 by Douglas C. Wu, PhD, MD, and Mitchel P. Goldman, MD Drs. Wu and Goldman are with Cosmetic Laser Dermatology in San Diego, California.
by Douglas C. Wu, PhD, MD, and Mitchel P. Goldman, MD Drs. Wu and Goldman are with Cosmetic Laser Dermatology in San Diego, California.
Funding: No funding was provided.
Disclosures: Drs. Wu and Goldman have no conflicts of interest relevant to the content of this article.
Abstract: Background. Fractionated, ablative lasers are usually associated with post-treatment erythema, edema, and crusting, which can last from 5 to 14 days. Conjugated linolenic acid, an omega-5 fatty acid, has significant antioxidant and anti-inflammatory properties, and has been shown to stimulate keratinocyte proliferation and epidermal regeneration. By modulating the early inflammatory milieu and directly affecting skin structure and function, conjugated linolenic acid might therefore shorten downtime following fractionated ablative laser resurfacing of the face.
Objective. To evaluate the efficacy and subject satisfaction of a topical regimen containing conjugated linolenic acid derived from pomegranate seed extract in accelerating wound healing and improving skin quality following fractionated ablative laser resurfacing of the face.
Materials and Methods. Thirty-four subjects were enrolled and received fractionated CO2 laser resurfacing. Subjects were randomized to use the test healing regimen (n=24) or 1% dimethicone ointment (n=10) post-procedure. The primary endpoint was the degree of erythema, edema, crusting, and exudation evaluated by a blinded clinician at post-treatment Days 1, 3, 7, 10, 14, and 30. Secondary endpoints included a blinded evaluator assessment of the degree of wrinkling and elastosis using the Fitzpatrick-Goldman Wrinkle and Elastosis Scale; subject-assessed degree of pain, itching, tightness, oozing, and crusting; and subject overall satisfaction.
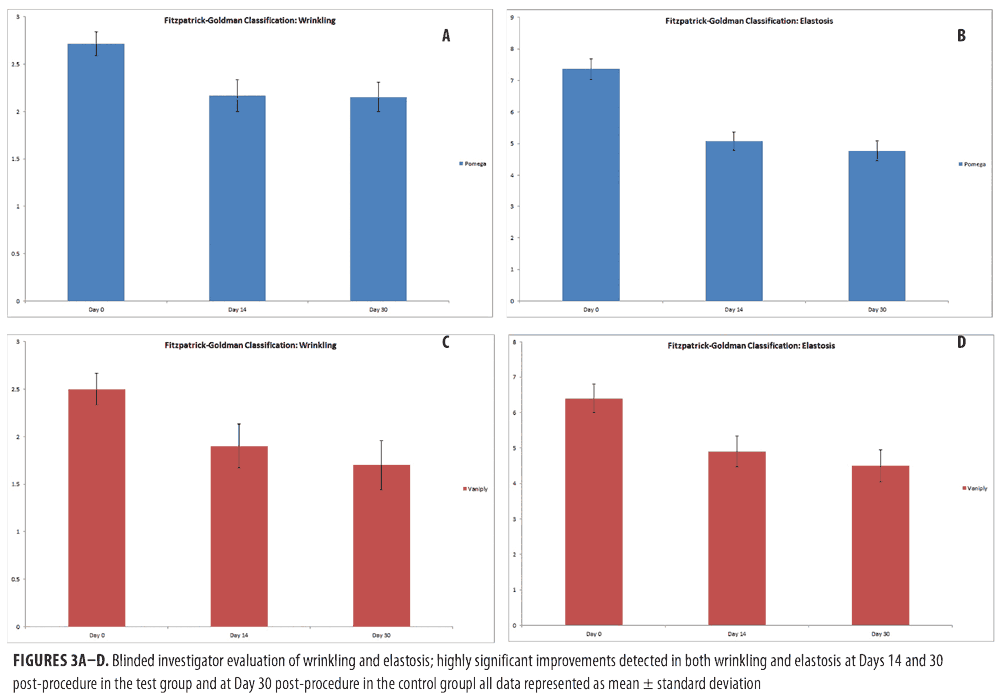
Results. Subjects who applied the topical conjugated linolenic acid healing regimen experienced significantly reduced edema on post-procedure Day 3 (p=0.04), and itching on Days 1 and 3 (p=0.03 and p=0.04). Both regimens produced significant improvements in wrinkling and elastosis at Days 14 and 30 post-treatment, with conjugated linolenic acid outperforming placebo in improvements in wrinkling at Day 14. Both regimens were well tolerated with no statistical differences in adverse events or subject satisfaction.
Conclusion. The topical conjugated linolenic acid formulation outperformed placebo by decreasing acute pruritus and edema, and enabling a faster positive outcome in wrinkle improvement. Additionally, topical conjugated linolenic acid does not raise any safety or tolerability issues as compared to current standard of care.
Keywords: Conjugated linolenic acid, ablative resurfacing, CO2 laser, wound healing, post-laser healing.
J Clin Aesthet Dermatol. 2017;10(10):12–17
Introduction
Ablative resurfacing remains the gold standard for improving the appearance of rhytides, textural irregularities, dyspigmentation and sallowness of the skin. Confluent ablative techniques have proven to be very effective but are associated with prolonged healing and subsequent downtime.1–3 Fractionated ablative lasers offer efficacy with less downtime, but discovering improved healing regimens is important.
Cutaneous wound healing progresses through five continuous phases: coagulation, inflammation, cellular proliferation, remodeling and maturation. Although this cycle might take a year or longer to complete,4 the early inflammatory phase, which typically occurs within the first one to two weeks, plays a significant role in determining the final outcome. Excessive or prolonged inflammatory stimuli result in a poorer quality of wound healing and increased risk for adverse scarring.5 In order to optimize the wound healing response, appropriate inflammatory modulation combined with adequate moisture balance are essential.6, 7
Conjugated linolenic acid (CLA) is an 18 carbon fatty acid possessing three double bonds, with the first double bond located between the fifth and sixth carbons. As an omega-5 fatty acid, it has significant antioxidant and anti-inflammatory properties.8–11 Additionally, experimental models have demonstrated the ability of CLA to reduce keratinocyte production of inflammatory mediators, such as arachidonic acid, prostaglandin, and histamine, resulting in a reduction of pruritus in the setting of atopic dermatitis.12–14 When used as a dietary supplement, CLA induces significant changes in skin composition by altering the adipocyte composition of the subcutaneous layer.15–16 Taken together, these data suggest that CLA might have a positive impact on cutaneous wound healing by modulating the early inflammatory milieu and directly affecting skin structure and function. However, due to the CLA molecule being highly unstable, it has not been available in a product for topical administration that could deliver the compound to the skin in its active form. Recent technological innovations have now led to the development of a topical CLA formulation that doubles as both a delivery system and a highly stable preservative system that lacks the conventional chemical-based preservative deck (Pomega Inc., San Anselmo, California).
In this prospective, randomized, standard-controlled, evaluator-blind study, we assessed the safety and efficacy of a topical healing regimen containing CLA derived from the extract of the pomegranate seed (Pomega MDTM, Pomega Inc., San Anselmo, California) in the setting of fractionated ablative laser resurfacing of the face.
Materials and Methods
Study design. This was a prospective, randomized, standard-controlled, evaluator-blind, institutional review board (IRB)-approved clinical trial that was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization. After obtaining informed consent to participate in the study, as well as photoconsent for publicaiton of photos, 34 otherwise healthy subjects 18 to 75 years of age with skin Fitzpatrick phototypes between I–IV were enrolled. Subjects were excluded if they were pregnant or breastfeeding, had undergone any ablative or non-ablative energy device treatment of the face within the past three months, or had a history of hypertrophic or keloidal scarring.
Fractionated ablative laser resurfacing. All subjects underwent a standard fractionated CO2 laser resurfacing treatment to the face (UltraPulse®, Lumenis, Yokneam, Israel). Based on the subjects’ pre-existing wrinkling and elastosis, a combination of Active FXTM and Deep FXTM modes were utilized. Prior to laser resurfacing, both topical anesthesia via a compounded ointment of 7% lidocaine and 7% tetracaine and local nerve blocks to the supratrochlear, supraorbital, infraorbital, and submental regions were applied. Additional light, conscious sedation consisting of 3 to 5mg intravenous (IV) midazolam and 50 to 75mcg IV fentanyl was also administered. The settings used for Active FXTM treatments were 100 to 125mJ pulse energy and a computer pattern generator (CPG) density of 3 to 6. Deep FXTM settings ranged from 15- to 20-percent density and 20 to 25mJ pulse energy. Following laser resurfacing, either test product or standard-of-care control were applied to the treated areas and subjects were monitored for an additional 30 minutes to ensure stability post-procedure prior to discharge.
Study intervention. Following ablative laser resurfacing of the face, 24 subjects were randomized to receive the test healing regimen, with 10 randomized to receive 1% dimethicone ointment (Vaniply, Pharmaceutical Specialties Inc., Rochester, Minnesota) as the control. The test regimen consisted of a two-phase system with CLA from pomegranate seed oil being the main active ingredient in each phase. The first phase was applied for up to 14 days post-procedure and consisted of a thick occlusive ointment. The second phase was applied for the following two weeks and consisted of a concentrated repair serum and a healing cream. The control group applied 1% dimethicone ointment throughout the 28-day duration.
Evaluation of efficacy, safety, and satisfaction. A blinded evaluator assessed wrinkling and elastosis at baseline and at the end of trial, utilizing the validated 3- and 9-point Fitzpatrick-Goldman Wrinkle and Elastosis Scale.3 Erythema, edema, crusting, and exudation were also evaluated on a standardized 5-point scale at regular time intervals throughout the trial.
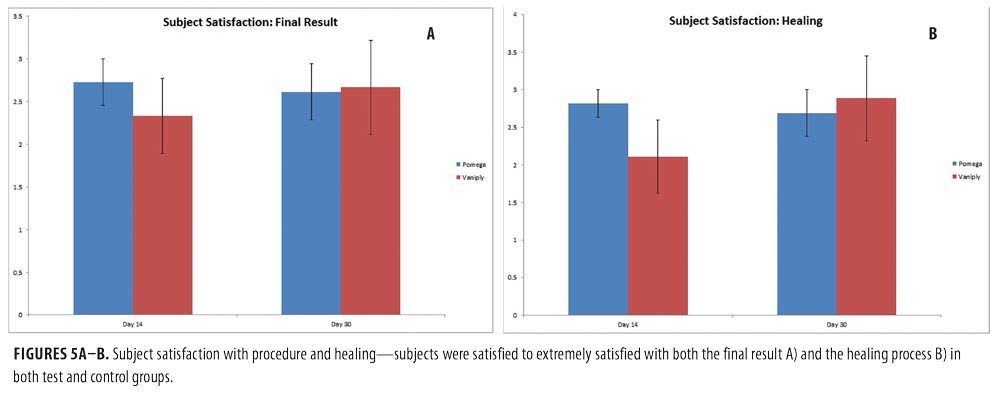
Subjects were asked to evaluate pain, itching, tightness, oozing, and crusting on a standardized 10-point visual-analog scale at regular intervals throughout the trial. At the end of the trial, subjects rated their satisfaction in terms of healing and overall cosmetic result on a standardized 5-point scale.
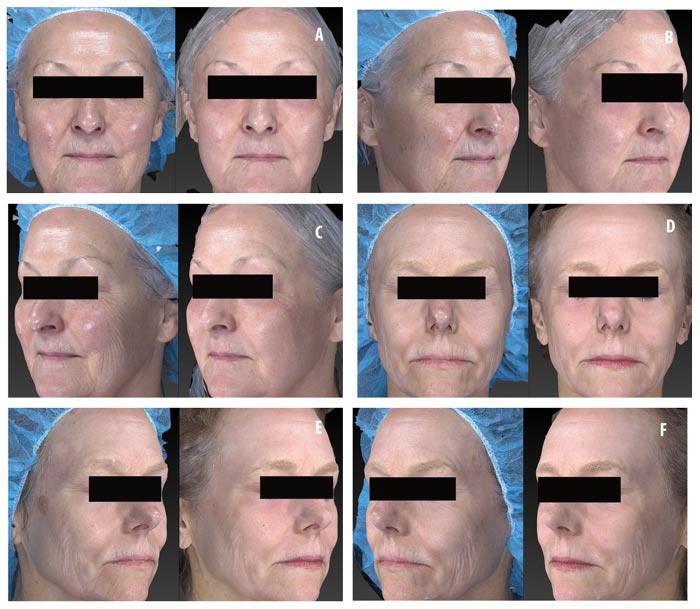
Photographic documentation. All digital images were taken under identical settings using the stationary Canfield Vectra 3D imaging system (Canfield Inc., Parsippany, New Jersey) to ensure comparability and to eliminate slight variations in lighting, position, and camera angle that are unavoidable with standard 2D photography.
Statistical analysis. Statistical evaluation was performed with Microsoft Excel 2013. The two-tailed Student’s t test was utilized to determine the difference in means between groups. All data are represented as mean ± standard deviation (SD).
Results
Subject demographics. Mean subject age and skin type were equivalent between test and control groups (Table 1).
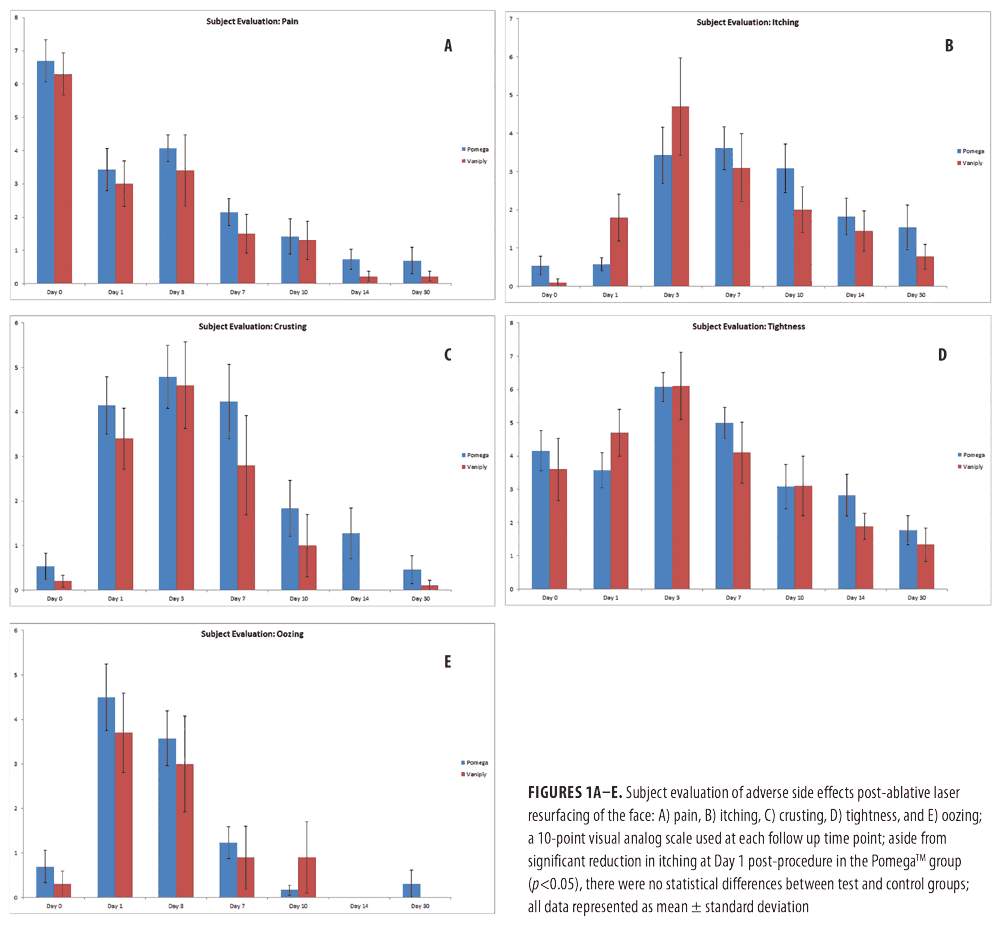
Safety and tolerability of topical CLA. Both physician and subject evaluations were analyzed to determine the safety and tolerability of the topical CLA healing regimen as compared with the control. Subjects were asked to evaluate pain, itching, tightness, oozing, and crusting on a 10-point visual analog scale. Overall, the topical CLA healing regimen was very well tolerated with no increased adverse side effects compared to bland 1% dimethicone ointment (Figure 1). Subjects who applied the topical CLA healing regimen experienced significantly reduced itching on Day 1 and Day 3 post-procedure.
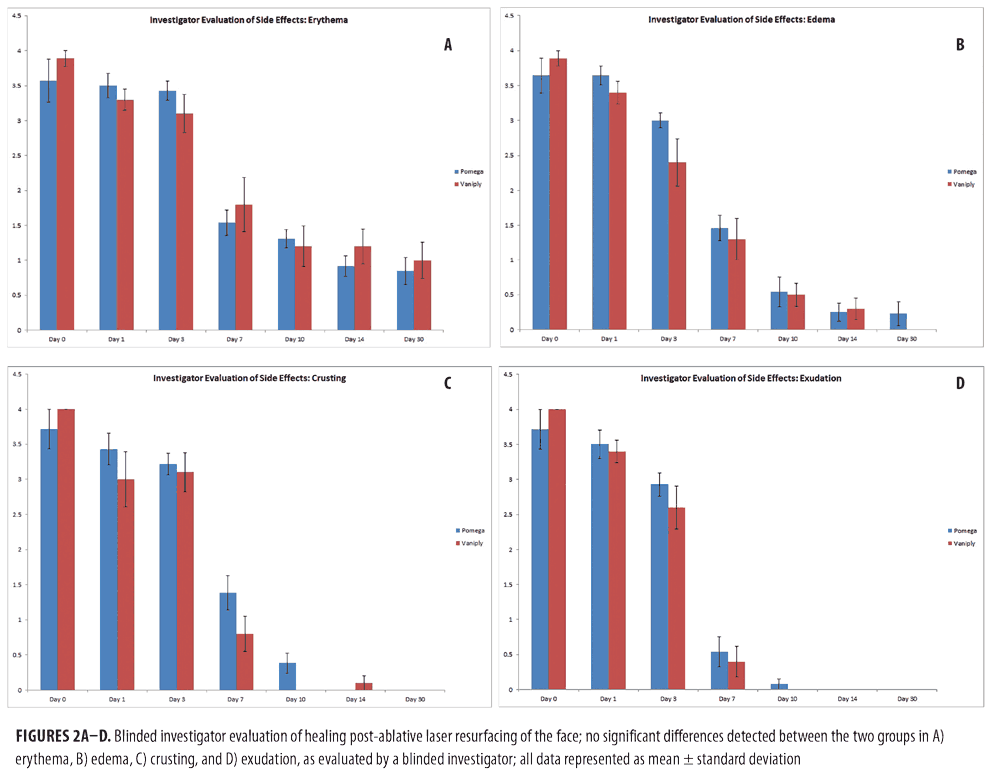
Physician assessments of erythema, edema, crusting, and exudation revealed no statistical difference between the two post-procedure healing regimens (Figure 2).
Efficacy of topical CLA post-ablative laser resurfacing. A 3-point wrinkling and 9-point elastosis scale was used to evaluate the effectiveness of topical CLA in promoting post-procedure healing as compared to control. Both test and control regimens proved excellent at promoting safe and effective healing with highly significant improvements in wrinkling and elastosis noted at two and four weeks post-resurfacing (Figure 3). The topical CLA healing regimen outperformed the control with significant improvements in wrinkling seen as early as two weeks post-procedure. The end results remained similar between the two groups (Figure 4). Subjects were satisfied to extremely satisfied with the outcomes of both healing regimens (Figure 5).
Discussion
Improvements in “healability” represent important advances in achieving optimal outcomes following ablative procedures. In this study, we demonstrated the safety and efficacy of a novel anti-inflammatory, anti-oxidant topical healing regimen based on conjugated linolenic acid derived from pomegranate oil extract. Whereas this compound had previously demonstrated experimental efficacy in a range of inflammatory disorders, the data presented in this study are the first to explore the role of CLA when applied directly to damaged and inflamed skin in a clinical setting.8–13 In these preliminary data, topical CLA does not raise any safety or tolerability issues when compared to current standard of care. Furthermore, the topical CLA formulation outperformed current wound care standards by decreasing acute pruritus and enabling a faster positive outcome in wrinkle improvement.
There are several features of this topical healing regimen that potentially contributed to the positive results. Firstly, the anti-inflammatory and anti-oxidant nature of the CLA and other omega-5 fatty acids might have facilitated the acute stages of wound healing. Secondly, the occlusive ointment-based delivery system enabled regulation of temperature, humidity, and moisture balance within the local wound healing environment resulting in superior healing. Finally, the unique, natural preservative system likely contributed to the excellent safety and tolerability profile.
The positive results of this study give rise to consideration of the applicability of topical CLA in other disorders of impaired cutaneous barrier function and increased cutaneous inflammation. Pilot studies suggest a potential positive impact of CLA on atopic dermatitis and rosacea.13,14 Since the present study has shown that this compound is safe on laser-damaged skin, which had all barrier function stripped and intense inflammatory conditions iatrogenically applied, other dermatitides, such as atopic dermatitis and radiation dermatitis, might also benefit from the healing and anti-inflammatory capabilities of topical CLA.
Limitations. The limitations of this study include the relatively small sample size, as well as the slight variations in laser treatment settings. Given that this was a clinical aesthetic study, the unique features of each individual subject were taken into account when selecting the most appropriate treatment settings. The treating and evaluating investigators were blinded to the post-procedure regimen throughout the duration of the study.
References
- Fitzpatrick RE, Williams B, and Goldman MP, Preoperative anesthesia and postoperative considerations in laser resurfacing. Semin Cutan Med Surg. 1996;15(3): 170–176.
- Ratner D, Tse Y, Marchell N, et al. Cutaneous laser resurfacing. J Am Acad Dermatol. 1999;41(3 Pt 1):365–389; quiz 390–392.
- Fitzpatrick RE, Goldman MP, Satur NM, Tope WD. Pulsed carbon dioxide laser resurfacing of photo-aged facial skin. Arch Dermatol. 1996;132(4):395-402.
- Manuskiatti W, Fitzpatrick RE, Goldman MP. Long-term effectiveness and side effects of carbon dioxide laser resurfacing for photoaged facial skin. J Am Acad Dermatol. 1999;40(3):401–411.
- Qian LW, Fourcaudot AB, Yamane K, et al. Exacerbated and prolonged inflammation impairs wound healing and increases scarring. Wound Repair Regen. 2016;24(1):26–34.
- Weiss RA, Goldman MP. Interpenetrating polymer network wound dressing versus petrolatum following facial CO2 laser resurfacing: a bilateral comparison. Dermatol Surg. 2001;27(5): 449–451.
- Goldman MP, Roberts TL 3rd, Skover G, et al. Optimizing wound healing in the face after laser abrasion. J Am Acad Dermatol. 2002;46(3):399–407.
- Boussetta T, Raad H, Lettéron P, et al. Punicic acid a conjugated linolenic acid inhibits TNFalpha-induced neutrophil hyperactivation and protects from experimental colon inflammation in rats. PLoS One. 2009;4(7): e6458.
- Costantini S, Rusolo F1, De Vito V, et al. Potential anti-inflammatory effects of the hydrophilic fraction of pomegranate (Punica granatum L.) seed oil on breast cancer cell lines. Molecules. 2014;19(6): 8644–8660.
- Coursodon-Boyiddle CF, Snarrenberg CL, Adkins-Rieck CK, et al. Pomegranate seed oil reduces intestinal damage in a rat model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2012;303(6): G744–G751.
- Hontecillas R, O’Shea M, Einerhand A, et al. Activation of PPAR gamma and alpha by punicic acid ameliorates glucose tolerance and suppresses obesity-related inflammation. J Am Coll Nutr. 2009;28(2):184–195.
- Liu KL, Belury MA. Conjugated linoleic acid reduces arachidonic acid content and PGE2 synthesis in murine keratinocytes. Cancer Lett. 1998;127(1–2): 15–22.
- Ishiguro K, Oku H, Suitani A, Yamamoto Y. Effects of conjugated linoleic acid on anaphylaxis and allergic pruritus. Biol Pharm Bull. 2002;25(12):1655–1657.
- Noli C, Carta G, Cordeddu L, et al. Conjugated linoleic acid and black currant seed oil in the treatment of canine atopic dermatitis: a preliminary report. Vet J. 2007;173(2): 413–421.
- Oikawa D, Nakanishi T, Nakamura Y, et al. Dietary CLA and DHA modify skin properties in mice. Lipids. 2003;38(6):609–614.
- Oikawa D, Nakanishi T, Nakamura YN, et al. Modification of skin composition by conjugated linoleic acid alone or with combination of other fatty acids in mice. Br J Nutr. 2005;94(2): 275–281.

