J Clin Aesthet Dermatol. 2019;12(6):17–24
 by James Q. Del Rosso, DO, FAOCD, FAAD; Emil Tanghetti, MD, FAAD; Guy Webster, MD, PhD, FAAD; Linda Stein Gold, MD, FAAD; Diane Thiboutot, MD, FAAD; and Richard L. Gallo, MD, PhD, FAAD
by James Q. Del Rosso, DO, FAOCD, FAAD; Emil Tanghetti, MD, FAAD; Guy Webster, MD, PhD, FAAD; Linda Stein Gold, MD, FAAD; Diane Thiboutot, MD, FAAD; and Richard L. Gallo, MD, PhD, FAAD
Dr. Del Rosso is Adjunct Clinical Professor of Dermatology at Touro University, Nevada in Henderson, Nevada; and Research Director at JDR Dermatology Research, Clinical Dermatology, Thomas Dermatology in Las Vegas, Nevada. Dr. Tanghetti is with the Center for Dermatology and Laser Surgery in Sacramento, California. Dr. Webster is with Jefferson Medical College in Hockessin, Delaware. Dr. Stein Gold is the Director of Dermatology, Clinical Research, and Division Head of Dermatology at Henry Ford Health System in Detroit and West Bloomfield, Michigan. Dr. Thiboutot is Professor of Dermatology at Penn State University College of Medicine in Hershey, Pennsylvania. Dr. Gallo is Chief, Division of Dermatology, and Professor of Medicine and Pediatrics at the University of California, San Diego in San Diego, California.
FUNDING: The American Acne & Rosacea Society received no direct funding for this manuscript. No corporate benefactor of the AARS was involved in any aspect of the above actions, none were involved in any discussions related to the decision to submit the manuscript for publication or any of its contents, nor did any AARS corporate benefactor or related agency contribute to manuscript review or content.
DISCLOSURES: : Dr. Del Rosso is an investigator, advisor, and/or speaker for Aclaris Therapeutics, Almirall, BiopharmX, EPI Health, Foamix, Galderma Laboratories, LEO Pharma, Mayne Pharma, Ortho Dermatologics, and Sol-Gel Technologies. Dr. Stein Gold is an investigator, advis or, and speaker for Galderma and Ortho Dermatologics and an investigator and advisor for Foamix Pharmaceuticals and Sol-Gel Technologies. Dr. Tanghetti is an investigator, advisor, and/or speaker for Accure, Galderma Laboratories, Hologic, Novartis Pharmaceuticals, Ortho Dermatologics, and Pfizer. Dr. Thiboutot is an investigator, advisor, and/or speaker for BiopharmX, Botanix, Cassiopea, Foamix, Galderma Laboratories, and Novartis Pharmaceuticals. Dr. Webster is an investigator, advisor and/or speaker for Aclaris Therapeutics, Allergan, BMS, Cutanea, Dermira, Galderma, GSK, Janssen, Ortho Dermatologics, and Sienna
ABSTRACT: Importance. Previous consensus articles on rosacea from the American Acne and Rosacea Society (AARS) have focused on pathophysiology, clinical assessment based on phenotypic expressions of rosacea, management guidelines, discussions of individual medical therapies, and reviews of physical modalities. Pathophysiologic mechanisms believed to be operative in rosacea have been covered extensively in the literature.
Objective.This article updates the previously published consensus recommendations from the AARS on the management of rosacea, including systematic literature and evidence-based reviews of available therapeutic agents and physical modalities.
Observations. This article includes discussions of available published data on topical ivermectin, topical oxymetazoline, combination therapy approaches, and physical devices for the management of rosacea. Consistent with what many publications on rosacea currently emphasize, clinicians are encouraged to define the clinical manifestations present in the patient and to select therapies that correlate with the optimal treatment of those manifestations. There are less data available on how to optimally integrate therapies; however, it appears that rationally selected medical therapies can be utilized concurrently.
Conclusion. Due to the multifactorial pathogenesis of rosacea, its clinical presentation is heterogeneous. Rosacea is a chronic and recurrent inflammatory disorder, and clinical manifestations often vary in nature and severity over time, which might necessitate an adjustment in treatment. As new data become available, rosacea management approaches should be updated.
KEYWORDS: Alpha-agonist, erythema, inflammation, rosacea
Recognized as one of the most common and clinically characteristic facial skin disorders, rosacea is an inflammatory dermatosis with a reported prevalence of at least 10 percent among Caucasian adults; it also affects several other racial groups, including Latin-American, African-American, African, and Asian people.1–4 The diagnosis of rosacea is made clinically, based on visible assessment and patient history, after other causes of facial erythema and/or papulopustular skin lesions have been excluded,2,5 including contact dermatitis, seborrheic dermatitis, photodamage, acne vulgaris, cutaneous lupus, and carcinoid syndrome.
The classification of rosacea in both clinical practice and research previously utilized subtype designations as described by Wilkin et al in 20025 from the National Rosacea Society. However, the current recommendations from multiple organizations with interest in the diagnosis and treatment of rosacea suggest characterizing patients with rosacea by individual clinical manifestations and symptoms that are present at the time of examination.2,6–8 As rosacea is a phenotypically heterogeneous disease, this might include central facial erythema without papulopustular (PP) lesions; central facial erythema with PP lesions; the presence of phymatous changes, ocular signs, and symptoms; extensive presence of facial telangiectasias; and marked, persistent, nontransient facial erythema that remains between flares of rosacea and might exhibit severe intermittent flares of acute vasodilation (flushing of rosacea).6,7 Manifestations at various time points in a single patient might differ depending on whether the rosacea is flared or quiescent, the age of the patient, the duration of his or her disease, the frequency and magnitude of rosacea flares, and associated symptomatology.6,8,9
Previous consensus articles on rosacea from the American Acne & Rosacea Society (AARS) focused on pathophysiology, clinical assessment based on phenotypic expressions of rosacea, management guidelines, discussions of individual medical therapies, and reviews of physical modalities.6,10–13 Pathophysiologic mechanisms believed to be operative in rosacea have been covered extensively in the literature.14–16 The goal of this article is to update the previously published consensus recommendations from the AARS on the management of rosacea, including a review of therapeutic agents and formulations that have become available since the previous publications and a discussion of newer information on physical modalities.
The hope is that the current management recommendations, based on currently available evidence and clinical experience, can serve as a guide to clinicians. In all of the studies referenced in this article, unless otherwise specified, recognized inclusion criteria, exclusion criteria, washout periods of any previous relevant therapies, and tolerability/safety assessments were incorporated and accepted methods for endpoint evaluations were used (e.g., Investigator Global Assessment [IGA], lesion counts, tolerability/safety assessments).
Rosacea Management Recommendations
Topical ivermectin. Ivermectin (IVM) is an avermectin derivative that has been used extensively for many years in human and veterinary medicine due to its antiparasitic activity and anti-inflammatory properties.17 The favorable safety profiles of both oral and topical IVM have been correlated with its inability to cross the human blood-brain barrier (BBB) while exhibiting a high affinity for invertebrate neuronal ion channels, allowing for its selective activity against many parasitic organisms.17 With regard to rosacea, especially in the presence of PP lesions, the anti-inflammatory properties of IVM that appear to correlate with rosacea pathophysiology are of specific investigative interest. The reduction of Demodex mite proliferation, which appears to have a role as a trigger factor in a subgroup of patients with rosacea, is another targeted area of research.18–20
IVM and rosacea pathophysiology. Avermectin derivatives, including IVM, have been associated with anti-inflammatory effects in multiple in-vitro studies; however, the correlation of these effects with rosacea is unknown.17,21,22 Recently, a single-center, single-treatment pilot study assessed once-daily application of IVM 1% cream on the facial skin of 20 subjects with papulopustular rosacea (PPR). Over a 12-week treatment period, investigators observed marked clinical improvement through dual mechanisms of action.23 In addition to assessing standard clinical parameters, this study utilized real-time polymerase chain reaction (RT-PCR) and immunofluorescence staining to evaluate multiple inflammatory/immune tissue biomarkers; the study also evaluated Demodex mite density via skin surface biopsies. Gene expression levels for multiple biomarkers (e.g., LL-37 [cathelicidin], interleukin [IL]-8, toll-like receptor [TLR]-4, human beta-defensin [HBD]-3) were significantly downregulated following 12 weeks of topical IVM use (p<0.05); mean mite density also was significantly reduced (p<0.001). All 20 subjects were reported to improve clinically, with 80 percent (16/20) achieving “clear” or “almost clear” results according to Investigator’s Global Assessment (IGA) score.23
Topical IVM clinical studies. Once-daily IVM 1% cream (Soolantra® Cream, 1%; Galderma Laboratories LP, Fort Worth, Texas) was shown to be significantly more effective than vehicle (n=461) in two pivotal, Phase III, 12-week, double-blind, randomized, controlled trials of adults (N=910) with moderate-to-severe PPR (p<0.001).24 In a 16-week, investigator-blinded, randomized, controlled trial of adults with moderate-to-severe PPR, IVM once daily (N=478) demonstrated significant superiority in efficacy compared to metronidazole 0.75% cream applied twice daily (n=484) (p<0.001).25 An extension assessment of the 16-week study evaluated time to rosacea relapse and maintenance of remission over 36 weeks.26 In this extension study, IVM cream once daily (n=399) was compared to metronidazole 0.75% cream twice daily (n=365). Both agents were used intermittently for flares in their respective study groups until subjects achieved an IGA score of “clear” or “almost clear”; if new flares occurred, these treatments were restarted until PPR was controlled again, as described above. Median time to first relapse was significantly longer in the IVM group (115 days) than in the metronidazole group (85 days) (p=0.0365; Kaplan-Meier plot analysis), and median days free of treatment was higher with IVM use compared to metronidazole use (196 days vs. 169.5 days; p=0.026).26
Favorable tolerability and safety profiles of IVM 1% cream have also been established in a long-term (52-week) safety study, with low reported rates of cutaneous tolerability reactions (<2% overall), comparable skin tolerability rates to those of metronidazole 0.75% cream and vehicle, and no observed systemic safety signals.27
Clinical application of topical ivermectin in rosacea. IVM 1% cream has been shown to be an effective, well-tolerated, and safe treatment for PPR in adults in several randomized, controlled trials of subjects with moderate-to-severe disease and in a case series (N=34) from clinical practice.24–29 A systematic meta-analysis of 19 randomized, clinical trials reported that IVM 1% cream once daily appears to be more effective than, and at least as tolerable/safe as, other available topical agents used to treat PPR;30 however, no true head-to-head comparative studies currently exist, with the exception of studies comparing IVM 1% cream to metronidazole 0.75% cream.30 Based on a review of four randomized, controlled trials (N=1,366) comparing IVM 1% cream to metronidazole 0.75% cream, achieving a study endpoint of “clear” based on IGA assessment optimized remission of rosacea; the median time to relapse was greater than eight months in subjects achieving an IGA rating of “clear,” compared with three months for those rated as “almost clear” (p<0.0001).31
Available data support the use of IVM 1% cream as an option for treatment of PPR as a monotherapy, as well as in combination with a topical alpha1-agonist for treatment of the persistent nontransient facial erythema component of PPR.28,32–33
Topical oxymetazoline. Oxymetazoline 1% cream, applied once daily, is a topical alpha1-agonist that was approved by the United States Food and Drug Administration (FDA) for the treatment of persistent facial erythema of rosacea in adults.34 Morning application is recommended to allow for reduction of the facial erythema during the day; a noticeable onset of effect generally occurs within 1 to 3 hours after application, with a duration of effect usually observed over 8 to 10 hours. In a Phase II, four-week, double-blind, randomized, controlled trial of adult subjects with moderate-to-severe persistent facial erythema due to rosacea (N=356), oxymetazoline HCI cream (Rhofade® Cream, 1%; Aclaris Therapeutics, Inc., Wayne, Pennsylvania) demonstrated optimal dosing at one percent, compared to 0.5-percent and 1.5-percent concentrations, when applied once or twice daily; safety and application-site skin tolerability were considered favorable and were similar among all study groups.35
Topical oxymetazoline clinical studies. Two Phase III, four-week, double-blind, randomized, controlled trials compared oxymetazoline 1% cream to vehicle, both applied once daily, in adult subjects with moderate-to-severe persistent facial erythema due to rosacea at baseline (N=885; 1:1).36,37 In both pivotal studies, oxymetazoline 1% cream demonstrated significant superiority to vehicle in reaching the primary study endpoint—achieving at least a two-grade reduction in erythema—which was rated separately by investigator and patient at the end of the study (p<0.001 in both studies). Digital image analysis evaluating erythema reduction also favored once-daily application of oxymetazoline 1% cream over once-daily application of vehicle (p<0.001).36
A long-term (52 weeks), open-label study evaluated the use of oxymetazoline 1% cream once daily for moderate-to-severe persistent facial erythema of rosacea in adults (N=440).38 Overall, this study demonstrated sustained efficacy, tolerability, and safety over the 52 week duration of the study. Discontinuation of treatment, due mostly to application-site adverse events (AEs), occurred in 3.2 percent of subjects, with no systemic safety signals demonstrated; no clinically relevant changes in skin blanching (i.e., over-whitening), inflammatory (PP) lesions, or telangiectasias were noted.38
The FDA-approved protocol designs used in the pivotal randomized, controlled trials evaluating both brimonidine 0.33% gel and oxymetazoline 1% cream were very similar.39,40 However, the studies evaluating oxymetazoline 1% cream included additional follow-up steps to assess worsening of facial erythema, such as rebound after discontinuation.37–39 Data from the clinical studies and the approved package insert for oxymetazoline 1% cream did not report post-treatment rebound or worsening of facial erythema of rosacea.34,36–39 AEs reported during treatment phases showed that application-site erythema occurred in one percent of subjects treated with oxymetazoline 1% cream compared to 0.4 percent in vehicle-treated subjects in the pivotal randomized, controlled trials and in two percent of oxymetazoline 1% cream-treated subjects in the long-term study.34,36–39 These data support that treatment-related worsening of facial erythema (defined as rebound in pivotal clinical studies) noted during active use and/or after discontinuation of once-daily oxymetazoline 1% cream is uncommon.
Clinical application of topical oxymetazoline in rosacea. Oxymetazoline 1% cream may be used for the management of persistent, nontransient, facial erythema of rosacea in adults who present with or without PP lesions.36–38 In patients with PPR, oxymetazoline 1% cream has been successfully utilized for the reduction of persistent facial erythema along with concurrent use of an agent that reduces PP lesions and perilesional erythema (e.g., topical metronidazole, topical azelaic acid, topical IVM, oral doxycycline).38
Topical azelaic acid (AzA). AzA 15% gel (Finacea® Gel, 15%; LEO Pharma Inc., Madison, New Jersey), applied twice daily, is a well-established treatment for PPR.10,42–45 AzA has been used as a monotherapy, primarily in cases of mild-to-moderate severity, or in combination with oral doxycycline (including sub-antibiotic dose doxycycline) in patients with severe PPR.44,46 More recently, twice-daily AzA 15% foam (Finacea® Foam, 15%; LEO Pharma Inc., Madison, New Jersey) was approved by the FDA for the treatment PPR in adults, with studies reporting efficacy and safety similar to that observed in the twice-daily AzA 15% gel studies.47–49 The foam vehicle is a lipid-rich, hydrophilic oil-in-water emulsion.47,50
Phase III, 12-week, randomized, controlled trials compared AzA 15% gel and AzA 15% foam, both applied twice daily, to their respective vehicles in adult subjects with facial PPR.42,48–50 Baseline demographics and disease-related characteristics (i.e., lesion counts, IGAs) were similar in these studies. In the Phase III studies evaluating AzA 15% foam (n=484), application site pain (e.g., stinging, burning) occurred in 3.5 percent and pruritus in 1.4 percent of AzA-treated subjects, all of whom, based on study protocol, were instructed to use gentle skin care products.48–50 In the AzA 15% gel Phase III studies, the most commonly reported treatment-related AEs were burning, stinging, and/or tingling (29%) and pruritus (11%), with no recommendations given regarding skin care during these studies.42 Although there are no comparative head-to-head studies of AzA 15% foam versus AzA 15% gel, these data support the concept that proper skin care is a vital component of rosacea management and that vehicle formulation can play an important role in mitigating application-site AEs.50
Combination topical therapy. When treating patients with PPR, an important clinical consideration is how to optimally integrate a topical alpha-agonist, used to treat persistent facial erythema of rosacea, with a topical agent, used to treat PP lesions and perilesional erythema. This question was investigated in a multicenter, 12-week, double-blind, randomized, controlled trial that evaluated subjects with moderate-to-severe PPR characterized by marked persistent facial erythema and PP lesions (N=190).33 Enrolled subjects were randomized to one of three groups:
• Active group 1—brimonidine 0.33% gel, applied once daily in the morning (AM) and IVM 1% cream, applied once daily in the evening (PM), both for 12 weeks (n=49)
• Active group 2—gel vehicle (once daily AM, Weeks 1–4), brimonidine 0.33% gel (once daily AM, Weeks 1–8), and IVM 1% cream (once daily PM, Weeks 1–12) (n=46)
• Vehicle group— gel vehicle (once daily AM) and cream vehicle (once daily PM) for 12 weeks (n=95).
Over the duration of the study, gentle skin care was controlled with a specific cleanser, moisturizer, and sunscreen provided to all subjects. Significantly superior efficacy based on IGA ratings of “clear” or “almost clear” ratings for the reduction in facial erythema and decrease in PP lesions was greatest in the active groups (combined 55.8%) compared to the vehicle group (36.8%) at Week 12 (p=0.007).33 Treatment success was greater in Active Group 1 (IGA “clear” or “almost clear,” 61.2%), which received both active treatments for all 12 weeks, compared to Active Group 2 (50%), in which use of brimonidine 0.33% gel was delayed until Week 5. Skin tolerability favorable in all study groups.33
Clinical relevance of combination therapy data. The reductions in facial erythema and PP lesion counts in this topical combination study33 supports the results of other studies demonstrating the additive therapeutic benefit of combining alpha-agonist therapy with an agent that reduces PP lesions. The best therapeutic outcome was noted when both topical agents were used throughout the study; however, delaying the use of the topical alpha-agonist for the first four weeks of treatment was still associated with marked clinical improvement by Week 12.33 In addition to parameters assessed by the investigator (e.g., IGA, lesion counts), study subjects in the active groups also reported greater improvements than those in the vehicle group. Lastly, the use of proper skin care appears to be an integral component of successful rosacea management.
Physical modalities (device therapy). Consensus recommendations from the AARS on use of physical modalities for the treatment of rosacea were reviewed in detail in previous publications.7,8,10,11,13 An important benefit of device treatment for rosacea is that the therapeutic effects are generally seen over a limited number of treatment sessions, which are in contrast to the need for daily treatment over extended periods of time with topical or oral medication. Once an endpoint of an acceptable therapeutic effect is achieved, the results are typically maintained for a number of years. Concurrent medical therapy is often used to complement device treatments.
Telangiectasias/diffuse facial erythema. Since improvements in telangiectasias and facial erythema of rosacea were reported with use of the pulsed-dye laser (PDL), this laser continues to be an important modality in rosacea treatment.51 Later generations of PDL have incorporated a different pulse format, which largely eliminated the marked bruising observed after treatment with early PDL devices.
Intense pulsed light (IPL) devices have also been used successfully to treat both the facial erythema and dilated facial vessels associated with rosacea.52 Studies have demonstrated comparable efficacy between updated PDL and IPL devices.53,54
Early studies with long-pulsed 532-nm neodymium-doped yttrium aluminum garnet (Nd:YAG) laser demonstrated efficacy in treating telangiectasia.55 More recent studies using a more powerful 532-nm laser reported excellent results when treating telangiectasia and diffuse erythema in patients with rosacea, which were comparable to those seen with PDL devices.56 Importantly, the use of lasers, IPL devices, and PDLs have shown superior results treating telangiectatic vessels compared to results achieved treating diffuse facial erythema of rosacea, although both have shown response.57
Electrocautery has been employed for many years at low settings to treat visible dilated blood vessels associated with rosacea. While treatment can be successful when performed carefully using a fine-point tip, there is a risk of nonspecific thermal damage that can produce small linear or punctate scars.37
PP lesions. Data on the use of lasers and light devices for the treatment of papules and pustules (PP) of rosacea suggest they can be helpful.51 However, the study methodology used to collect these data failed to capture PP lesion counts or clinical descriptions of rosacea in a controlled manner. Additional well-designed studies evaluating the use of devices for treatment of PPR are needed.
Combination use of a topical alpha-agonist and device therapy. Data are limited on the use of topical alpha-agonist therapy in combination with IPL or specific lasers for the treatment of rosacea. One of the authors of this article (ET), who has extensive experience with the use of devices for rosacea, suggests that the use of a topical alpha-agonist and physical devices are complementary. The natural appearance and the degree of improvement of diffuse facial erythema with use of either topical brimonidine or topical oxymetazoline usually produces a better visible facial appearance than the partial improvement typically seen with devices alone. The partial response achieved when using laser/light devices to treat diffuse facial erythema, combined with the excellent results seen with these devices when treating telangiectasia51–57 (which are not responsive to the use of a topical alpha-agonist), suggest that a topical alpha-agonist can be initiated after laser and light treatments. There have been some early studies that suggest that the use of an alpha-agonist immediately following treatment with these devices diminishes the pulse treatment erythema that commonly occurs with these devices.58 Hopefully, further studies will help determine whether use of a topical alpha-agonist will change or compromise the therapeutic effects of the device. Additionally, there are studies in progress that are evaluating the use of alpha-agonists to compliment device treatments when used a few days after treatment, as well as literature supporting the potential inhibition of vascular endothelial growth factor with brimonidine, which suggests a potential additive effect of device treatment followed by the use of a topical alpha-agonist.59 At this point, we do not have sufficient data regarding the complimentary use of these agents with laser and light devices to make evidence-based treatment recommendations.
Adverse effects associated with the use of an ablative device followed directly by the use of a topical alpha-agonist have been observed.60 Potentially, a treatment with any device that damages the epidermal barrier can result in increased percutaneous absorption of a topically applied alpha-agonist, increasing the risk of hypotension. Studies exploring the safe and complimentary use of devices and topical alpha-agonist therapy are important and much needed.
Microfocused ultrasound and bipolar radiofrequency. There are a number of devices that cause nonselective vascular damage that hold some promise for success in the treatment of rosacea. Microfocused ultrasound with visualization (MFU-V) and bipolar radiofrequency pins have been shown to improve the diffuse facial erythema associated with rosacea.61 Data from the study evaluating MFU-V technology in patients with rosacea was generated using the same rigorous parameters as those used in the alpha-agonist pivotal clinical trials, which bolsters the investigators’ findings. Moving forward, clinical studies evaluating the efficacy and safety of devices for the treatment of rosacea could generate better quality data by incorporating validated assessment methods, such as the IGA, Clinician’s Erythema Assessment, telangiectasia grading score, inflammatory lesion counts, standardized side effect assessments, and patient efficacy evaluations, especially when the number of study participants is limited or a split-faced study design is being utilized.
Consensus Recommendations for the Management of Rosacea
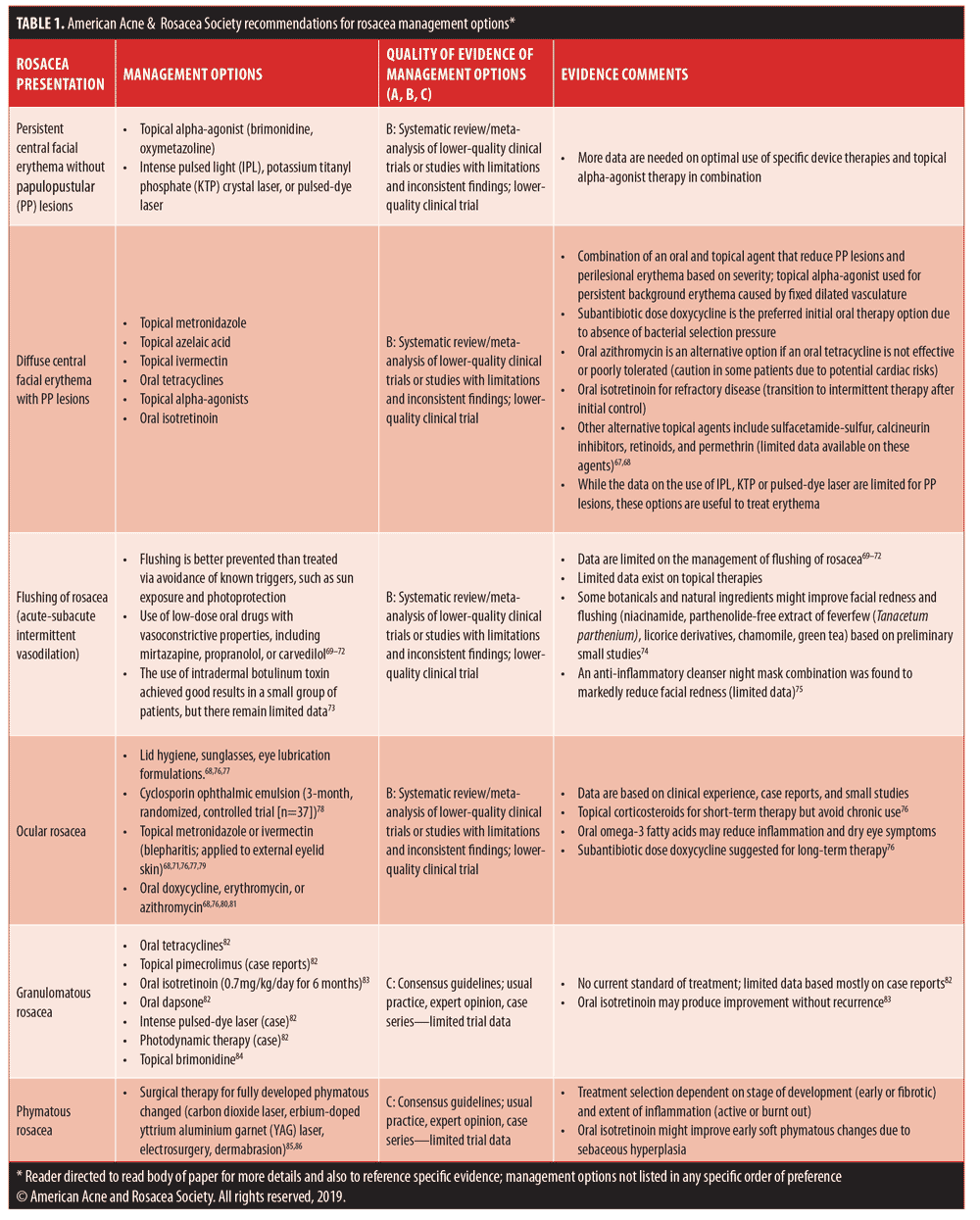
The already published guidelines for rosacea management primarily focus on incorporating medical and/or device therapies that are correlated with the visible manifestations of rosacea.7,8,10–13,62–66 In all cases, proper skin care, photoprotection, and avoidance of patient-specific rosacea triggers are suggested. How therapies are used, either concurrently or in a staggered fashion, might be considered by some to be more art than science, as clinical studies and outcomes data are currently lacking. However, some combination approaches have been addressed in the literature.33,46,58 These include the initial use of topical metronidazole or topical azelaic acid concurrently with oral doxycycline for treatment of severe PPR with transition to topical therapy alone after adequate response is achieved; topical brimonidine and topical ivermectin for treatment of PPR with diffuse persistent facial erythema of at least moderate severity; and combination treatment with potassium titanyl phosphate laser and topical brimonidine for diffuse persistent facial erythema of rosacea.36,46,58 Table 1 depicts consensus recommendations from the AARS on rosacea management correlated with clinical manifestations observed at the time of presentation.7,8,10–13,62–76
Summary
This article provides an update to previously published consensus recommendations from the AARS on rosacea management, including discussions of topical ivermectin, topical oxymetazoline, combination therapy approaches, and physical devices. Consistent with what many publications on rosacea currently emphasize, clinicians are encouraged to define the clinical manifestations currently present in each individual patient and to select therapies that correlate with the optimal treatment of those manifestations. There are less data available on how to optimally combine therapies; however, it appears that rationally selected medical therapies can be utilized concurrently. As the pathophysiology of rosacea is multifactorial, the clinical presentation of rosacea is heterogeneous. Rosacea is a chronic and recurrent inflammatory disorder, and clinical manifestations often vary in their nature and severity over time. This might necessitate an adjustment in management. As new data become available, management approaches should be updated.
Acknowledgments
The authors would like to acknowledge the American Acne & Rosacea Society Board of Directors Drs. Julie Harper, Mark Jackson, Bethanee Schlosser, Jonathan Weiss, Joshua Zeichner, and Emmy Graber for their review and approval of and contribution to this manuscript. Administrative and editorial assistance was provided by Stacey Moore, Executive Director of the American Acne & Rosacea Society.
References
- van Zuuren E. Rosacea. N Engl J Med. 2017;377(18):1754–1764.
- Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78(1):148–155.
- Tan J, Berg M. Rosacea: current state of epidemiology. J Am Acad Dermatol. 2013;69:6 (Suppl 1):S27–S35.
- Al-Dabagh A, Davis SA, McMichael AJ, Feldman SR. Rosacea in skin of color: not a rare diagnosis. Dermatol Online J. 2014;20(10). pii: 13030/qt1mv9r0ss.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46(4):584–587.
- Del Rosso JQ, Gallo RL, Tanghetti E, et al. An evaluation of potential correlations between pathophysiologic mechanisms, clinical manifestations, and management of rosacea. Cutis. 2013;91(3 Suppl):1–8.
- Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 1: a status report on the disease state, general measures, and adjunctive skin care. Cutis. 2013;92(5):234–240.
- Tan J, Almeida LM, Bewley A, et al. Updating the diagnosis, classification, and assessment of rosacea: recommendations from the global Rosacea Consensus (ROSCO) panel. Br J Dermatol. 2017;176(2):431–438.
- Two AM, Wu W, Gallo RL, Hata TR. Rosacea: part I. Introduction, categorization, histology, pathogenesis, and risk factors. J Am Acad Dermatol. 2015;72(5):749–758.
- Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 2: a status report on topical agents. Cutis. 2013;92(6):277–284.
- Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 3: a status report on systemic therapies. Cutis. 2014;93(1):18–28.
- Tanghetti E, Del Rosso JQ, Thiboutot D, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 4: a status report on physical modalities and devices. Cutis. 2014;93(2):71–76.
- Del Rosso JQ, Thiboutot D, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 5: a guide on the management of rosacea. Cutis. 2014;93(3):134–138.
- Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(Suppl 1):S15–S26.
- Ahn CS, Huang WW. Rosacea pathogenesis. Dermatol Clin. 2018;36(2):81–86.
- Steinhoff M, Schmelz M, Schauber J. Facial erythema of rosacea—aetiology, different pathophysiologies and treatment options. Acta Derm Venereol. 2016;96(5):579–586.
- Kircik LH, Del Rosso JQ, Layton AM, Schauber J. Over 25 years of clinical experience with ivermectin: an overview of safety for an increasing number of indications. J Drugs Dermatol. 2016;15(3):325–332.
- Del Rosso JQ. Topical ivermectin: data supporting dual modes of action in rosacea. J Clin Aesthet Dermatol. 2017;10(9):39–42.
- Casas C, Paul C, Lahfa M, et al. Quantification of Demodex folliculorum by PCR in rosacea and its relationship to skin innate immune activation. Exp Dermatol. 2012;21(12):906–910.
- Aroni K, Tsagroni E, Lazaris AC, et al. Rosacea: a clinicopathological approach. Dermatology. 2004;209(3):177–182.
- Ci X, Li H, Yu Q, et al. Avermectin exerts anti-inflammatory effect by downregulating the nuclear transcription factor kappa-B and mitogen-activated protein kinase activation pathway. Fundam Clin Pharmacol. 2009;23(4):449–455.
- Yan S, Ci X, et al. Anti-inflammatory effects of ivermectin in mouse model of allergic asthma. Inflamm Res. 2011;60(6):589–596.
- Schaller M, Gonser L, et al. Dual anti-inflammatory and anti-parasitic action of topical ivermectin 1% in papulopustular rosacea. J Eur Acad Dermatol Venereol. 2017;31(11):1907–1911.
- Stein L, Kircik L, et al. Efficacy and safety of ivermectin 1% cream in treatment of papulopustular rosacea: results of two randomized, double-blind, vehicle-controlled pivotal studies. J Drugs Dermatol. 2014;13(3): 316–323.
- Taieb A, Ortonne JP, et al. Superiority of ivermectin 1% cream over metronidazole 0·75% cream in treating inflammatory lesions of rosacea: a randomized, investigator-blinded trial. Br J Dermatol. 2015;172(4):1103–1110.
- Taieb A, Khemis A, et al. Maintenance of remission following successful treatment of papulopustular rosacea with ivermectin 1% cream vs. metronidazole 0.75% cream: 36-week extension of the ATTRACT randomized study. J Eur Acad Dermatol Venereol. 2016;30(5):829–836.
- Stein-Gold L, Kircik L, et al. Long-term safety of ivermectin 1% cream vs azelaic acid 15% gel in treating inflammatory lesions of rosacea: results of two 40-week controlled, investigator-blinded trials. J Drugs Dermatol. 2014;13(11):1380–1386.
- van Zuuren EJ, Fedorowicz Z, Carter B, et al. Interventions for rosacea. Cochrane Database Syst Rev. 2015;(4):CD003262.
- Mendieta E, Gundin L. Treatment of rosacea with topical ivermectin cream: a series of 34 cases. Dermatol Online J. 2016;22(8). pii: 13030/qt9ks1c48n.
- Siddiqui K, Stein Gold L, Gill J. The efficacy, safety, and tolerability of ivermectin compared with current topical treatments for the inflammatory lesions of rosacea: a network meta-analysis. Springerplus. 2016;5(1):1151.
- Webster G, Schaller M, Tan J, et al. Defining treatment success in rosacea as “clear” may provide multiple patient benefits: results of a pooled analysis. J Dermatolog Treat. 2017;28(5):469–474.
- Sahni DR, Feldman SR, Taylor SL. Ivermectin 1% (CD5024) for the treatment of rosacea. Expert Opin Pharmacother. 2018;19(5):511–516.
- Gold LS, Papp K, Lynde C, et al. Treatment of rosacea with concomitant use of topical ivermectin 1% cream and brimonidine 0.33% gel: a randomized, vehicle-controlled study. J Drugs Dermatol. 2017;16(9):909–916.
- Del Rosso JQ. Topical alpha-agonist therapy for persistent facial erythema of rosacea and the addition of oxymetazoline to the treatment armamentarium: where are we now?. J Clin Aesthet Dermatol. 2017;10(7):28–32.
- DuBois J, Dover JS, Jones TM, et al. Phase II randomized, dose-ranging study of oxymetazoline cream for treatment of persistent facial erythema associated with rosacea. J Drugs Dermatol. 2018;17(3):308–316.
- Baumann L, Goldberg DJ, Stein-Gold L, et al. Pivotal trial of the efficacy and safety of oxymetazoline cream 1.0% for the treatment of persistent facial erythema associated with rosacea: findings from the second REVEAL trial. J Drugs Dermatol. 2018;17(3):290–298.
- Kircik LH, DuBois J, et al. Pivotal trial of the efficacy and safety of oxymetazoline cream 1.0% for the treatment of persistent facial erythema associated with rosacea: findings from the first REVEAL trial. J Drugs Dermatol. 2018;1;17(1): 97–105.
- Draelos ZD, Gold MH, Weiss RA, et al. Efficacy and safety of oxymetazoline 1.0% for treatment of persistent facial erythema associated with rosacea: findings from the 52-week open-label REVEAL trial. J Am Acad Dermatol. 2018;78(6):1156–1163.
- Rhofade (oxymetazoline HCI) cream 1% [package insert]. Aclaris Therapeutics, Inc., Wayne, Pennsylvania, November 2018.
- Mirvaso (brimonidine) topical gel 0.33% [package insert]. Galderma Laboratories, LP, Fort Worth, TX; November 2017.
- Del Rosso JQ. Management of facial erythema of rosacea: what is the role of topical ?-adrenergic receptor agonist therapy?. J Am Acad Dermatol. 2013;69(6 Suppl 1):S44–S56.
- Thiboutot D, Thieroff-Ekerdt R, Graupe K. Efficacy and safety of azelaic acid (15%) gel as a new treatment for papulopustular rosacea: results from two vehicle controlled, randomized phase III studies. J Am Acad Dermatol. 2003;48:836–845.
- Two AM, Wu W, Gallo RL, Hata TR. Rosacea: part II. Topical and systemic therapies in the treatment of rosacea. J Am Acad Dermatol. 2015;72(5): 761–770; quiz 771–772.
- Del Rosso JQ, Kircik LH. Update on the management of rosacea: a status report on the current role and new horizons with topical azelaic acid. J Drugs Dermatol. 2014;13(12):S101–S107.
- Finacea (azelaic acid) gel 15% [package insert]. LEO Pharma Inc., Seven Giralda Farms, Madison, New Jersey; August 2018.
- Bhatia ND, Del Rosso JQ. Optimal management of papulopustular rosacea: rationale for combination therapy. J Drugs Dermatol. 2012;11(7):838–844.
- Finacea (azelaic acid) foam 15% [package insert].LEO Pharma Inc., Seven Giralda Farms, Madison, New Jersey; August 2018.
- Draelos ZD, Elewski B, Staedtler G, Havlickova B. Azelaic acid foam 15% in the treatment of papulopustular rosacea: a randomized, double-blind, vehicle-controlled study. Cutis. 2013;92(6):306–317.
- Draelos ZD, Elewski BE, et al. A phase 3 randomized, double-blind, vehicle-controlled trial of azelaic acid foam 15% in the treatment of papulopustular rosacea. Cutis. 2015;96(1):54–61.
- Del Rosso JQ. Azelaic acid topical formulations: differentiation of 15% gel and 15% foam. J Clin Aesthet Dermatol. 2017;10(3):37–40.
- Lowe NJ, Behr KL, Fitzpatrick R, et al. Flash lamp pumped dye laser for rosacea-associated telangiectasia and erythema. J Dermatol Surg Oncol. 1991;17(6):522–525.
- Schroeter CA, Haaf-von Below S, Neumann HA. Effective treatment of rosacea using intense pulsed light systems. Dermatol Surg. 2005;31(10):1285–1289.
- Neuhaus IM, Zane LT, Tope WD. Comparative efficacy of nonpurpuragenic pulsed-dye laser and intense pulsed light for erythematotelangiectatic rosacea. Dermatol Surg. 2009;35(6):920–927.
- Tanghetti EA. Split-face randomized treatment of facial telangiectasia comparing pulsed-dye laser and an intense pulsed light hand piece. Lasers Surg Med. 2011;44(2):97–102.
- Adrian RM, Tanghetti EA. Long pulse 532-nm laser treatment of facial telangiectasia. Dermatol Surg. 1998;24(1):71–74.
- Uebelhoer NS, Bogle MA, Stewart B, et al. A split-face comparison study of pulsed 532-nm KTP laser and 595-nm pulsed-dye laser in the treatment of facial telangiectasias and diffuse telangiectatic facial erythema. Dermatol Surg. 2007;33(4):441–448.
- Bernstein EF. The pulse-dyed laser for the treatment of cutaneous conditions. G Ital Dermatol Venerol. 2009;144(5): 557–572.
- Hofmann MA, Kokolakis G. A case report of combination treatment with potassium-titanyl phosphate laser and brimonidinetopical gel in erythematotelangiectatic rosacea. J Cosmet Laser Ther. 2017;19(4):222–224.
- Kusari J, Padillo E, Zhou SX, et al. Effect of Brimonidine on retinal and choroidal neovascularization in a mouse model of retinopathy of prematurity and laser-treated rats. Invest Ophthalmol Vis Sci. 2011; 8 (52):5424–5431.
- Lee SJ, Kim H, Kim HS. Topical brimonidine tartrate 0.33% gel on post-laser erythema: our experience and review of the literature. Derm Surg. 2018;44(1):144–147.
- Schlessinger J, George R, Lupin M, Amato D. Evaluation of the safety and efficacy of microfocused ultrasound with visualization (MFU-V) for the treatment of signs and symptoms of erythematotelangiectatic rosacea—final data (ePoster). Presented at the American Society for Laser Medicine and Surgery annual meeting; April 5–9, 2017; San Diego, CA.
- Elewski BE, Draelos Z, Dréno B, et al. Rosacea— global diversity and optimized outcome: proposed international consensus from the Rosacea International Expert Group. J Eur Acad Dermatol Venereol. 2011;25(2):188–200.
- Schaller M, Almeida LM, Bewley A, et al. Rosacea treatment update: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176(2):465–471.
- Reinholz M, Tietze JK, Kilian K, et al. Rosacea—S1 guideline. J Dtsch Dermatol Ges. 2013;11(8): 768–780.
- Asai Y, Tan J, Baibergenova A, et al. Canadian clinical practice guidelines for rosacea. J Cutan Med Surg. 2016;20(5):432–445.
- Del Rosso JQ, Baldwin H, Webster G; American Acne & Rosacea Society. American Acne & Rosacea Society Rosacea medical management guidelines. J Drugs Dermatol. 2008;7(6):531–533.
- McGregor SP, Alinia H, Snyder A, et al. A review of the current modalities for the treatment for papulopustular rosacea. Dermatol Clin. 2018;36:135–150.
- Berth-Jones J. Rosacea. In: Lebwohl MG, Heymann WR, Berth-Jones J, Coulson I, Eds. Treatment of Skin Disease: Comprehensive Therapeutic Strategies, 4th Edition. Philadelphia, PA: Elsevier-Saunders; 2014: 684–691.
- Park JM, Mun JH, Song M, et al. Propranolol, doxycycline and combination therapy for the treatment of rosacea. J Dermatol. 2015;42(1): 64–69.
- Pietschke K, Schaller M. Long-term management of distinct facial flushing and persistent erythema of rosacea by treatment with carvedilol. J Dermatolog Treat. 2018;29(3):310–313.
- Rivero AL, Whitfield M. An update on the treatment of rosacea. Aust Prescr. 2018;41(1): 20–24.
- Cline A, McGregor S, Feldman SR. Medical management of facial redness in rosacea. Dermatol Clin. 2018;36(2):151–159.
- Park KY, Hyun MY Seong SY, et al. Botulinum toxin for the treatment of refractory erythema and flushing of rosacea. Dermatol. 2015;230(4): 299–301.
- Kallis PJ, Price A, Dosal JR, Nichols AJ, Keri J. A biologically based approach to acne and rosacea. J Drugs Dermatol. 2018;17(6):611–617.
- Draelos ZD, Donald A. The effect of an anti-inflammatory botanical cleanser/ night mask combination on facial redness reduction. J Drugs Dermatol. 2017(6):671–676.
- Vieira AC, Mannis MJ. Ocular rosacea: common and commonly missed. J Am Acad Dermatol. 2013;69(6 Suppl 1):S36–S41.
- Schaller M, Almeida L, Bewley A et al. Rosacea treatment update: Recommendations from the global ROSacea Consensus (ROSCO) panel. Br J Dermatol. 2017;176(2):465–471.
- Schecher BA, Katz RS, Friedman LS. Efficacy of topical cyclosporine for treatment of ocular rosacea. Adv Ther. 2009;26(6):651–659.
- Schaller M, Pietschke K. Successful therapy of ocular rosacea with topical ivermectin. Br J Dermatol. 2018; 179(2):520–521.
- Abokwidir M, Feldman SR. Rosacea management. Skin Appendage Disord. 2016;2(1–2):26–34.
- Lam-Franco L, Perfecto-Avalos Y, Patino-Ramirez BE, Rodriguez Garcia A. Il-1a and MMP-9 tear levels of patients with active ocular rosacea before and after treatment with systemic azithromycin or doxycycline. Ophthalmc Res. 2018;60(2):109–114.
- Lee GL, Zirwas MJ. Granulomatous roswacea and periorificial dermatitis: controversies and review of management and treatment. Dermatol Clin. 2015;33(3):447–455.
- Rallis E, Korfitis C. Isotretinoin for the treatment of granulomatous rosacea: case report and review of the literature. J Cutan Med Surg. 2012:16(6):438–441.
- Kok WL, Oon HH, Giam YC. A case of granulomatous rosacea of the face. Singapore Med J. 2018;59(4):228–229.
- LaRosa C, Chiaravelloti A, Jinna S, et al. Laser treatment of medical skin disease in woman. Int J Womens Dermatol. 2017;3(3):131–139.
- Tanghetti E, Del Rosso JQ, Thiboutot D, et al. Consensus recommendations from the American acne & rosacea society on the management of rosacea, part 4: a status report on physical modalities and devices. Cutis. 2014;93(2):71–76.

