J Clin Aesthet Dermatol. 2019;12(10):16–23
 by Linda Stein Gold, MD; Sunil Dhawan, MD; Jonathan Weiss, MD; Zoe Diana Draelos, MD; Herman Ellman, MD; and Iain Stuart, PhD
by Linda Stein Gold, MD; Sunil Dhawan, MD; Jonathan Weiss, MD; Zoe Diana Draelos, MD; Herman Ellman, MD; and Iain Stuart, PhD
Dr. Stein Gold is with the Henry Ford Health System in Detroit, Michigan. Dr. Dhawan is with the Center for Dermatology Clinical Research, Inc., in Fremont, California. Dr. Weiss is with Gwinnett Dermatology in Snellville, Georgia. Dr. Draelos is with Dermatology Consulting Services in High Point, North Carolina. Dr. Ellman was formerly (Ret.) with Foamix Pharmaceuticals Inc. in Bridgewater, New Jersey. Dr. Stuart is with Foamix Pharmaceuticals Inc. in Bridgewater, New Jersey.
FUNDING: This study was sponsored by Foamix Pharmaceuticals Inc.
DISCLOSURES: Dr. Stein Gold is an advisor and investigator for Foamix, Galderma, LEO, Novartis, and Valeant; an investigator for Janssen, AbbVie, and Solgel; and an advisor and investigator for Novartis. Dr. Dhawan is an investigator for Foamix, AbbVie, Galderma, Lilly, Moberg, Solgel, Incyte, Valeant, Novartis, and Dermira and a speaker for Pfizer and Dermira. Dr. Weiss is a principal investigator for AbbVie, Aclaris, Endo, Foamix, Galderma, LEO, Moberg, Promius, and Valeant; a speaker for AbbVie and Ortho Dermatologics; a consultant for Aclaris and LEO; and an advisor for Foamix, Galderma, and Valeant. Dr. Draelos is a principal investigator and advisor for Foamix. Dr. Ellman is a former employee and stockholder of Foamix. Dr. Stuart is an employee and stockholder of Foamix.
ABSTRACT: Objective. This study sought to evaluate the long-term safety and efficacy of FMX101 4% topical minocycline foam for the treatment of moderate-to-severe acne.
Design. This was an open-label extension of two double-blind studies, Study 04 and Study 05.
Setting. Subjects were enrolled at 35 sites in the United States and one site in the Dominican Republic.
Participants. Eligible subjects who completed 12 weeks of double-blind treatment with FMX101 4% or vehicle in those studies could continue for an additional nine months of open-label treatment with FMX101 4%. Eligibility required an Investigator’s Global Assessment (IGA) score that had not worsened when compared to baseline at the 12-week visit.
Measurements. Safety, efficacy, and satisfaction assessments were performed.
Results. Of the 961 subjects enrolled in Study 04 (n=466) and Study 05 (n=495), 657 subjects entered the open-label phase (Study 04: n=284; Study 05: n=373), with 414 subjects completing the study (Study 04, n=172; Study 05, n = 242). Treatment-emergent adverse events (TEAEs) were similar to those in the double-blind studies. No serious TEAEs led to subject discontinuation. At Week 52, for facial tolerability assessment, over 95 percent of subjects had no or only mild signs and symptoms. Through Week 52, there were ongoing reductions in inflammatory lesions and increasing IGA treatment success.
Conclusion. FMX101 4% minocycline foam appeared to be safe, effective, and well-tolerated for up to 52 weeks in the treatment of moderate-to-severe acne.
KEYWORDS: Acne vulgaris, minocycline, open-label, randomized, safety
Acne vulgaris is the most common dermatologic disorder in the United States, affecting approximately 85 percent of the adolescent population, although it can persist into adulthood, therefore affecting almost all age groups.1–4 Mild acne is typically managed with topical therapies, whereas systemic antibiotics—in particular, tetracyclines—are a mainstay of treatment for moderate-to-severe acne.5–7 As a chronic disease, acne often requires prolonged treatment.8 Oral minocycline and doxycycline are recommended by the American Academy of Dermatology as first-line therapies; however, these drugs can be associated with significant systemic adverse events.2,9 Resistance is another factor limiting the use of antibiotics in dermatology. Among antibiotics, tetracyclines, especially minocycline, have the lowest resistance rates, whereas increasing resistance has been reported with lincosamide or macrolide topical antibiotics, such as clindamycin and erythromycin, and their use has been accordingly curtailed.1,9
Minocycline is a semisynthetic second-generation tetracycline. It has anti-inflammatory and bacteriostatic properties and has been shown to be an effective treatment for moderate-to-severe acne.9 FMX101 4% is a topical foam formulation of minocycline developed for the treatment of acne of a moderate-to-severe intensity. Use of this foam formulation aims to circumvent the systemic side effects associated with the oral administration of minocycline while retaining its anti-inflammatory and bacteriostatic activity against the target anaerobic microbe, Cutibacterium acnes.9
Minocycline exposure with FMX101 4% versus oral minocycline was evaluated in a pharmacokinetic study involving subjects 18 years or older with a diagnosis of moderate-to-severe acne.2 Minimal systemic minocycline exposure was observed under the FMX101 4% posology, with mean exposure being 730 to 765 times lower than that seen with oral minocycline.2 Similarly, low systemic minocycline exposure levels were observed in a comparable study, evaluating FMX101 4% only in subjects 9 to 17 years of age.10
The efficacy and safety of FMX101 4% in treating moderate-to-severe acne have been previously reported in three Phase III studies (Study 04, Study 05, and Study 22).9,11 In all three studies, FMX101 4% demonstrated statistically significant reductions in both inflammatory and noninflammatory lesions from baseline. In Study 05 and Study 22 specifically, statistically superior rates of treatment success, defined as scores of 0 (“clear”) or 1 (“almost clear”) on the Investigator’s Global Assessment (IGA), compared to the vehicle, occurred at Week 12. In addition, FMX101 4% was found to be safe and well-tolerated during up to 12 weeks of daily application.
To assess the long-term safety of FMX101 4% topical minocycline foam, an open-label extension phase of Study 04 and Study 05 was conducted, in which FMX101 4% was applied daily over the course of an additional 40 weeks. Efficacy assessment was carried out at all visits during the open-label phase, allowing for the evaluation of the continuation of effects of FMX101 4% in subjects with moderate-to-severe acne over a total of 52 weeks of treatment.
Methods
Study design. Study 04 and Study 05 were identical Phase III, randomized, double-blind, two-part, vehicle-controlled studies. In part 1, male and female subjects aged nine years or older were eligible; they were required to have lesion counts within predetermined criteria and IGA scores for acne severity of 3 (“moderate”) or 4 (“severe”) (Table 1). Excluded were females who were pregnant, lactating, or planning a pregnancy, and individuals with any dermatologic condition of the face or facial hair that could interfere with clinical evaluations, such as acne conglobate, acne fulminans, or secondary acne (e.g., chloracne, drug-induced acne). Eligible subjects were randomized 2:1 to receive FMX101 4% or vehicle, self-applied once daily for 12 weeks, in the double-blind phase. At Week 12, subjects from either treatment arm were invited to continue into the open-label phase (part 2) for an additional 40 weeks, provided their acne had not worsened relative to their baseline IGA assessment. In the open-label phase, treatment was guided by each subject’s clinical response. Treatment could be stopped if there was clinical improvement or a resolution of acne or could be restarted if acne recurred or worsened, as subjects remained in the study even if treatment was stopped. Throughout the open-label phase of the study, concomitant acne medications were permitted.
Both studies were conducted in compliance with the principles of the Declaration of Helsinki and of Good Clinical Practice, and conformed with all applicable regulatory requirements, per the 2016 revised guidelines of the International Conference for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. The study protocol was approved by an institutional review board or an independent ethics committee at each site; all patients provided written informed consent.
Safety endpoints. The majority of the open-label safety and efficacy assessments were carried out at all visits (treatment Weeks 16, 22, 28, 34, 40, 46, and 52). Treatment weeks were calculated from the baseline of the study’s double-blind phase (part 1). Laboratory testing was carried out at Weeks 28 and 52. Safety assessments included adverse events, vital signs, dermal tolerability, and laboratory testing; subject satisfaction was assessed by a questionnaire administered at Week 52. Although not a primary objective of this phase of the studies, efficacy in the open-label phase was assessed and reported as 1) the absolute change in inflammatory and noninflammatory lesion counts from baseline of the double-blind phase; and 2) the portion (percentage) of subjects achieving IGA treatment success.
Statistical analysis. All safety parameters were assessed among all subjects who entered the open-label safety extension phase of either study and used at least one dose of the study product (safety population). Safety assessments were based on descriptive statistics; no statistical tests were performed. No comparator statistical testing was performed for the open-label efficacy data, and all data are presented from observed cases only, with no imputation for missing data.
Results
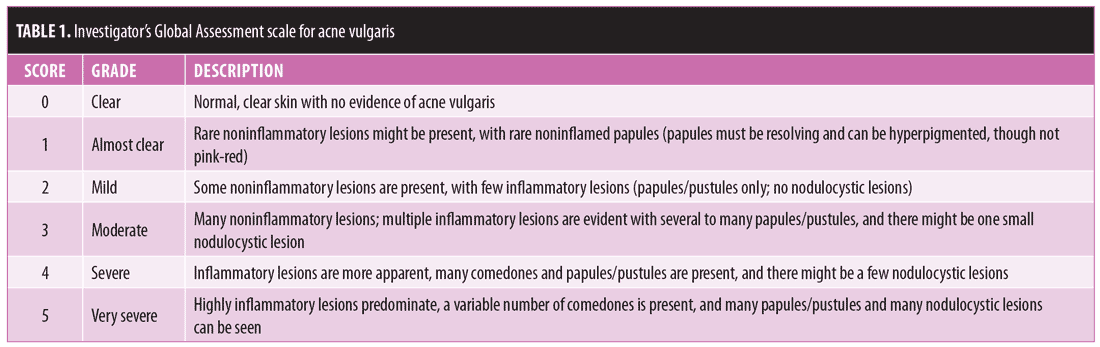
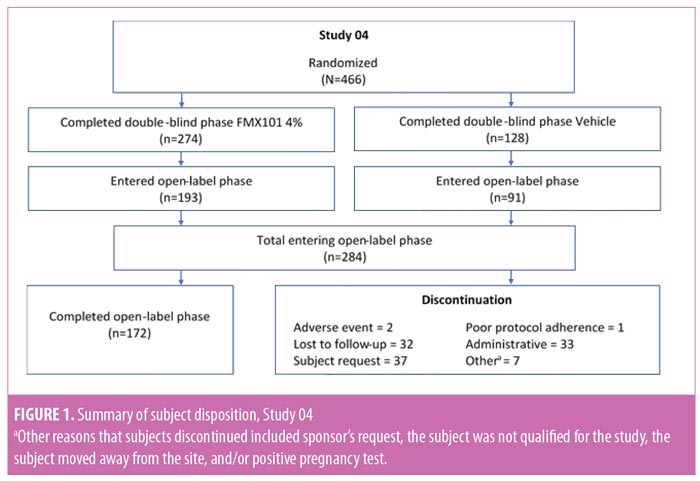
Baseline characteristics and subject disposition. Of the 961 subjects enrolled in Study 04 (n=466) and Study 05 (n=495), 657 subjects entered the open-label phase. From Study 04, 193 and 91 subjects were from the FMX101 4% and vehicle arms, respectively, while from Study 05, 256 and 117 subjects were from the FMX101 4% and vehicle arms, respectively. A total of 414 subjects completed the open-label phase (Study 04, n=172; Study 05, n=242), of which 291 subjects completed 52 weeks of FMX101 4% (Study 04, n=122; Study 05, n=169). Overall, 243 subjects discontinued the study (Study 04, n=112; Study 05, n=131), with seven subjects discontinuing due to a treatment-emergent adverse event (TEAE). Figures 1 and 2 show the disposition of subjects.
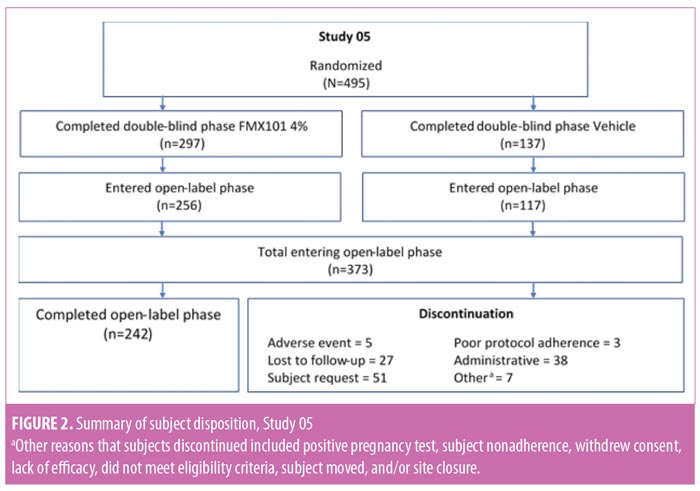
There were no significant differences in baseline demographics or disease characteristics between the two treatment groups in either study (Table 2). The mean subject age ranged between 19.8 and 20.3 years, and the majority of subjects were female and white. The mean inflammatory lesion count for subjects upon entering the open-label phase of Study 04 was 18.2 for the FMX101 4% group and 19.0 for the vehicle foam group; for Study 05, these numbers, respectively, were 18.2 and 21.8.
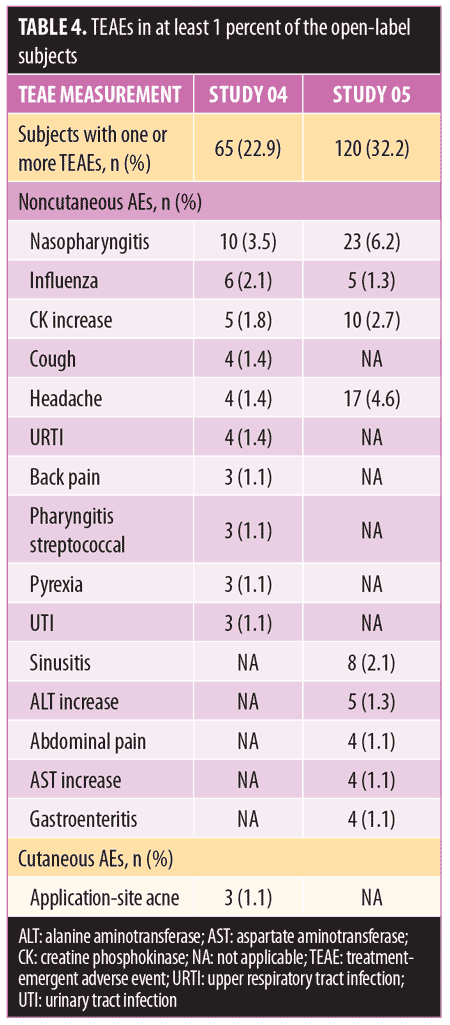
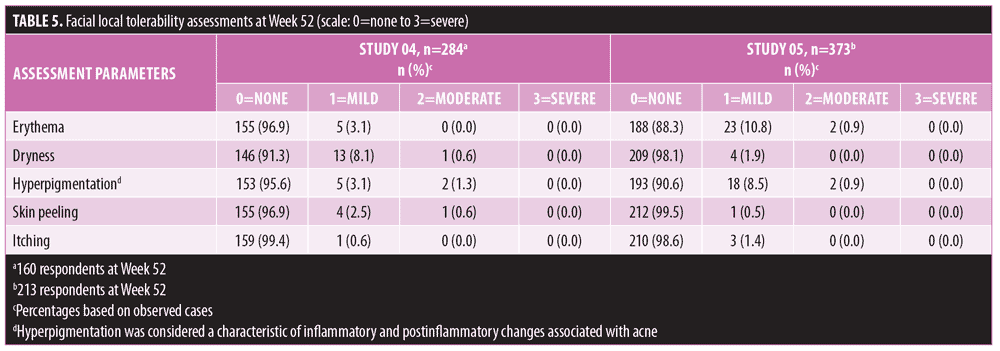
Long-term safety and tolerability. FMX101 4% minocycline foam was generally safe and well-tolerated. A total of 132 TEAEs were reported in 65 (22.9%) subjects in Study 04, while 207 TEAEs were reported in 120 (32.2%) subjects in Study 05 (Table 3). The most common TEAEs (occurring in 1% or less of subjects) in Study 04 were nasopharyngitis (3.5%), influenza (2.1%), and elevated creatine phosphokinase level (1.8%). In Study 05, the most common TEAEs (1% or less of subjects) were nasopharyngitis (6.2%), headache (4.6%), and elevated creatine phosphokinase level (2.7%) (Table 4). There were few treatment-related TEAEs, and all such events were considered mild to moderate in severity. Discontinuations due to TEAEs were reported for two subjects in Study 04 (application-site acne) and five subjects in Study 05 (application-site edema, application-site dermatitis) (Table 3). Three TEAEs leading to discontinuation were considered possibly (application-site edema and acne) or probably (application-site dermatitis) related to the study drug but were not considered serious in nature. Three serious TEAEs were reported (Study 04: pneumonia; Study 05: fatigue, head injury from fainting), with none being considered treatment-related or leading to discontinuation (Table 3). The most common cutaneous TEAE was application-site acne, reported in more than 1 percent of subjects in Study 04. Other application-site TEAEs included rash, application-site cyst, and dry skin. At the Week 52 assessment of dermal tolerability (face only), more than 95 percent of the subjects evaluated in the open-label phase of both studies had no or only mild severity scores for these assessments (Table 5).
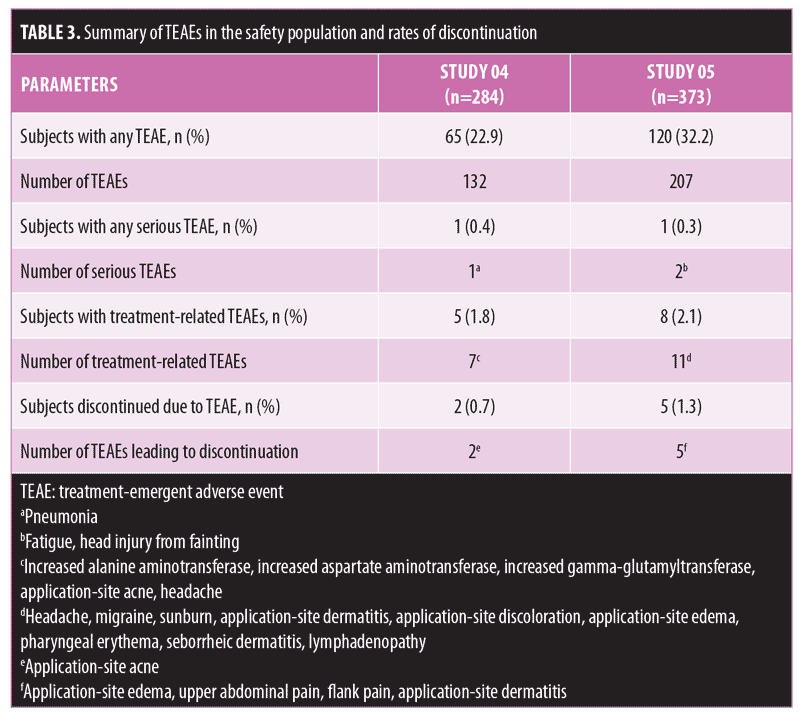
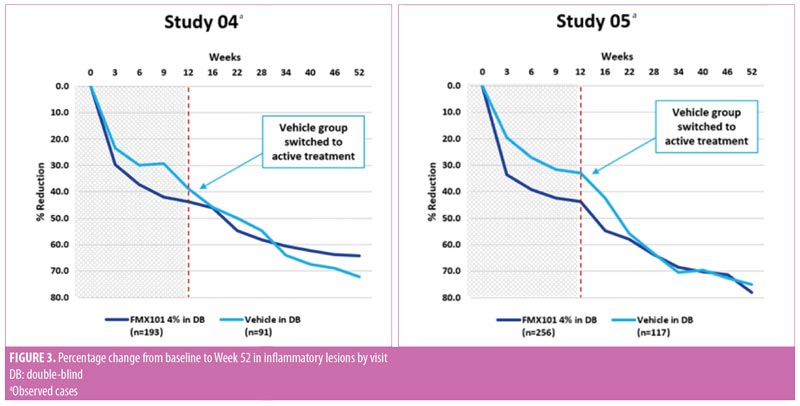
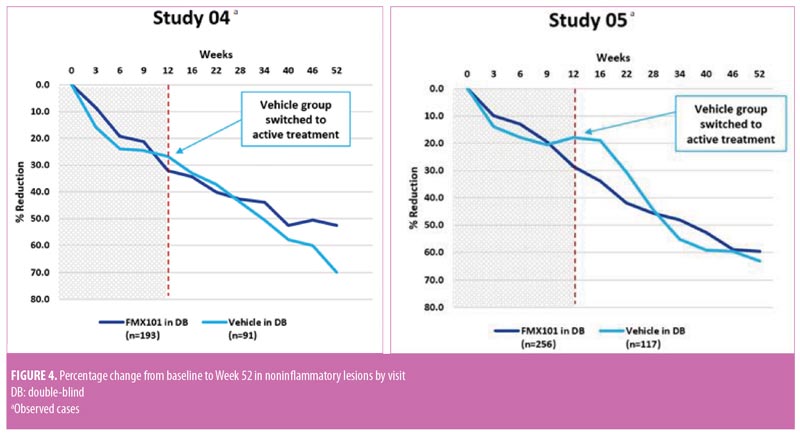
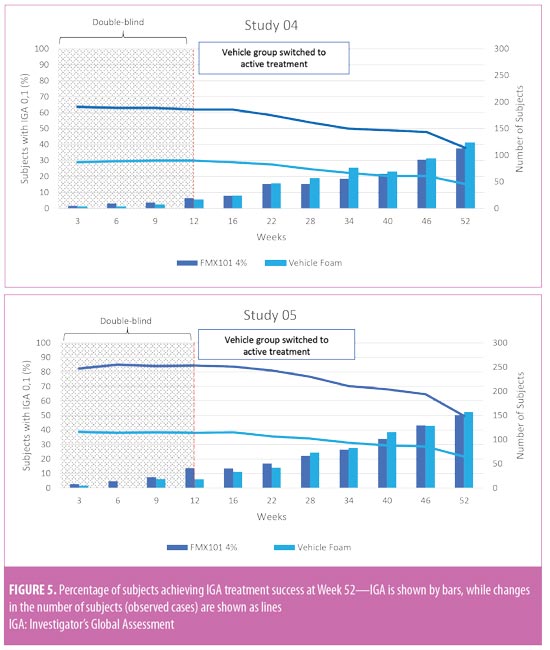
Long-term efficacy. In both studies, inflammatory and noninflammatory lesion counts continued to decrease throughout the open-label treatment time course, along with an increase in the portion of subjects achieving IGA treatment success. The Week 52 assessment showed a 64.3-percent reduction in inflammatory lesion count from baseline of the double-blind phase with FMX101 4% in Study 04 and a corresponding 78.0-percent reduction in Study 05 (Figure 3). The reduction from baseline of the double-blind phase in the noninflammatory lesion count was also maintained throughout and was 52.5 percent in Study 04 and 59.6 percent in Study 05 at Week 52 (Figure 4). The proportions of subjects achieving IGA treatment success at this time point were 37.7 percent in Study 04 and 50.3 percent in Study 05 (Figure 5).
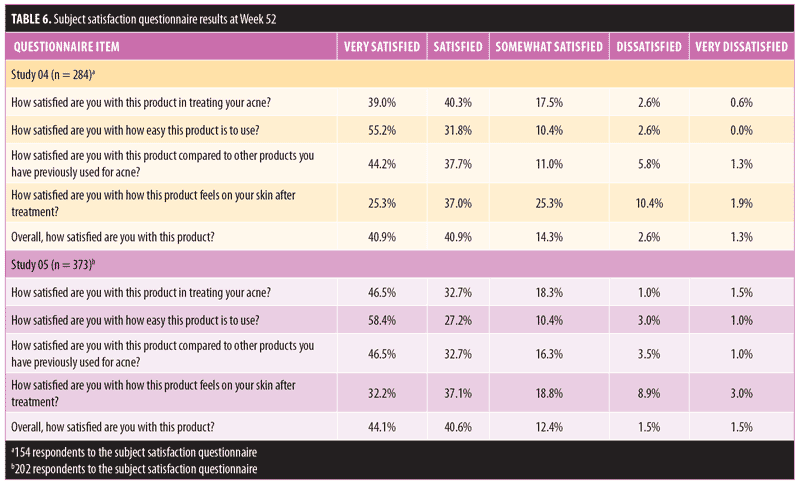
Subject satisfaction. Overall, there was a high rate of subject satisfaction with using FMX101 4% across both study groups. The majority of subjects (>80%) reported being overall satisfied or very satisfied with FMX101 4% (Table 6). In Study 04 and Study 05, 79.3 percent and 79.2 percent of subjects were, respectively, either satisfied or very satisfied with using FMX101 4% to treat their acne. More than 85 percent of all subjects were also satisfied or very satisfied with the ease of use of FMX101 4% (Study 04: 31.8% and 55.2%; Study 05: 27.2% and 58.4%), and approximately 80 percent of all subjects were also satisfied or very satisfied with how the product compared to treatments previously used (Study 04: 37.7% and 44.2%; Study 05: 32.7% and 46.5%).
Discussion
The efficacy, safety, and tolerability of FMX101 4% topical minocycline foam for the short-term treatment of moderate-to-severe acne were demonstrated in 1,377 subjects across three Phase III, 12-week studies (Study 04, Study 05, and Study 22). Results of the extension phase of Study 04 and Study 05 reported here indicated that the long-term use of FMX101 4% for up to 52 weeks appeared to be generally safe and well-tolerated, with few TEAEs. In relation to efficacy, FMX101 4% continued to be associated with a decreasing number of inflammatory lesions during the extended-treatment phase, as well as with an improvement in overall disease severity as measured by the IGA. In addition, the decrease in the number of noninflammatory lesions that was observed in the double-blind phase also continued throughout the extension phase.
The safety results during the open-label phase were similar to those observed during the double-blind phase of the studies. In both study phases, the overall incidence of TEAEs was comparable. In Study 04 and Study 05, most TEAEs were considered mild to moderate and unrelated to treatment. The type and frequency of TEAEs observed in all patients in the open-label phase were similar to those observed in the double-blind phases: nasopharyngitis, headache, and elevated creatine phosphokinase level were the most common.
An important observation was that the most concerning adverse events historically associated with oral minocycline were not seen with FMX101 4% over an extended-use period. For oral minocycline, TEAEs have been reported in 56 percent of patients and include fatigue, dizziness, and lupus-like syndrome; for oral tetracyclines, these are commonly photosensitivity and pigmentation of the skin, mucous membranes, and teeth.12,13 Pseudotumor cerebri (benign intracranial hypertension) has been associated with oral minocycline use in adolescents and adults, with clinical manifestations of headache and blurred vision.12 Although 1.4 percent of subjects in Study 04 and 4.6 percent of subjects in Study 05 reported headaches following treatment with FMX101 4%, these events were not considered serious and did not lead to study discontinuation.
The safety profile demonstrated by FMX101 4% was favorable. There were three serious TEAEs reported, but none of these was treatment-related or led to discontinuation of the study. The serious adverse events, which involved two subjects across both study groups (Study 04, pneumonia; Study 05, fatigue and head injury from fainting), were resolved prior to study completion. Very few subjects discontinued treatment due to TEAEs across the open-label treatment phase of both studies. All of the TEAEs implicated in discontinuation were mild to moderate in severity, and only three (application-site acne, Study 04; application-site edema and application-site dermatitis, Study 05) were considered possibly related to treatment.
Patient adherence to acne therapy is known to be low, estimated at approximately 50%.8,14 Adherence can be compromised by such factors as patient frustration due to a lack of clinical response or delayed response and by general dissatisfaction with the treatment experience or the occurrence of adverse events deemed unacceptable.8,14 In the current study, several outcomes—overall favorable tolerability profile, continued reduction in lesion counts, and steady improvement in the rate of IGA treatment success—suggested that use of an effective topical foam product might have had an impact on patient adherence to therapy. Satisfaction levels support this notion, considering that nearly 80 percent of all subjects were generally satisfied or very satisfied with FMX101 4% for treating their acne during the open-label phase; these findings were also consistent with the high satisfaction levels noted in the double-blind phase.9
In the Phase III studies, dermal tolerability assessments at Week 12 showed that more than 95 percent of the subjects reported no or mild symptoms in the treatment area. This trend continued in the open-label phase, with more than 95 percent of subjects reporting no or mild severity scores for these assessments at Week 52. Dryness (8.7%) and hyperpigmentation (4.4%) were the most commonly reported symptoms in Study 04, while erythema (11.7%) and hyperpigmentation (9.4%) were the most common in Study 05, although the majority of the cases were mild. Hyperpigmentation was considered a characteristic of the inflammatory and postinflammatory changes associated with acne and not the rare phenomenon of cutaneous discoloration (silver/blue/gray) that can occur from long-term treatment with oral tetracyclines.
Limitations. Because of the nature of open-label studies, no inference can be made on comparability, due to the absence of a study control. Therefore, no comparator analysis of efficacy was carried out for data from the 40-week open-label phase. The results of the current study suggest that the efficacy of FMX101 4% can continue to develop beyond an initial 12 weeks of therapy, as evidenced by the results from subjects undergoing 52 weeks of FMX101 4% treatment and from subjects who were assigned to vehicle groups during the double-blind phase and then switched to FMX101 4% at Week 12 upon entering the open-label phase. The potential clinical outcome in patients with mild acne could be worth investigating, in light of these observations. By protocol requirement, the majority of subjects in this study (87%) had moderate acne (n=573), with the remaining (13%) having severe acne (n=84).
Subjects were permitted to use concomitant medications, either prescription or over-the-counter, and were able to discontinue or recommence the study at any time during the 40-week open-label phase. Concomitant medications most frequently used at any time in the study and either indicated for acne treatment or known to have therapeutic benefit in acne were preparations of tretinoin (1.8%), adapalene (1.7%), amoxicillin (1.7%), salicylic acid (1.4%), and azithromycin (1.1%). Consequently, the results might not provide an accurate representation of the efficacy and safety in all patients solely using FMX101 4% for 40 consecutive weeks. Nonetheless, the study design, coupled with its favorable clinical outcome, might reflect a more holistic approach to acne treatment and, in doing so, provide some insight into the potentially achievable clinical outcomes in a real-world setting.
Conclusion
The results of this open-label extension phase of Study 04 and Study 05 demonstrated that FMX101 4% topical minocycline foam appeared to be safe, effective, and well-tolerated for the long-term treatment of acne. The safety profile for an additional 40 weeks of daily application was similar to that seen after 12 weeks of use in the double-blind phase of these studies. Taken in its entirety, results from the clinical program evaluating the overall safety and efficacy of FMX101 4% provide a strong evidential foundation for a new topical treatment option for patients with moderate-to-severe acne.
Acknowledgments
The authors would like to thank the patients and investigators who participated in the study. Writing assistance for this manuscript was provided by p-value communications.
References
- Biswal I, Gaind R, Kumar N, et al. In vitro antimicrobial susceptibility patterns of Propionibacterium acnes isolated from patients with acne vulgaris. J Infect Dev Ctries. 2016;10(10):1 140–1145.
- Jones MT, Ellman H, deVries T. Pharmacokinetic comparison of once-daily topical minocycline foam 4% vs oral minocycline for moderate-to-severe acne. J Drugs Dermatol. 2017;16(10):1022.
- Pena S, Hill D, Feldman SR. Use of topical retinoids by dermatologists and non-dermatologists in the management of acne vulgaris. J Am Acad Dermatol. 2016;74(6):1252–1254.
- Thiboutot DM, Dréeno B, Abanmi A, et al. Practical management of acne for clinicians: an international consensus from the Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2018;78(2S1):S1–S23.e1.
- Mendoza N, Hernandez PO, Tyring SK, et al. Antimicrobial susceptibility of Propionibacterium acnes isolates from acne patients in Colombia. Int J Dermatol. 2013;52(6):688–692.
- Barbieri JS, James WD, Margolis DJ. Trends in prescribing behavior of systemic agents used in the treatment of acne among dermatologists and nondermatologists: a retrospective analysis 2004-2013. J Am Acad Dermatol. 2016;77(3):456–463.
- Odsbu I, Selmer R, Lundborg CS, Blix HS. Increased prescribing of systemic tetracyclines and isotretinoin for treatment of acne. J Antimicrob Chemother. 2017;72:1510–1515.
- Dreno B, Thiboutot D, Gollnick H, et al. Large-scale worldwide observational study of adherence with acne therapy. Int J Dermatol. 2010;49(4):448–456.
- Gold LS, Dhawan S, Weiss J, et al. A novel topical minocycline foam for the treatment of moderate-to-severe acne vulgaris: results of 2 randomized, double-blind, phase 3 studies. J Am Acad Dermatol. 2019;80(1):168–177.
- Jones TM, Ellman H, deVries T. Pharmacokinetic evaluation of once-daily topical 4% minocycline foam in adult and pediatric subjects with moderate-to-severe acne in two Phase 1 studies. Skin. 2018; 2:S34.
- Raoof J, Hooper D, Moore A, et al. Efficacy and safety of a novel topical minocycline foam for the treatment of moderate-to-severe acne vulgaris: a phase 3 study. J Am Acad Dermatol. 2019 Jun 1. pii: S0190-9622(19)30882-5.
- Solodyn [package insert]. Scottsdale, AZ: Medicis; March 2011.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5): 945–973.e33.
- Snyder S, Crandell I, Davis SA, Feldman SR. Medical adherence to acne therapy: a systematic review. Am J Clin Dermatol. 2014;15(2):87–94.

