J Clin Aesthet Dermatol. 2019;12(2):20–24
 by Gabriela Casabona, MD
by Gabriela Casabona, MD
Dr. Casabona is Scientific Director at Ocean Clinic in Marbella, Spain.
FUNDING: Funding for this study was provided by Merz Pharmaceuticals GmbH, Frankfurt am Main, Germany.
DISCLOSURES: The author has no conflicts of interest relevant to the content of this article.
Abstract: Objective. Stretch marks (striae) can be distressing for many people, and currently available treatments are less than optimal. A previous study showed that the use of a calcium hydroxylapatite (CaHA) filler followed by the topical application of 20% ascorbic acid solution and microneedling significantly improved striae appearance. We sought to evaluate additional improvements in atrophy and color of striae following treatment with microfocused ultrasound with visualization (MFU-V) in subjects who received previous treatments using CaHA, 20% ascorbic acid, and microneedling.
Participants.Subjects with remaining skin atrophy (N=20) were treated with MFU-V using transducers with a frequency of 7MHz and 10MHz and a focal depth of 3.0 mm and 1.5 mm, respectively, applied in a cross-hatch pattern.
Measurements.Efficacy was based on changes in Manchester Scar Scale scores.
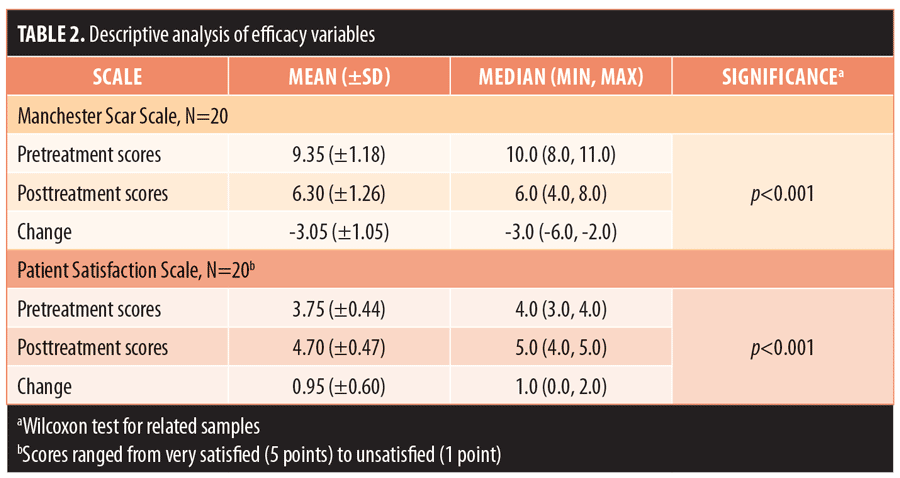
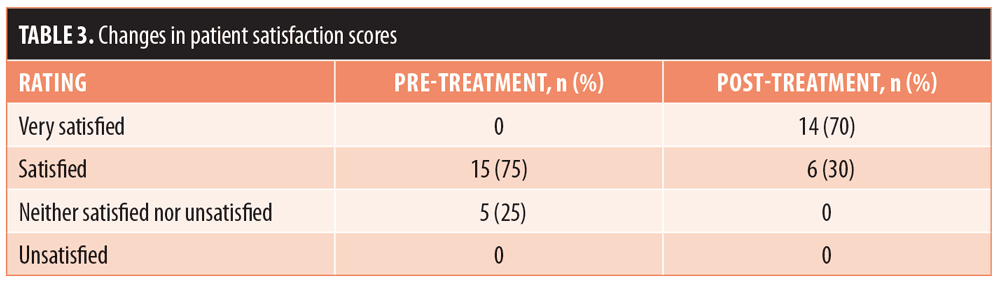
Results.At Day 90 post-treatment, the mean (± standard deviation) baseline Manchester Scar Scale score decreased from 9.35 (±1.18) to 6.30 (±1.26) (p<0.001) and the mean Patient Satisfaction Scale score increased from 3.75 (±0.44) to 4.70 (±0.47) (p<0.001). Most subjects (70%) were very satisfied with their results. No adverse events were reported.
Conclusion.The combined use of CaHA, 20% ascorbic acid, and microneedling appears to be effective in improving the appearance of striae. Additional esthetic improvements were seen with the application of MFU-V.
KEYWORDS: Striae, stretch marks, microfocused ultrasound, microneedling, topical ascorbic acid
Introduction
The etiology of stretch marks (striae distensae) remains unclear. Possible mechanisms for the development of striae include mechanical stretching of the skin, hormonal alterations, and structural skin changes. Evidence for mechanical stretching is the perpendicular position of stretch marks in relation to skin direction,1 with a greater prevalence among younger people whose skin is less able to stretch.2
Striae often occur during altered hormonal conditions. Adrenocorticotrophic hormone and cortisol appear to promote fibroblast activity, leading to increased protein catabolism and changes in collagen and elastin fibers.2 Accordingly, stretch marks often occur during adolescence, pregnancy and obesity,3,4 Cushing’s syndrome5 and chronic steroid use.2,4 Structural changes in the skin have been linked to a genetic propensity for developing stretch marks.6 The expression of collagen, elastin, and fibronectin is decreased in striae tissue, with changes in fibroblast metabolism.7,8
The prevalence of stretch marks ranges from 11 to 88 percent, and they commonly affect the abdomen, breasts, thighs, and buttocks.2 Among susceptible populations, the prevalence is reported to range from 43 to 88 percent for pregnant women, 86 percent for adolescents, and 43 percent for individuals with obesity.2 African-American women are more severely affected than Caucasian women.9
Newly formed stretch marks are red (striae rubrae), eventually fading into permanent, hypopigmented, atrophic areas (striae albae).2 Depending on their anatomical location, the appearance of stretch marks can pose a significant psychological burden for some patients10 and have a negative impact on quality of life measures.11
Although there are commercial creams, lotions, and oils that claim to prevent stretch marks,4 the published literature reports no evidence that these remedies provide any benefit.4,12,13 One systematic literature review described six clinical trials that evaluated the use of topical products for preventing stretch marks during pregnancy.14 The authors concluded that there was no high-quality evidence supporting the use of any of the topical preparations for preventing stretch marks. Treatments for existing striae have been less than optimal, as they cannot be completely removed.15
Microfocused ultrasound with visualization (MFU-V) (Ultherapy®, Merz Aesthetics, Raleigh, North Carolina) has been shown to safely and effectively tighten and lift lax skin on the face and neck.16 The use of ultrasound visualization at depths of up to 8mm below the skin surface enables accurate treatment of targeted tissues and avoidance of nontarget tissues, such as bone, helping to improve the safety of the technology. MFU-V heats tissue to approximately 60 to 70 degrees Celsius, producing small (<1mm3) zones of thermal coagulation to a depth of up to 5mm within the mid-to-deep reticular layer of the dermis and subdermis, while sparing overlying skin layers.17 Delivering MFU-V to targeted areas in the superficial muscular aponeurotic system and platysma results in immediate collagen contraction and initiates neocollagenesis and collagen remodeling.18
A recent retrospective study previously assessed the effectiveness of combined treatment with a calcium hydroxylapatite (CaHA) filler (Radiesse®, Merz North America, Raleigh, North Carolina), topical application of a 20% ascorbic acid solution, and microneedling for treating new (red) and old (white) striae on various anatomical areas.19 Additional treatments with ascorbic acid and microneedling only were repeated twice at 30-day intervals. Among the enrolled subjects (N=35), 30 (86%) were very satisfied or satisfied with their treatment results one month after the last treatment session. Histology studies demonstrated an increase in the quantity and quality of collagen and elastin fibers in the dermis targeted with this combination treatment.
The objective of the present retrospective, nonrandomized study was to assess the safety and effectiveness of MFU-V for further improving the appearance of moderate-to-severe stretch marks previously treated with CaHA, ascorbic acid solution, and microneedling. The combined use of CaHA and MFU-V is consistent with recently adopted guidelines for combined aesthetic interventions.20
Materials and Methods
Study subjects. Healthy female subjects aged 18 to 55 years who were previously treated with CaHA, topical 20% ascorbic acid solution, and microneedling, as described above, were provided an opportunity to receive additional treatment with MFU-V in an effort to achieve additional improvement in striae appearance. Enrolled subjects (N=20) were required to have recent or old, moderate-to-severe stretch marks on the buttocks, thighs, breasts, or abdomen and a body mass index of less than 35kg/m2. Subjects were required to have not undergone any other treatment for striae during the previous 90 days.
Procedure. MFU-V was delivered using transducers with a frequency of 7MHz at a focal depth of 3.0mm and a frequency of 10MHz at a focal depth of 1.5mm. Each transducer was used to deliver up to 75 parallel treatment lines,
2 to 3mm apart, to each treated area in a cross-hatched pattern. Two-dimensional digital images were obtained prior to treatment and at 90 days post-treatment.
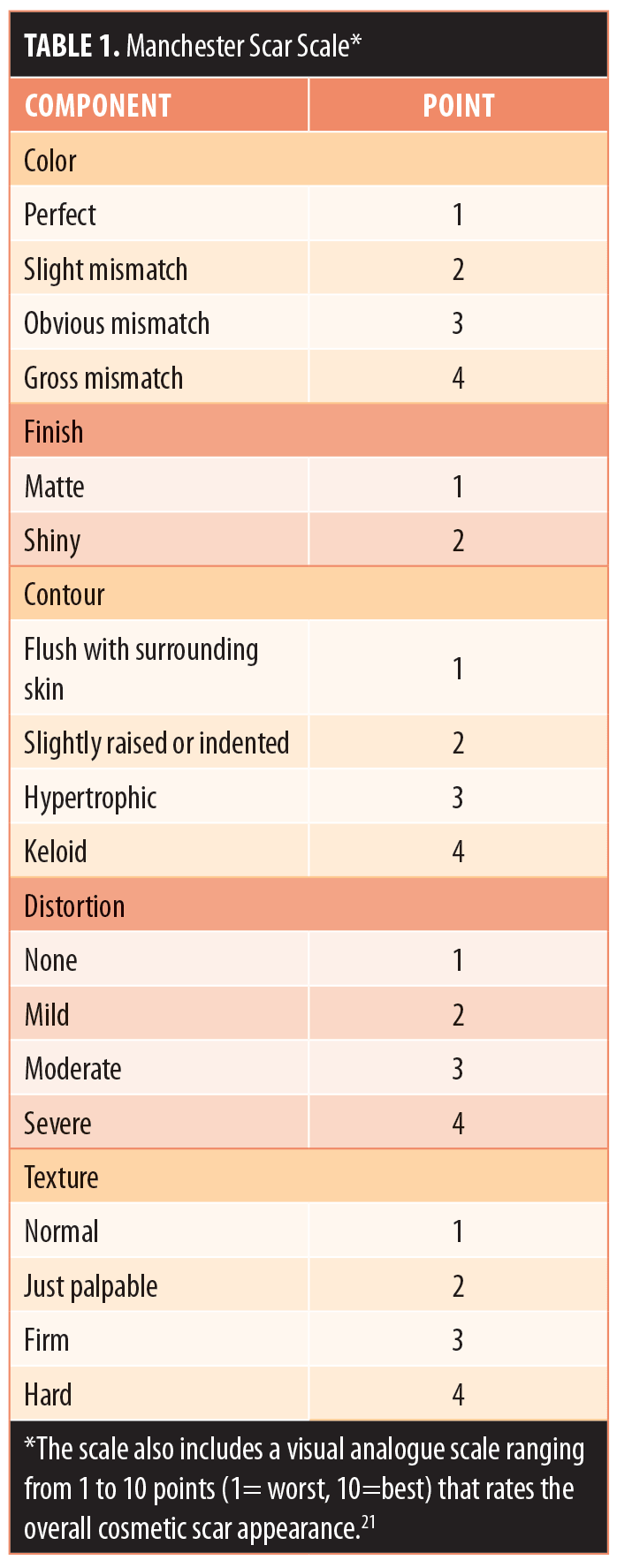
The 90-day effectiveness measures included changes in baseline Manchester Scar Scale scores.21 There are five components to this scale: color, contour, distortion, texture, and finish. All components were scored on a scale of 1 to 4, except for finish, which was scored either 1 (matte) or 2 (shiny) (Table 1). Manchester Scar Scale scores range from a possible high of 18 points to a low of five points, with lower scores indicating better aesthetic appearance (the 10-point visual analog scale was not used) (Table 1).21 Subject satisfaction with the aesthetic results was assessed at 90 days via a satisfaction questionnaire. Four possible responses ranged from very satisfied (5 points) to unsatisfied (1 point). Safety assessments included physical examination of the treated area for the presence of erythema or edema and reports of adverse events.
Statistical analysis. Numerical data were described using descriptive statistics. The Wilcoxon test for related samples was used to compare pretreatment Manchester Scar Scale scores and Patient Satisfaction Scale scores with scores obtained at Day 90. The level of significance for statistical tests was five percent (p<0.05). Statistical reporting was performed using the SAS® System for Windows, version 9.2 (SAS Institute Inc., Cary, North Carolina).
Results
The 20 enrolled subjects received MFU-V for striae on their breasts (n=2; 10%), buttocks (n=3; 15%), thighs (n=7; 35%), or abdomen (n=8; 40%), and all patients completed the 90-day evaluation. The mean (± standard deviation [SD]) pretreatment Manchester Scar Scale score decreased from 9.35 (±1.18) to 6.30 (±1.26) after 90 days (p<0.001) (Table 2). Separately, the mean Patient Satisfaction Scale score increased from 3.75 (±0.44) to 4.70 (±0.47) at 90 days (p<0.001). Although the majority of subjects (75%) were satisfied with the appearance of their striae prior to MVU-F treatment, most (70%) became very satisfied 90 days post-treatment with MFU-V (Table 3). There were no reported adverse events. Figures 1 and 2 are examples of improvements seen in the striae of study subjects.
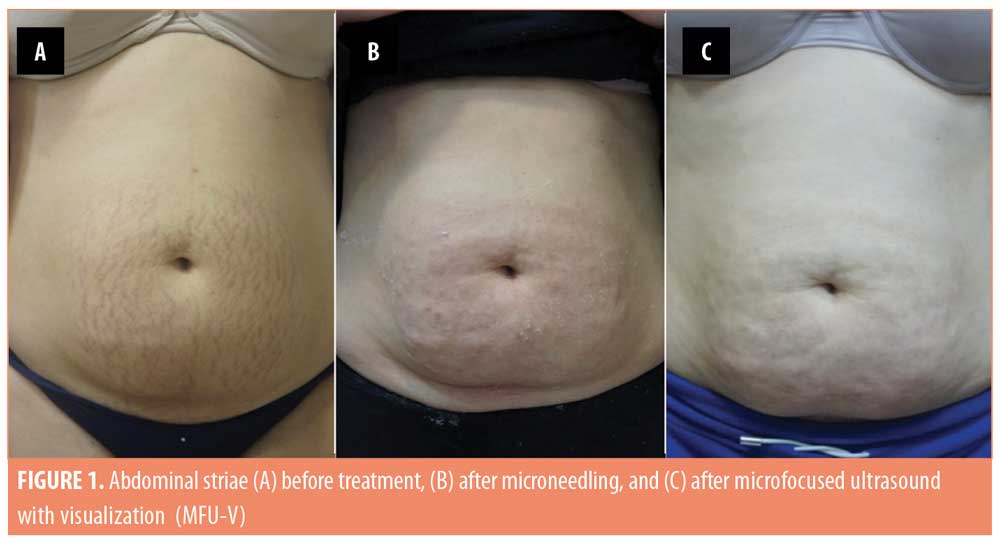
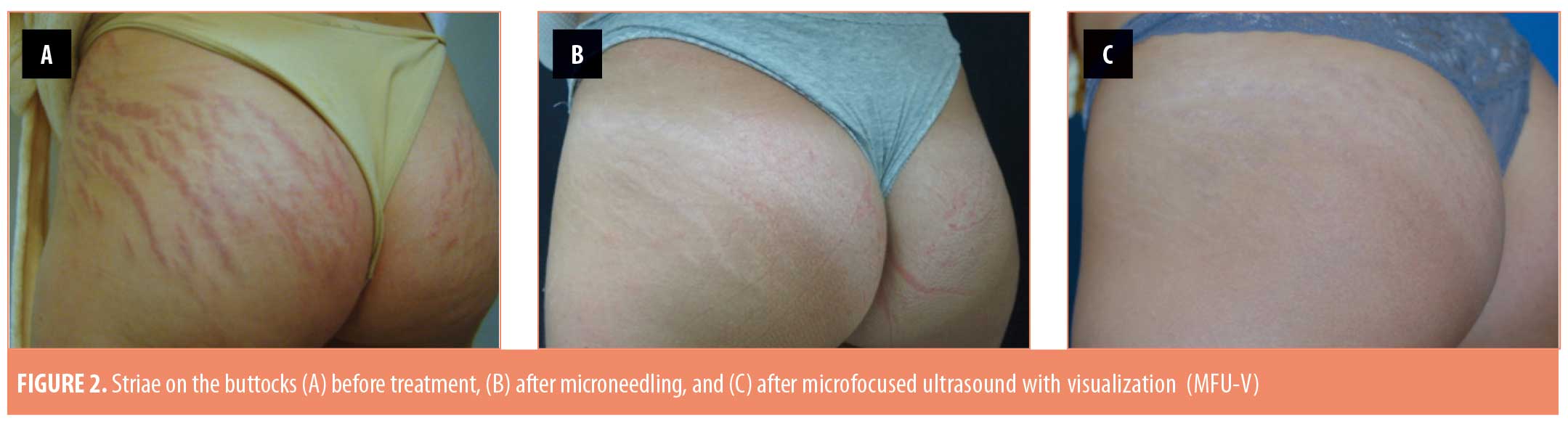
Discussion
Currently, there are no effective topical treatments available for preventing stretch marks,14 and the effectiveness of available products used to treat existing stretch marks is very limited. Treatment with topical tretinoin was reported to improve the appearance of stretch marks in two small studies.22,23 Alpha-hydroxy acid peels, such as glycolic acid, increase collagen expression in the skin24 and have also been reported to improve striae.25 The beneficial effects of topical ascorbic acid additionally include neocollagenesis.26
Use of laser- and light-emitting devices, such as nonablative fractional lasers27,28 and fractional CO2 lasers,29,30 for the treatment of striae has gained recent attention. These techniques; however, require multiple treatments over extended periods of time to achieve improvements in the appearance of striae. For example, nonablative fractional erbium fiber lasers typically require 3 to 4 treatments at 4- to 5-week intervals, 27,28 and fractional CO2 lasers generally require five sessions at 2- to 4-week intervals.29,30 Lasers achieve their beneficial effects by contracting old collagen and stimulating the production of new collagen;31,32 however, laser therapies are not suitable for all skin types.33 Reported adverse events include transient erythema and severe edema,28 mild postinflammatory hyperpigmentation,27,30 and mild-to-moderate acne.27
Microneedling has recently emerged as a new method for treating stretch marks, and was shown to be beneficial after three treatments at monthly intervals.34 The results of two subsequent studies indicate microneedling is superior to CO2 laser application35 and microdermabrasion with sonophoresis36 for treating striae. Microneedling stimulates the release of growth factors and induces collagen production,37 and it has been used in combination with glycolic acid peels for treating atrophic acne scars.38
In addition to long-lasting correction of moderate-to-severe facial wrinkles and folds,39 CaHA has also been shown to be effective in treating blemishes such as acne scars.40,41 CaHA filler provides immediate volume to atrophic areas and also stimulates long-term neocollagenesis surrounding filler microspheres,42 which might contribute to the overall improvement in the appearance of treated areas.43 CaHA results in active, physiologic remodeling of the extracellular matrix by increasing elastin and stimulating a two-step process whereby collagen type I gradually replaces collagen type III.44
A recent report demonstrated the safety and effectiveness of combining CaHA with microneedling and topical ascorbic acid for treating red and white striae on buttocks, thighs, knees, abdomen, and breasts.19 CaHA filler was diluted 1:1 with lidocaine 2% without epinephrine. A maximum of 3.0mL of filler was injected into each patient at all skin depths and immediately followed by microneedling and topical application of 20% ascorbic acid. Microneedling with ascorbic acid was repeated twice more at one-month intervals. One month after the final treatment, mean baseline Manchester Scar Scale scores decreased from 12.0 to 7.1. More than 85 percent of subjects reported satisfaction with their results.
MFU energy heats tissue in the mid-to-deep reticular layer of the dermis and subdermis to produce small points of thermal coagulation.17,18,45 The goal is to elevate the local temperature to a point at which collagen fibers become denatured and contract.46,47 In addition, de-novo collagen synthesis occurs within the areas of thermal tissue coagulation.48,49 Although MFU-V is approved for treating skin laxity in the eyebrow area and rhytides on the lower face and décolletage areas,50 it has also been used for skin tightening in other areas including the abdomen,51 thighs,51,52 upper arms,51,52 elbows,53 knees,51,52 and buttocks.51,54 Many of these areas are highly susceptible to the formation of stretch marks.
Similarly, the injection of CaHA has been shown to decrease skin flaccidity and increase skin density and thickness in the abdomen, thighs, and upper arms.55 Not surprisingly, increased collagen and better aesthetic results have been demonstrated when more than one type of therapy is used for treating stretch marks, such as combining fractionated microneedle radiofrequency and fractional carbon dioxide laser.56 Expert consensus supports the use of combined treatments for facial rejuvenation,57 and position statements have been developed for the use of multiple procedures for treating the upper arms, abdomen, buttocks, and knees.20
The safety and efficacy combining MFU-V with CaHA for treating skin laxity and improving the appearance of cellulite on the buttocks and thighs has been previously demonstrated.58 Immediately after MFU-V, subjects received treatment with 1.5mL CaHA diluted in a 1:1 manner with 1.5mL of 2% lidocaine solution. Using a 25-gauge, 48-mm-long cannula, 3.0mL of diluted CaHA was injected into the subdermis using a microdroplet fanning technique (1mL per treatment site with 10 lines of 0.1mL per line) to cover the same area as the MFU-V.
After 90 days, there was a significant improvement in cellulite appearance (i.e., 4.5 points on the 15-point Cellulite Severity Scale). Nearly all of the subjects (95%) reported satisfaction with their treatment results. Histology studies demonstrated neocollagenesis in treated skin.
While 75 percent of enrolled subjects with red and white striae in the current study were satisfied following the combined treatment with CaHA, topical ascorbic acid, and microneedling, satisfaction improved further following the additional treatment with MFU-V (in that 70% were very satisfied). These results are further supported by other investigators who recently demonstrated the safety and efficacy of combining several aesthetic treatments, including botulinum toxin, temporary and semipermanent dermal fillers, microfocused ultrasound, and other energy-based treatments for facial rejuvenation.59,60
Conclusion
The currently available treatments for improving the appearance of stretch marks are less than optimal. The combined use of CaHA with a 20% topical ascorbic acid solution and a microneedling procedure known to increase collagen production appears to be an effective treatment for the appearance of stretch marks. Post-treatment application of MFU-V appears to offer additional benefits and improve outcomes.
Acknowledgment
The author acknowledges Carl Hornfeldt for his editorial assistance with the preparation of this manuscript.
References
- Shuster S. The cause of striae distensae. Acta Derm Venereol Suppl (Stockh). 1979;59(85):161–169.
- Al-Himdani S, Ud-Din S, Gilmore S, et al. Striae distensae: a comprehensive review and evidence-based evaluation of prophylaxis and treatment. Br J Dermatol. 2014;170(3):527–547.
- García Hidalgo L. Dermatological complications of obesity. Am J Clin Dermatol. 2002;3(7):497–506.
- Ud-Din S, McGeorge D, Bayat A. Topical management of striae distensae (stretch marks): prevention and therapy of striae rubrae and albae. J Eur Acad Dermatol Venereol. 2016;30(2):211–222.
- Nieman LK. Cushing’s syndrome: update on signs, symptoms and biochemical screening. Eur J Endocrinol. 2015;173(4):M33–M38.
- Di Lernia V, Bonci A, Cattania M, et al. Striae distensae (rubrae) in monozygotic twins. Pediatr Dermatol. 2001;18(3):261–262.
- Tung JY, Kiefer AK, Mullins M, et al. Genome-wide association analysis implicates elastic microfibrils in the development of nonsyndromic striae distensae. J Invest Dermatol. 2013;133(11):2628–2631.
- Lee KS, Rho YJ, Jang SI, et al. Decreased expression of collagen and fibronectin genes in striae distensae tissue. Clin Exp Dermatol. 1994;19(4):285–288.
- Elbuluk N, Kang S, Hamilton T. Differences in clinical features and risk factors for striae distensae in African American and white women. J Am Acad Dermatol. 2009;60(3):62.
- Kordi M, Rashidi Fakari F, Mazloum SR, et al. Quality of life evaluation in Iranian postpartum women with and without striae gravidarum. Iran J Psychiatry Behav Sci. 2016;10(2):e3993.
- Yamaguchi K, Suganuma N, Ohashi K. Quality of life evaluation in Japanese pregnant women with striae gravidarum: a cross-sectional study. BMC Res Notes. 2012;5:450.
- Moore J, Kelsberg G, Safranek S. Clinical inquiry: do any topical agents help prevent or reduce stretch marks?. J Fam Pract. 2012;61(12):757–758.
- Korgavkar K, Wang F. Stretch marks during pregnancy: a review of topical prevention. Br J Dermatol. 2015;172(3):606–615.
- Brennan M, Young G, Devane D. Topical preparations for preventing stretch marks in pregnancy. Cochrane Database Syst Rev. 2012;11:CD000066.
- Mayo Foundation for Medical Education and Research (MFMER). Diseases & conditions: stretch marks. Available: http://www.mayoclinic.org/diseases-conditions/stretch-marks/home/ovc-20169154. Accessed January 11, 2017.
- Alam M, White LE, Martin N, et al. Ultrasound tightening of facial and neck skin: a rater-blinded prospective cohort study. J Am Acad Dermatol. 2010;62(2):262–269.
- Laubach HJ, Makin IR, Barthe PG, et al. Intense focused ultrasound: evaluation of a new treatment modality for precise microcoagulation within the skin. Dermatol Surg. 2008;34(5):727–734.
- White WM, Makin IR, Barthe PG, et al. Selective creation of thermal injury zones in the superficial musculoaponeurotic system using intense ultrasound therapy: a new target for noninvasive facial rejuvenation. Arch Facial Plast Surg. 2007;9(1):22–29.
- Casabona G, Marchese P. Calcium hydroxylapatite dermal filler combined with microneedling and topical ascorbic acid: a novel and effective method of treating stretch marks. Plast Reconstr Surg Glob Open. 2017;5(9):e1474.
- Fabi SG, Burgess C, Carruthers A, et al. Consensus recommendations for combined aesthetic interventions using botulinum toxin, fillers, and microfocused ultrasound in the neck, décolletage, hands, and other areas of the body. Dermatol Surg. 2016;42(10):1199–1208.
- Beausang E, Floyd H, Dunn KW, et al. A new quantitative scale for clinical scar assessment. Plast Reconstr Surg. 1998;102(6):1954–1961.
- Elson ML. Treatment of striae distensae with topical tretinoin. J Dermatol Surg Oncol. 1990;16(3):267–270.
- Rangel O, Arias I, García E, et al. Topical tretinoin 0.1% for pregnancy-related abdominal striae: an open-label, multicenter, prospective study. Adv Ther. 2001;18(4):181–186.
- Bernstein EF, Lee J, Brown DB, et al. Glycolic acid treatment increases type I collagen mRNA and hyaluronic acid content of human skin. Dermatol Surg. 2001;27(5):429–433.
- Ash K, Lord J, Zukowski M, et al. Comparison of topical therapy for striae alba (20% glycolic acid/0.05% tretinoin versus 20% glycolic acid/10% L-ascorbic acid). Dermatol Surg. 1998;24(8):849-856.
- Stamford NP. Stability, transdermal penetration, and cutaneous effects of ascorbic acid and its derivatives. J Cosmet Dermatol. 2012;11(4):310–317.
- Malekzad F, Shakoei S, Ayatollahi A, et al. The safety and efficacy of the 1540 nm non-ablative fractional XD probe of Star Lux 500 device in the treatment of striae alba: before–after study. J Lasers Med Sci. 2014;5(4):194–198.
- Tretti Clementoni M, Lavagno R. A novel 1565 nm non-ablative fractional device for stretch marks: a preliminary report. J Cosmet Laser Ther. 2015;17(3):148–155.
- El Taieb MA, Ibrahim AK. Fractional CO2 laser versus intense pulsed light in treating striae distensae. Indian J Dermatol. 2016;61(2):174–180.
- Naein FF, Soghrati M. Fractional CO2 laser as an effective modality in treatment of striae alba in skin types III and IV. J Res Med Sci. 2012;17(10):928–933.
- Hantash BM, Bedi VP, Kapadia B, et al. In vivo histological evaluation of a novel ablative fractional resurfacing device. Lasers Surg Med. 2007;39(2):96–107.
- Preissig J, Hamilton K, Markus R. Current laser resurfacing technologies: a review that delves beneath the surface. Semin Plast Surg. 2012;26(3):109–116.
- Davis EC, Callender VD. Aesthetic dermatology for aging ethnic skin. Dermatol Surg. 2011;37(7):
901–917. - Park KY, Kim HK, Kim SE, et al. Treatment of striae distensae using needling therapy: a pilot study. Dermatol Surg. 2012;38(11):1823–1828.
- Khater MH, Khattab FM, Abdelhaleem MR. Treatment of striae distensae with needling therapy versus CO2 fractional laser. J Cosmet Laser Ther. 2016;18(2):75–79.
- Nassar A, Ghomey S, El Gohary Y, et al. Treatment of striae distensae with needling therapy versus microdermabrasion with sonophoresis. J Cosmet Laser Ther. 2016;18(6):330–334.
- Hou A, Cohen B, Haimovic A, et al. Microneedling: a comprehensive review. Dermatol Surg. 2017;43(3):321–339.
- Sharad J. Combination of microneedling and glycolic acid peels for the treatment of acne scars in dark skin. J Cosmet Dermatol. 2011;10(4):317–323.
- Jacovella PF. Use of calcium hydroxylapatite (Radiesse) for facial augmentation. Clin Interv Aging. 2008;3(1):161–174.
- Wollina U, Goldman A. Fillers for the improvement in acne scars. Clin Cosmet Investig Dermatol. 2015;8:493–499.
- Goldberg DJ, Amin S, Hussain M. Acne scar correction using calcium hydroxylapatite in a carrier-based gel. J Cosmet Laser Ther. 2006;8(3):134–136.
- Marmur ES, Phelps R, Goldberg DJ. Clinical, histologic and electron microscopic findings after injection of a calcium hydroxylapatite filler. J Cosmet Laser Ther. 2004;6(4):223–226.
- Berlin AL, Hussain M, Goldberg DJ. Calcium hydroxylapatite filler for facial rejuvenation: a histologic and immunohistochemical analysis. Dermatol Surg. 2008;34 Suppl 1:S64–S67.
- Yutskovskaya Y, Kogan E, Leshunov E. A randomized, split-face, histomorphologic study comparing a volumetric calcium hydroxylapatite and a hyaluronic acid-based dermal filler. J Drugs Dermatol. 2014;13(9):1047–1052.
- Gliklich RE, White WM, Slayton MH, et al. Clinical pilot study of intense ultrasound therapy to deep dermal facial skin and subcutaneous tissues. Arch Facial Plast Surg. 2007;9(2):88–95.
- Ferraro GA, De Francesco F, Nicoletti G, et al. Histologic effects of external ultrasound-assisted lipectomy on adipose tissue. Aesthetic Plast Surg. 2008;32(1):111–115.
- Hayashi K, Thabit G 3rd, Massa KL, et al. The effect of thermal heating on the length and histological properties of the glenohumeral joint capsule. Am J Sports Med. 1997;25(1):107–112.
- Misell LM, Emson CL, Turner SM, et al. Microfocused ultrasound therapy stimulates collagen synthesis in human skin. J Clin Aesthet Dermatol. In press.
- Casabona G, Michalany N. Microfocused ultrasound with visualization and fillers for increased neocollagenesis: clinical and histological evaluation. Dermatol Surg. 2014;40 Suppl 12:S194–S198.
- Wulkan AJ, Fabi SG, Green JB. Microfocused ultrasound for facial photorejuvenation: a review. Facial Plast Surg. 2016;32(3):269-275.
- Sasaki G, Tevez A. Microfocused ultrasound for nonablative skin and subdermal tightening to the periorbitum and body sites: preliminary report on eighty-two patients. J Cosmet Dermatol Sci Appl. 2012;2(2A):109–116.
- Alster TS, Tanzi EL. Noninvasive lifting of arm, thigh, and knee skin with transcutaneous intense focused ultrasound. Dermatol Surg. 2012;38(5):754–759.
- Rokhsar C, Schnebelen W, West A, et al. Safety and efficacy of microfocused ultrasound in tightening of lax elbow skin. Dermatol Surg. 2015;41(7):821–826.
- Goldberg DJ, Hornfeldt CS. Safety and efficacy of microfocused ultrasound to lift, tighten, and smooth the buttocks. Dermatol Sur. 2014;40(10):1113–1117.
- Cogorno Wasylkowski V. Body vectoring technique with Radiesse® for tightening of the abdomen, thighs, and brachial zone. Clin Cosmet Investig Dermatol. 2015;8:267–273.
- Ryu HW, Kim SA, Jung HR, et al. Clinical improvement of striae distensae in Korean patients using a combination of fractionated microneedle radiofrequency and fractional carbon dioxide laser. Dermatol Surg. 2013;39(10):1452–1458.
- Carruthers J, Burgess C, Day D, et al. Consensus recommendations for combined aesthetic interventions in the face using botulinum toxin, fillers, and energy-based devices. Dermatol Surg. 2016;42(5):586–597.
- Casabona G, Pereira G. Combination treatment using microfocused ultrasound with visualization and calcium hydroxylapatite to improve skin laxity and the appearance of cellulite on buttocks and thighs. Plast Reconstr Surg Glob Open. 2017;5:e1388.
- Fabi SG, Goldman MP, Mills DC, et al. Combining microfocused ultrasound with botulinum toxin and temporary and semi-permanent dermal fillers: safety and current use. Dermatol Surg. 2016;42 Suppl 2:S168–S176.
- Langelier N, Beleznay K, Woodward J. Rejuvenation of the upper face and periocular region: combining neuromodulator, facial filler, laser, light, and energy-based therapies for optimal results. Dermatol Surg. 2016;42 Supple 2:S77–S82.

