 by Chankiat Songsantiphap, MD, and Pravit Asawanonda, MD, DSc
by Chankiat Songsantiphap, MD, and Pravit Asawanonda, MD, DSc
Drs. Songsantiphap and Asawanonda are with the Division of Dermatology in the Department of Medicine at Chulalongkorn University in Bangkok, Thailand.
ABSTRACT: Background. There are limited number of topical agents for the treatment of common warts. Few reports show efficacy of zinc oxide for such indication.
Objectives. We sought to evaluate the efficacy of topical 15% zinc oxide ointment for the reduction in size of common warts.
Materials and Methods. Sixteen patients with two comparable palmar warts or verruca vulgaris were randomized to receive either 15% zinc oxide ointment or placebo three times a day for four weeks. Diameter, surface area, and volume change of warts in both groups were used as objective assessments, while patient and physician assessments were also recorded.
Results. At Week 4, zinc oxide significantly reduced the median surface area compared to baseline (P<0.037). However, when the median percent changes between groups were compared, there were no statistically significant differences.
Conclusions. Zinc oxide can reduce the size of common hand warts after four weeks. We suggest that it can be used as an adjunctive therapy to enhance the efficacy of other treatments.
KEYWORDS: Zinc, common warts, verruca vulgaris, human papillomavirus
FUNDING: Funding for this study was provided by the Ratchadapisek Sompoch Endowment Fund (RA 60/022).
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
J Clin Aesthet Dermatol. 2019;12(9):26–31
Among immunocompetent individuals, cutaneous viral warts are usually harmless and can spontaneously resolve within months or years. A number of factors, such as host immunity, human papillomavirus (HPV) type,1,2 and site of infection all influence the rate of resolution.3 There are numerous treatments for cutaneous viral warts, ranging from physical or destructive methods, such as cryotherapy or electrocautery, to the less complicated, non-scarring, and more practical measures that are suitable for children and patients with multiple lesions, such as topical treatment.
Impaired immune response and slow clearance of papillomavirus result from the HPV mechanisms to evade the host immunity. The primary mechanism is avoidance of antigen presentation by inhibition of cell lysis and delayed cell differentiation. Moreover, some specific anti-inflammatory cytokines, such as interferon (IFN)-? and IFN? are also inhibited by HPV non-structural proteins. Such mechanisms allow papillomavirus to survive in their host cells.4 Therefore, immunotherapy has been used to treat recalcitrant or latent cutaneous viral warts.5,6 Methods such as imiquimod, diphenylcyclopropenone (DCP) and intralesional candida, mumps, and tuberculin antigen, among others, have been used in both immunocompetent and immunocompromised patients.7 The main mechanisms are induction and activation of the immune response, either innate or adaptive, to attack virus-infected epidermal cells.8
Zinc might be categorized as a form of immunotherapy, considering its role in the regulation of the immune system when used alone or as an adjuvant therapy. It has actions on macrophage and neutrophil functions, natural killer cell/phagocytic activity, and various inflammatory cytokines9 and moreover, directly regulates the interaction between host cells and viral components.10 Although the exact mechanism still remains unclear, there are reports in the literature of using zinc effectively in various topical and oral forms as well as in concentrations for treatment of cutaneous viral warts with promising outcomes.11–15
Due to its availability, cost, and low chance of complication, topical zinc might be an option for treatment of cutaneous viral warts. In general, even when aggressive physical modalities are used, several sessions might be required to completely cure the warts. This can result in extended downtime, especially when multiple lesions on the hands or weight bearing areas of the feet are treated. The objective of this study is to investigate the efficacy of 15% topical zinc oxide in reducing the size of cutaneous viral warts within four weeks compared to placebo.
Materials and Methods
The study was conducted in accordance with the Declaration of Helsinki. The protocol was approved by the Institutional Review Board of Faculty of Medicine at Chulalongkorn University in Bangkok, Thailand (approval number 732/59). All subjects were recruited at an outpatient dermatology clinic of King Chulalongkorn Memorial Hospital in Bangkok, from April to November 2017. The subjects were informed about the objectives and the method of the study and signed informed consents were obtained prior to enrollment.
Inclusion and exclusion criteria. Subjects older than 18 years of age, with at least two similar palmar warts or verruca vulgaris on either one or both hands were eligible. The wart diameter had to range from 2 to 8 millimeters, and the total number of lesions had to be at least two but no more than 15. All included subjects reported no previous treatments with zinc preparations. The washout period for previous wart treatment was at least four weeks. Patients were excluded from the study if they had recalcitrant warts, defined as warts unresponsive to at least five treatments in the past six months.16 In addition, patients with known allergies to any formulation of zinc, those who were categorized as immunocompromised due to acquired immunodeficiency syndrome (AIDS), poorly controlled diabetes mellitus, chronic kidney disease, or treatment with topical or systemic immunosuppressive agents, as well as pregnant or lactating women were excluded from the study.
Two comparable warts (i.e., warts whose diameters differed no more than two millimeters) distanced at least 1cm apart were identified on each subject by a principal investigator (CS). Zinc oxide 15% ointment and hydrophilic cream, acting as the placebo, were randomized by block allocation (block of 2) as Drug 1 and 2 by a research assistant and applied three times per day to designated Lesions 1 and 2 for four weeks. Both drugs were compounded and packaged in similar tubes by the department of pharmacy at King Chulalongkorn Memorial Hospital. At baseline and Weeks 2 and 4, both lesions were photographed using dermoscopy (Dermlite DL4, 3Gen Inc., San Juan Capistrano, California) attached to the iPhone® 6 (Apple Inc., Cupertino, California). Another set of photographs for volume assessment was taken with the Antera 3D® camera (Miravex, Dublin, Ireland). The camera was placed parallel to the lesions. The brightness of each capture had to be more than 95% degree; white and black balance were also corrected. The exact locations for both types of photography were recorded at every visit.
Characteristics of each subject were recorded at their first visit. Adherence and adverse effects were recorded at Weeks 2 and 4.
Objective Measurements. Diameter and surface area. Photographs taken by dermoscope/iPhone® 6 were analyzed for diameters by Adobe Illustrator CS6® (Adobe System Inc., San Jose, California) with line segment tool function. The exact scales were standardized. The diameter of each wart was measured three times, in millimeters. To determine the size of the lesions, the surface area was calculated based on a formula of surface area, ?r2.
Volume. Antera 3D® software was used to automatically evaluate the volume in mm3. Measurements were performed again in triplicates and mean/median values calculated.
Physician’s Global Assessment. Three blinded dermatologists evaluated the global change of lesions by comparing close-up digital photographs from dermoscopy taken at Weeks 2 and 4 to the pictures taken at baseline (Week 0). We used consensus grading scales as follows: Grade 1, 1 to 25-percent improvement (fair improvement); Grade 2, 25 to 50-percent improvement (good improvement); Grade 3, 50 to 75-percent improvement; Grade 4, 75 to 100-percent improvement. Grades 3 and 4 were collectively reported as excellent improvement. Corresponding minus scales were applicable in case of worsening.
Participant’s Assessment. At Week 4, after completing the study, the subjects assessed, via questionnaire, their satisfaction, perception, and acceptance of using both drugs. This questionnaire comprised two parts. The first part evaluated the ease of drug application and response to treatment. Grading scales were as follows: Grade 0, no improvement (unsatisfied); Grade 1, 1 to 25-percent improvement (marginally satisfied); Grade 2, 25 to 50-percent improvement (moderately satisfied); Grade 3, 50 to 75-percent improvement (very satisfied); Grade 4, 75 to 100-percent improvement (extremely satisfied). The second part consisted of a single yes/no question regarding the patient-perceived acceptability of both preparations. All participants were blinded throughout the study.
Application and adherence. At Week 0, both warts were pared once by CS using a scalpel. Subjects were instructed to apply Drug 1 or 2 on the chosen lesion, three times per day, every day, using cotton-tip applicators (CTA). After, the lesions were occluded with a bandage (3M Thailand Co., Lat Lum Kaew, Pathum Thani) for at least one hour. For drug adherence assessments, one CTA was used for each drug application and an adequate amount of drug was defined as when complete coverage was achieved on the surface of the lesions. Subjects were asked to return all used tubes and remaining CTAs at each visit so that the tubes could be weighted to determine adherence. The drop out criteria applied to patients who switched the treatment or did not apply each drug more than 15 times, according to their diaries and CTA counts.
Safety and adverse events. At Weeks 2 and 4, subjects were asked about any adverse events from using either drugs. Any subjects who had severe side effects, such as severe contact dermatitis, would be excluded from the study.
Sample size and statistical analysis. To detect a clinically significant 25-percent reduction in size, between 15% zinc oxide ointment and placebo, assuming the 90% of power and a 5% two-sided type I error, 16 lesions were required on each arm. Baseline characteristics and side effects were summarized using descriptive statistics (number with percent or median with interquartile range [IQR]). Objective measurements were analyzed as median or median percent change from Week 0 to Week 4, compared by Wilcoxon signed-rank test or Generalize Equation Estimate (GEE). Proportion of percent change and physician’s and patient’s assessment were analyzed by Mc-Nemar test. All statistical analyses were done with Stata program (version 13.1, StataCorp, College station, Texas). The difference was considered statistically significant when P<0.05.
Results
Seventeen patients participated in this study. Sixteen patients completed the study, as one patient was unable to attend further follow-up appointments beyond the second visit. Table 1 shows the demographic characteristics of the participants with almost equal sex representation (7 men [43.7%], 9 women [56.3%]) and median age was 29 (IQR=24.5–40) years. Almost all participants reported no underlying diseases (2 patients [12.5%] reported having porokeratosis and hand eczema), and few reported previous treatments for their warts (4 cryotherapy [25%], 1 electrocautery [6.25%]). Median duration of disease was 12 months (IQR=3–12).
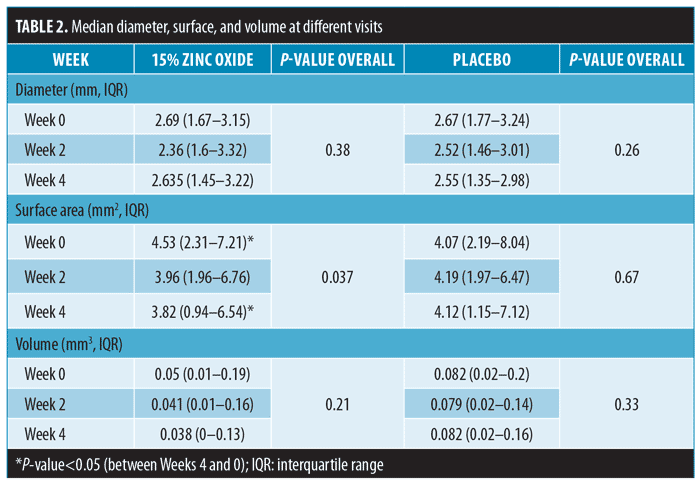
Diameters and surface areas. By objective measurement, median baseline wart diameters between zinc oxide group (2.69mm) and placebo (2.67mm) were comparable. The lesions in the zinc oxide group had higher baseline values in surface area (4.53mm2 vs. 4.07mm2 in placebo group). Within the zinc oxide group, the median surface area at Week 4 was significantly smaller (3.82mm2 [IQR=0.94–6.54]) compared to baseline (4.53mm2 [IQR=2.31 – 7.21]) (P=0.037). Notably, the placebo did not result in changes to either diameter or surface area (Table 2).
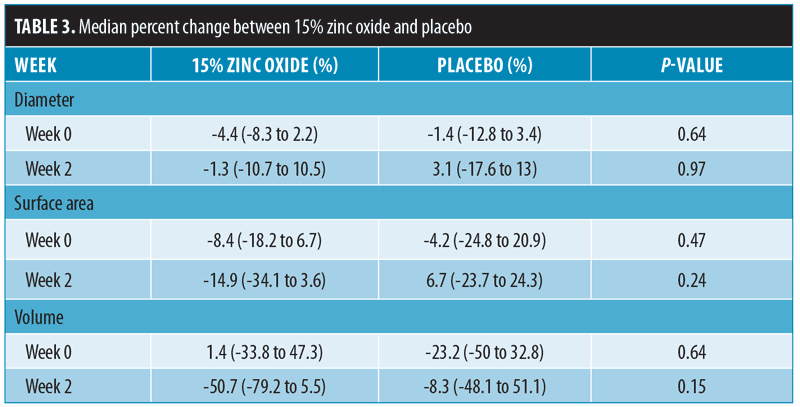
To compare between 15% zinc oxide and placebo, we calculated median percent change from the baseline to each follow-up time (Table 3). At Week 4, we found that in the zinc oxide group, the median percent change showed reductions in diameter (-1.3%) and surface area (-14.9%). On the contrary, both diameter and surface area had increased (+3.1% and +6.7%, respectively) in the placebo group. However, these differences were not statistically significant between two groups (P=0.97 and 0.24, respectively).
Volumes. The volumes of warts in the zinc oxide group also reduced from 0.05mm3 (IQR=0.01–0.19) at baseline to 0.041mm3 (IQR=0.01–0.16) at Week 2 and 0.038mm3 (IQR=0 – 0.13) at Week 4. However, these changes did not reach statistically significant levels. Again, the placebo did not result in notable changes in volumes (Table 2).
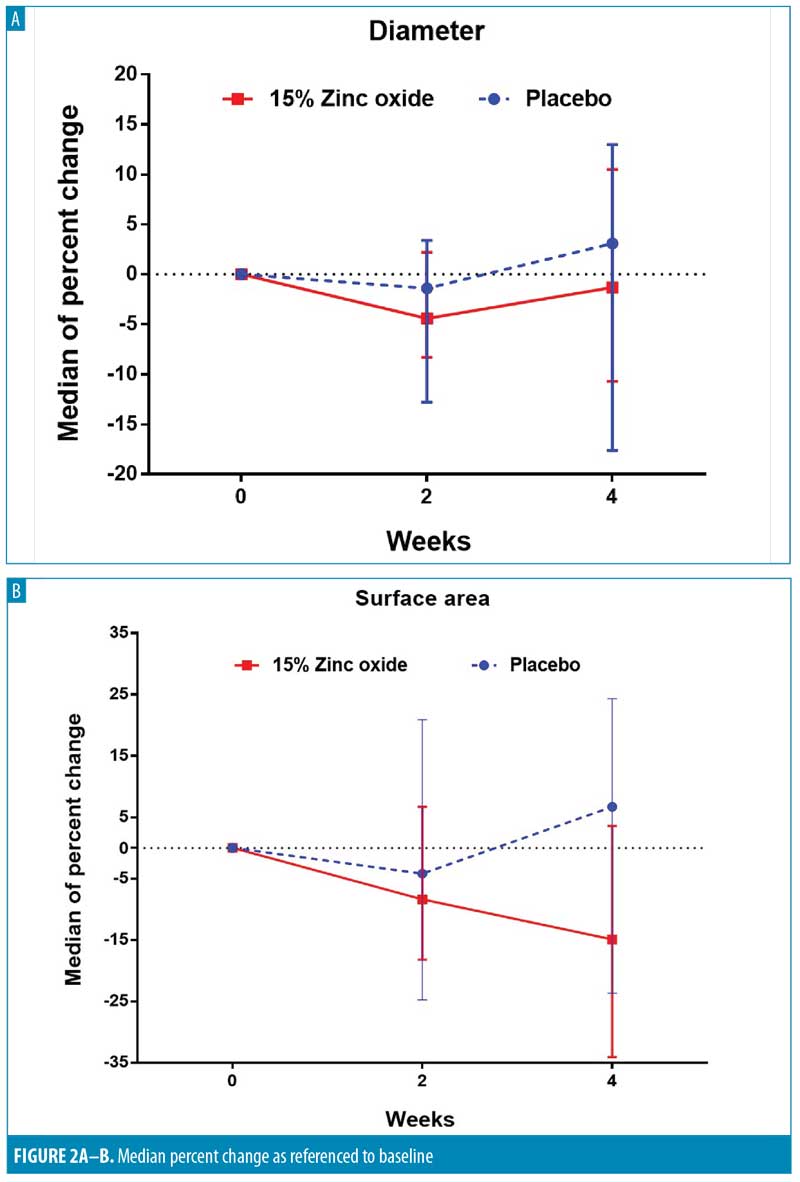
Similar to changes in diameters and surface areas, four weeks of zinc oxide resulted in sizeable reductions in wart volumes (-50.7%), whereas placebo resulted in only slight decrease (-8.3%). However, the difference did not reach statistically significant levels (P=0.15). Interestingly, we observed complete clearance from both treatments in one of the subjects (Figure1). When comparing the proportion of subjects with at least a 25-percent reduction at Week 4, there were twice as many subjects with at least a 25-percent reduction in volume from zinc oxide (62.5%) compared to the placebo (31.3%) (P=0.09) (Table 4).
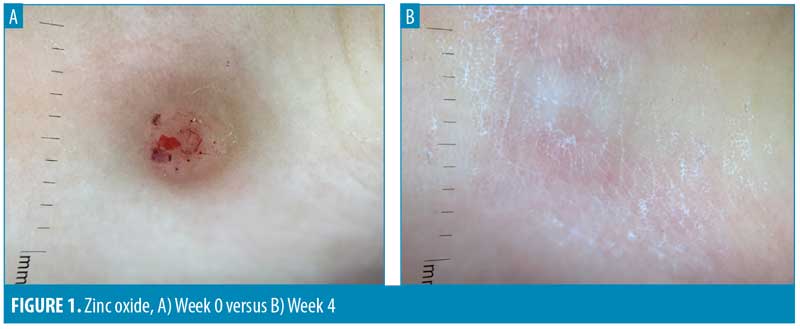
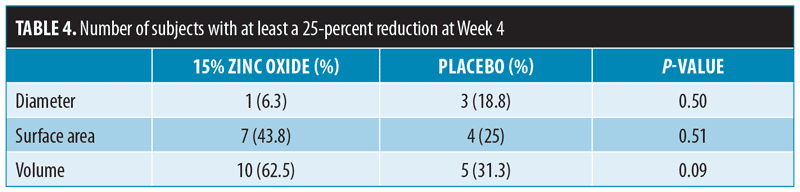
Figure 2 illustrates the percent changes of the three measurements during each follow-up visit as referenced to the baseline values. There were trends in reduction within zinc oxide group for diameter and surface area at two weeks. At Week 4, surface area and volume had gradually decreased. In the placebo group, increments were observed in all measurements at Week 4.
When expert physicians compared photographs taken at Week 4 to those taken at baseline, seven lesions (43.9%) treated with zinc oxide were rated as, “improved,” while nine lesions (56.3%) treated with the placebo were rated similarly. However, there were no statistical difference between the two groups (P=0.69). For patient-reported assessments regarding ease of use, 31.3 percent of participants reported being very satisfied with zinc oxide (31.3%) and 50 percent in the placebo group reported similarly. No adverse effects from zinc oxide or placebo were reported during four weeks of the treatment.
Discussion
Several treatments for warts provide variable responses, but no single treatment is considered the gold standard.8 This is especially true in patients with multiple lesions or lesions in difficult-to-treat locations, such as weight bearing areas. In young children with warts, conventional destructive therapies might cause undesirable complications. Topical and systemic immunotherapy have now become viable treatments for warts in these patients due to its nondestructive action, low incidence of pain and scarring, ease of use, and promising results. Zinc, both topical and oral, is categorized as one of those immunotherapeutic agents.7,8
Topical zinc in different formulations and concentrations has demonstrated efficacy for the treatment of common warts in only a few studies. In 2007, Khattar et al12 evaluated the efficacy of topical 20% zinc oxide compared to combination salicylic/lactic acid. No placebo was included. They found that zinc oxide resulted in a 50-percent clearance of warts after 12 weeks of use. However, no statistically significant difference between the groups was detected.Moreover, several types of warts were included, with many more flat warts in the zinc oxide group. It is well documented that HPV types greatly affect spontaneous resolution and response to treatment.1,2 Significant drop-outs also occurred. Additionally, treated warts were rubbed daily with emery stones, which could physically remove at least part of the warts. Sharquie et al15 evaluated the efficacy of 10% and 5% zinc sulfate in aqueous solutions for treating common and plane warts. There was no detail regarding how the subjects kept the solution on their lesions. Distilled water was used as control. The authors reported 85.7 percent clearance at Week 4 for plane warts versus 10 percent clearance in placebo group. However, only one of the nine patients with common warts achieved a full response using the 10% solution, while the remaining eight showed no improvement. They also reported that only 1 in 22 subjects who were treated with the 5% solution achieved full response. This, again, emphasizes the differing responses of various HPV types and suggests that concentrations of zinc can be an important determinant of outcome.
To our knowledge, our study is the first randomized, placebo-controlled trial investigating the efficacy of 15% topical zinc oxide for the treatment of common warts. We hypothesized that it is rather ambitious to expect any topical treatment to achieve complete clearance of common warts within four weeks, especially warts on the hands, which are notoriously difficult to treat. Instead, it might be of better use to reduce the size of the warts using the 15% topical zinc oxide, so that other destructive modalities would be easier to perform. In this study, we used three objective measurements to demonstrate zinc’s efficacy. We found that the median surface area was statistically significantly reduced at Week 4 in the zinc oxide group compared to baseline. The placebo treatment, on the other hand, resulted in a slight increase in surface area. However, when the median percent changes between the two groups were compared, there were no statistically significant differences. Zinc oxide also reduced wart diameter and volume after four weeks. Again, the changes did not reach statistically significant levels.
Impaired innate and adaptive responses are observed in HPV infection, including cell-mediated immunity. These mechanisms included the inhibition of IFN-? and IFN-? secretion, avoidance of antigen presentation by downregulation of major histocompatibility complex 1, impaired migration of Langerhans cells to the skin and delayed cell lysis and cell differentiation.4,17 To circumvent these immune aberrations, several immunotherapeutic agents have been used. For example, imiquimod increases the cellular levels of IFN-?, TNF-? and IL-6, which leads to strong antiviral properties.6 Its effects on keratinized, common warts are, however, much less impressive than when used for the genital counterparts. Intralesional vaccines (e.g. Bacille Calmette Guerin [BCG], mumps, measles, and rubella [MMR], or tuberculin) cause delayed hypersensitivity responses with activation of cell mediated and humoral immunity to the site of injection.7
Despite unclear mechanisms, some studies suggest that zinc is effective in treating many cutaneous infectious diseases.9 As shown in in-vivo and in-vitro studies, zinc can regulate cell development and function of the immune system, both innate and adaptive, especially cell mediated immunity. It can act as chemoattractant to polymorphonuclear cells (PMNs) and increases production of IL1?, IL-6 and TNF-? which are important for innate immune response.18 Furthermore, for adaptive response, zinc can facilitate T-cell receptor’s (TCR) functions in proliferation, enhanced production of IL-2 via activation of nuclear factor kappa B (NF-?B) or increases IFN-? production.19 All these lead to stimulation of Th1 response, which is essential for viral infected cell eradication. However, not much is known about the exact mechanism of action of zinc in the treatment of viral warts.
There were some possible factors which might have affected the treatment outcomes in our study. First, even with intact immune status, longer duration of treatment might be needed to provide enough time for immune activation. This corresponds to other immunotherapeutic treatments; for example, imiquimod takes 12 to 16 weeks to exert its full effects. BCG vaccine generally takes at least six weeks of treatment.7 Thus, it is rather impressive that within only four weeks, such an inexpensive treatment as zinc oxide could provide such a significant reduction, in surface area of common hand warts. One could argue that spontaneous resolution of warts does occur; however, this usually takes two months to two years.20
Second, penetration of zinc must be discussed. Since HPV infection mostly involves the entire epidermis, including the basal layer, topical drugs need to penetrate further below. With an average particle size of 0.1 to a few micrometers (>100 nm), topically applied zinc oxide might not penetrate the viable epidermis; however, the hydrolysis of zinc oxide on the skin’s surface can increase the penetration levels of zinc ions down to the basal layer of the epidermis.21 To avoid this issue, intralesional injections of 2% zinc sulfate were used with an 88-percent clearance rate of common warts after two months.22 We used scalpel paring at each participant’s baseline visit to enhance penetration, at least in the initial phase of the study. Occaisonal use of emery board or filing can also improve the treatment efficacy.
Finally, bandage occlusion might resemble duct tape wraps. De Haen et al23 reported 85 percent clearance of cutaneous warts from duct tape versus 60 percent clearance in warts treated with cryotherapy. Although there are some controversies about duct tape’s efficacy, we believe that this did not affect our results, as occlusion lasted only 1 to 2 hours at a time in our subjects.
The strengths of our study include being a randomized, triple-blinded, within-subject, placebo-controlled trial. We chose two comparable lesions on each subject to minimize any confounding factors, including age, individual immune response, HPV types, or host hygiene. Second, only common hand warts were enrolled, eliminating the variable responses from different types of warts. Third, we recruited only adult subjects, as it is well known that pediatric patients can demonstrate favorable natural course of disease and response to treatment. Our study population, albeit small, is more homogeneous than previous reports. We also employed objective measurements, which further substantiated our results and differed from previous studies, which mostly reported the outcome as complete or partial remission. Moreover, great adherence was achieved for both zinc oxide and placebo groups.
Limitations. There are some limitations to our study worth mentioning. We conducted this study in people from Thailand, which inherently cannot be generalized to other populations. The warts included were only on the hands; warts in other locations might respond differently. The various concentration and formulations of zinc might have different penetration profiles and deliver variable amounts of elemental zinc.
Conclusion
According to our study results, topical 15% zinc oxide might be effective reducing the size of common warts as monotherapy. Therefore, we believe that when used on difficult-to-treat warts prior to or as an adjunct to other treatments, it might increase the chance of wart clearance. Future studies involving the combination of topical zinc oxide 15% with other treatments, studies with a longer duration and larger sample sizes, and a cost effectiveness study might be warranted.
Acknowledgments
The authors wish to thank Drs. Kanyarat Kraivichian and Korbkarn Pongpairoj for their kind assistance in the assessment of clinical response through digital photography.
References
- Hogendoorn GK, Bruggink SC, de Koning MNC et al. Morphological characteristics and human papillomavirus genotype predict the treatment response in cutaneous warts. Br J Dermatol. 2018;178:253–260.
- Bruggink SC, Gussekloo J, de Koning MN, et al. HPV type in plantar warts influences natural course and treatment response: secondary analysis of a randomised controlled trial. J Clin Virol. 2013;57:227–232.
- Kwok CS, Gibbs S, Bennett C, Holland R, Abbott R. Topical treatments for cutaneous warts. Cochrane Database Syst Rev. 2012:CD001781.
- Frazer IH. Interaction of human papillomaviruses with the host immune system: a well evolved relationship. Virology. 2009;384:410–414.
- Jablnska S, Majewski S, Malejczyk J. [Immunology of HPV infections and mechanism of a latent infection]. Hautarzt. 1992;43:305–311.
- Czelusta AJ, Evans T, Arany I, Tyring SK. A guide to immunotherapy of genital warts: focus on interferon and imiquimod. BioDrugs. 1999;11:319–332.
- Thappa DM, Chiramel MJ. Evolving role of immunotherapy in the treatment of refractory warts. Indian Dermatol Online J. 2016;7:364–370.
- Sterling JC, Gibbs S, Haque Hussain SS, et al. British Association of Dermatologists’ guidelines for the management of cutaneous warts 2014. Br J Dermatol. 2014;171:696–712.
- Gupta M, Mahajan VK, Mehta KS, Chauhan PS. Zinc therapy in dermatology: a review. Dermatol Res Pract. 2014;2014:709152.
- Lazarczyk M, Favre M. Role of Zn2+ ions in host-virus interactions. J Virol. 2008;82:11486–11494.
- Akhavan S, Mohammadi SR, Modarres Gillani M, et al. Efficacy of combination therapy of oral zinc sulfate with imiquimod, podophyllin or cryotherapy in the treatment of vulvar warts. J Obstet Gynaecol Res. 2014;40:2110–2113.
- Khattar JA, Musharrafieh UM, Tamim H, Hamadeh GN. Topical zinc oxide vs. salicylic acid-lactic acid combination in the treatment of warts. Int J Dermatol. 2007;46:427–430.
- Khozeimeh F, Jabbari Azad F, Mahboubi Oskouei Y, et al. Intralesional immunotherapy compared to cryotherapy in the treatment of warts. Int J Dermatol. 2017;56(4):474–478.
- Sharquine KA, Al-Nuaimy AA. Treatment of viral warts by intralesional injection of zinc sulphate. Annals of Saudi Medicine. 2002;22:26–28.
- Sharquie KE, Khorsheed AA, Al-Nuaimy AA. Topical zinc sulphate solution for treatment of viral warts. Saudi Med J. 2007;28:1418–1421.
- Leung L. Recalcitrant nongenital warts. Aust Fam Physician. 2011;40:40–42.
- Stanley M. Immune responses to human papillomavirus. Vaccine. 2006;24 Suppl 1:S16–22.
- Bonaventura P, Benedetti G, Albarede F, Miossec P. Zinc and its role in immunity and inflammation. Autoimmun Rev. 2015;14:277–285.
- Hojyo S, Fukada T. Roles of zinc signaling in the immune system. J Immunol Res. 2016;2016:6762343.
- Pyrhonen S, Johansson E. Regression of warts. an immunological study. Lancet. 1975;1:592–596.
- Holmes AM, Song Z, Moghimi HR, Roberts MS. Relative penetration of zinc oxide and zinc ions into human skin after application of different zinc oxide formulations. ACS Nano. 2016;10: 1810–1819.
- Mohamed EE, Tawfik KM, Mahmoud AM. The clinical effectiveness of intralesional injection of 2% zinc sulfate solution in the treatment of common warts. Scientifica (Cairo). 2016;2016:1082979.
- de Haen M, Spigt MG, van Uden CJ, et al. Efficacy of duct tape vs placebo in the treatment of verruca vulgaris (warts) in primary school children. Arch Pediatr Adolesc Med. 2006;160:1121–1125.

