, and/or pruritus were noted in 6 to 37 percent of patients. Ninety-five percent of the parents who were interviewed reported they would use cantharidin therapy again to treat MC.
Limitations of use in dermatology. Beyond cases of marked blistering and/or pain that are sometimes observed, there are several limitations that confound routine use of topical cantharidin. First, there is no FDA-approved cantharidin product currently available; thus, raw material sources and topical formulations of cantharidin vary in consistency and availability.3,11 In 1962, the FDA removed cantharidin’s grandfathered drug status and required a new drug application (NDA) for cantharidin products, which led to the removal of previously existing cantharidin products from the United States marketplace.11 Subsequently, cantharidin formulations could only be obtained outside the United States or through compounding pharmacies. Lack of standardized formulation methods for topical cantharidin products have led to differences in vehicle composition, of which ether has been a typical additive ingredient. Additionally, application methods for topical cantharidin products have never been formally studied, though a wooden stick applicator appears to be the most commonly used method of applying topical cantharidin to individual MC lesions. Despite widespread use of topical cantharidin over the last several years, lack of large-scale RCTs, formulation inconsistencies, and nonstandardized methods of application, including the advantages and disadvantages of occlusion after application, have limited the evidence supporting its use.
Phase II, open-label, pilot study. The recognized efficacy of topical cantharidin established over several years of use coupled with the challenges created by the lack of an FDA-approved formulation have led researchers to develop a novel drug-device combination containing a standardized 0.7% w/v cantharidin solution (VP-102 solution) for treatment of MC.2 The results of a Phase II, open-label, pilot study evaluating this specific cantharidin 0.7% solution for the treatment of MC exhibited promising preliminary efficacy and safety results.16
Study inclusion and design. In the Phase II, open-label, 12-week pilot trial, 30 subjects with MC (aged 2–17 years, mean 5.8 years) were enrolled.2 Recognized exclusion criteria, such as systemic immunosuppression, and washout periods from previous treatments were incorporated. Institutional Review Board approval and signed patient consent (assent for minors) were obtained, and the study followed the human study guidelines set forth by the Declaration of Helsinki. Each study subject had up to 49 lesions treated with the active VP-102 solution, which was applied approximately every 21 days to any uncleared MC lesions for a maximum of four treatments. Subjects were instructed to wash the treatment area at either six hours (Cohort 1: 14/30 subjects) or 24 hours (Cohort 2: 16/30 subjects), or earlier if significant blistering occurred, following application of study product. Lesion counts were collected, and AEs were recorded throughout the study. The Children’s Dermatology Quality of Life Index (CDLQI) was measured at baseline and at the end of study (EOS). The primary efficacy endpoint was the percentage of subjects achieving total clearance by EOS (Day 84).
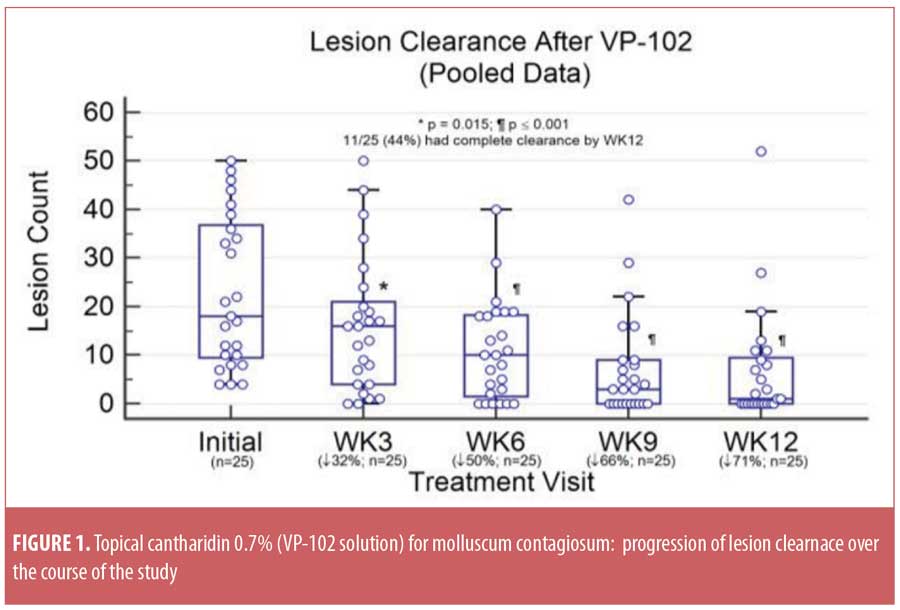
Study outcomes. The mean lesion count (±standard deviation [SD]) was reduced from 23.0±15.6 at baseline to 6.8±11.7 at EOS, based on data pooled from Cohorts 1 and 2 (p<0.0001). Total clearance of MC lesions was achieved in 11 of 25 subjects (44%), with five subjects withdrawing before EOS. The progression of MC lesion clearance is depicted in Figure 1 and demonstrates a continued decrease in MC lesions over time. Twenty-six subjects (86.7%) experienced at least one expected local AE, such as blistering or erythema. No serious or unexpected treatment-related AEs were observed. The mean CDLQI score improved markedly from 3.9±5.6 at baseline to 0.38±1.3 at EOS (p=0.01).
Conclusion
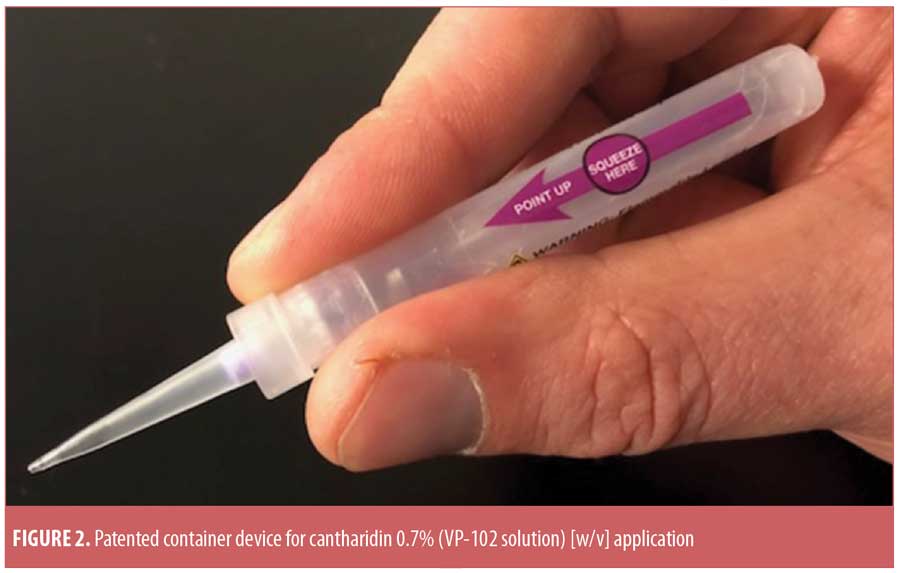
Although the Phase II pilot study outcomes are encouraging, especially considering that the results included data from children as young as two years of age, large-scale, Phase III studies assessing VP-102 treatment for MC are vital before firm conclusions regarding VP-102’s safety and efficacy can be established. It is important to note that the Phase II pilot study incorporated a wooden stick for local application of study drug to MC lesions. The product that will be evaluated for FDA approval, however, is a drug-device combination that packages the cantharidin 0.7% solution in a specially designed, patented delivery device, which allows more controlled application of the product to MC lesions (Figure 2). Developers hope this delivery device will facilitate the ease of application and reduce severity of AEs. Release of data from Phase III studies evaluating the VP-102 drug-device combination is anticipated during the first half 2019. This standardized, pharmaceutical-grade, ether-free formulation of cantharidin could potentially become a welcomed addition to the dermatologist’s armamentarium for the treatment of MC.
References
- Cantharidin. US National Center for Biotechnology Information. PubChem Compound Database; CID=5944, https://pubchem.ncbi.nlm.nih.gov/compound/5944. Accessed 17 Nov 2018.
- Guzman AK, Garelik JL, Cohen SR. Topical cantharidin revisited: a Phase 2 study investigating a commercially-viable formulation of cantharidin (VP-102) for the treatment of molluscum contagiosum. Poster presentation. Society for Pediatric Dermatology 43rd Annual Meeting. July 2018. Lake Tahoe, California.
- Javed A, Coulson I. Molluscum contagiosum. In: Lebwohl MG, Berth-Jones J, Heymann WR, Coulson I (eds). Treatment of Skin Disease: Comprehensive Therapeutic Strategies, 4th Edition. Philadelphia, PA: Elsevier-Saunders USA; 2014:460–463.
- Coloe J, Morrell DS. Cantharidin use among pediatric dermatologists in the treatment of molluscum contagiosum. Pediatr Dermatol. 2009;26:405-408.
- Pompei DT, Rezzadeh KS, Viola KV, et al. Cantharidin therapy: practice patterns and attitudes of health care providers. J Amer Acad Dermatol. 2013;68:1045–1046.
- Hurwitz S. Viral diseases of the skin. In: Hurwitz S (ed). Clinical Pediatric Dermatology: A Textbook of Skin Disorders of Childhood and Adolescence. Philadelphia, PA: WB Saunders Company; 2011: 38.
- Hussain SH, Treat JR, Yan AC. Infantile atopic dermatitis. In: Rudikoff D, Cohen SR, Scheinfeld N (eds). Atopic Dermatitis and Eczematous Disorders. Boca Raton, FL: CRC Press Taylor & Francis Group LLC; 2014:98.
- Mathes EF, Frieden IJ. Treatment of molluscum contagiosum with cantharidin: a practical approach. Pediatr Ann. 2010;39(3):124–128.
- Manti S, Amorini M, Cuppari C, et al. Filaggrin mutations and molluscum contagiosum skin infection in patients with atopic dermatitis. Ann Allergy Asthma Immunol. 2017;119(5):446-451.
- van der Wouden JC, van der Sande R, Kruithof E, et al. Interventions for cutaneous molluscum contagiosum. Cochrane Database Syst Rev. 2017;5:CD004767.
- Sheth PB, Landis MN. Topical and intralesional antiviral agents. In: Wolverton SE (ed). Comprehensive Dermatologic Drug Therapy, 3rd Edition. Philadelphia, PA: Saunders-Elsevier; 2012:479.
- Olsen JR, Gallacher J, Finlay AY, et al. Time to resolution and effect on quality of life of molluscum contagiosum in children in the UK: a prospective community cohort study. Lancet Infect Dis. 2015;15(2):190–195.
- Torbeck R, Pan M, DeMoll E, et al. Cantharidin: a comprehensive review of the clinical literature. Dermatol Online J. 2014 Jun 15;20(6). pii: 13030/qt45r512w0.
- Vakharia PP, Chopra R, Silverberg NB, et al. Efficacy and safety of topical cantharidin treatment for molluscum contagiosum and warts: a systematic review. Am J Clin Dermatol. 2018 Aug 10. doi: 10.1007/s40257-018-0375-4. [Epub ahead of print].
- Silverberg NB, Sidbury R, Mancini AJ. Childhood molluscum contagiosum: experience with cantharidin therapy in 300 patients. J Am Acad Dermatol. 2000;43(3):503-507.
- Globe Newswire. Verrica announces successful Phase II Innovate trial and complete enrollment of Phase III trials of VP-102 in molluscum contagiosum ahead of schedule. 12 Sep 2018. Data on file. Verrica Pharmaceuticals (West Chester, Pennsylvania).

