 J Clin Aesthet Dermatol. 2021;14(5):26–30.
J Clin Aesthet Dermatol. 2021;14(5):26–30.
by Renata Indelicato Zac, MD, MSc and Adilson da Costa, MD, MSc, PhD
Drs. Zac and Da Costa are with the Instituto de Assistência Médica ao Servidor Público Estadual de São Paulo/São Paulo’s Welfare Institute in São Paulo, Brazil.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Background: Androgenetic alopecia (AGA) is a common, genetically predisposed condition that begins after puberty and whose frequency increases with age; although biologically benign, AGA can impact patients both psychologically and socially, contributing to an impairment on their quality of life.
Objective: We sought to evaluate the psychological, social, and quality of life impairments inherent in women with AGA using 5% minoxidil daily for at least six months.
Design: Thirty-one women with diffuse central hair thinning and shaft miniaturization who were using 5% minoxidil daily for at least six months responded to a clinical questionnaire and underwent trichoscopy.
Results: 83.9 percent (n=26) of the participants reported they were satisfied with the 5% minoxidil treatment and its convenience. Hair loss influenced social life in 54.8 percent (n=17) of the respondents and choice of hair cut/hairstyle in 87.1 percent (n=27) of respondents. For 51.6 percent (n=16) hair loss was slightly increased, although it did not increase after beginning treatment. A frontoparietal pattern (74.2%, n=23), very low capillary density (61.3%, n=19), trichodynia (32.3%, n=10), and negative traction test (100%, n=31) were also observed. Miniaturization occurred in 100 percent (n=31) of patients, frontal/occipital hair thickness was reduced in 83.9 percent (n=26), and more than 10 percent of velus hair in the frontal area was observed in 83.9 percent (n=26) of patients. The number of hair shafts per follicular unit was reduced in 67.7 percent (n=21), and a higher frontal to occipital ratio of follicular units with one hair shaft was seen in 74.2 percent (n=23) of patients. Empty follicles, large numbers of peripillar brownish halo, scalp pigmentation, mild Ludwig’s baldness degree, and quality of life scores of 4±3.5 points were observed.
Conclusion: Our results indicate that patient satisfaction and quality of life of women with AGA on 5% topical minoxidil are high, although hair loss influences daily habits and social life.
Keywords: Androgenetic alopecia, minoxidil, patient satisfaction, quality of life
Androgenetic alopecia (AGA) is a common, genetically predisposed condition that begins after puberty and whose frequency increases with age,1 occurring in up to 40 percent of Caucasian women throughout their lives.2 Although this disease is physiologically benign, it can affect psychological and social experiences as well as quality of life.3 Women perceive hair loss at a greater extent than men because they feel social and personal pressure to not deviate from what is considered a normal feminine appearance.1
AGA is characterized by progressive shortening of the hair growth (i.e., anagen) phase in successive capillary cycles, increased kenogen (i.e., phase with empty follicles) duration and frequency, and progressive miniaturization of the hair follicle, where thick and long terminal hairs are replaced by thin and short hair shafts. This happens predominantly in the frontal and frontotemporal areas, and the frontal hair line might or might not be preserved.4,5
Hair follicles are structures with high proliferation rates that require a large supply of micronutrients.6 The most cited nutritional cause of hair loss is iron deficiency.6 Ferritin levels are a good initial marker of iron deficiency.6 In thyroid disease, alopecia is characterized by an increase in the number of dysplastic and broken hairs.7 In addition, hypothyroidism is associated with lower levels of sex hormone-binding globulin, which might result in an increase in free androgens and exacerbate AGA.7,8 Hair loss might be a symptom of zinc deficiency.9
The main goal in treating alopecia is to stop its progression and prevent future thinning.10 Topical minoxidil is an effective and safe treatment for female AGA (FAGA).11 The exact mechanism of action of minoxidil on hair growth is still uncertain, but is thought to be mediated by the opening of potassium channels, which leads to greater cutaneous blood flow and increased vascular endothelial growth factor (VEGF) levels and hair growth promoters in the dermal papilla. Moreover, it is also thought to increase the duration of the anagen phase and the hair diameter and stimulates early anagen in follicles that are in quenogen.12
The objective of the present study was to evaluate patient satisfaction and quality of life of women with AGA using 5% topical minoxidil, the impacts on their social life, and the epidemiological, clinical, and laboratory characteristics of this population.
Methods
This was a longitudinal study conducted between 2015 and 2018 at the author’s private clinic in the city of Belo Horizonte, Minas Gerais, Brazil. Thirty-one subjects, aged 18 to 60 years, using 5% minoxidil daily for at least six months were invited to respond to a questionnaire and undergo trichoscopy. Individuals were excluded if they did not agree to participate in the study, were pregnant, nursing, had apparent hair or scalp disease, clinical or biochemical evidence of androgen excess, or were using medications that could impact the characteristics of the hair (e.g., finasteride, cyproterone, drospirenone or chlormadinone).
The protocol and informed consent were established according to the Declaration of Helsinki and approved by the Ethics Committee of the University of Santo Amaro (UNISA) in São Paulo, SP, Brazil. Informed consent was obtained from all subjects before the beginning of this study.
Participants completed standardized questions to assess their epidemiological profile and psychological and social functioning and were assessed by the principal investigator with respect to clinical, tricoscopic and laboratory aspects.
The following data was collected:
- Patient-reported treatment satisfaction
- Patient-reported treatment convenience
- Patient-reported influence of alopecia on social life, personal life, and hairstyle
- Patient-reported hair loss perception and location
- Hair density
- Trichodynia
- Reduction in hair shaft thickness
- Number of velus hairs
- Number of hair shafts per follicular unit
- Empty follicles
- Peripilar signs
- Scalp pigmentation
- Participant age at the time of the study
- Phototype (i.e., leucodermic (Fitzpatrick Skin Types I and II), feodermic (Fitzpatrick Skin Types III and IV), and melanodermic (Fitzpatrick phototypes V and VI)]
- Skin type (i.e., dry, normal, or oily)
- Weight
- Number of children
- Marital status
- Smoking status
- Alcohol consumption
- Laboratory tests, such as hemoglobin, thyroid-stimulating hormone (TSH), ferritin, and zinc
- Dermatology Life Quality Index (DLQI) scores
The DLQI is a validated questionnaire with 10 questions related to events experienced by the patient in the week prior. The DLQI was developed to assess quality of life in patients with dermatological diseases. Each question has four answers, with the total score possible ranging from 0 to 30 points. Higher values indicate that the quality of life is more impaired.13
Macroscopic photographs of the scalp were taken in standard positions: front-parietal (i.e., head down), right lateral, left lateral, and posterior. To document the extension, the AGA was classified as Ludwig I to III. Trichoscopy was performed with 20× the magnification and two photos of the frontal, right temporal, left temporal, and occipital regions were taken, while one photo of the vertex was taken. In both, the Medicam™ device and Fotofinder™ software (Fotofinder Systems, Inc., Bad Birnbach, Bavaria, Germany) were used.
Qualitative variables are presented as absolute and relative frequencies, while numerical values were submitted to the Shapiro-Wilk normality test and presented as mean ± standard deviation (SD) values, in case of normality, or as median ± interquartile distance (ID) values otherwise. Fisher’s exact test was used to test the association between qualitative variables. To compare two groups, the Mann-Whitney U test for independent samples was used. Analyses were carried out using the free version of R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria) at a 5% level of significance.
Results
The mean age of the study population was 41.83±11.22 years, the mean weight was 61.81±11.61kg, 51.6 percent (n=16) were married and without children, 67.7 percent (n=21) were leucodermic, 61.3 percent (n=19) were of the eudermic biotype, the median clinical evolution time was 10±10.5 years, the mean hemoglobin concentration was 13.4±0.91g/dl, the mean TSH level was 1.79±1.04IU/L, the mean ferritin concentration was 76.30±70.83ng/mL, the mean zinc concentration was 92.90±16.68 mcg/dL, 53.3 percent (n=16) participated in occasional alcohol consumption, and only 6.7 percent (n=2) were smokers (Table 1).
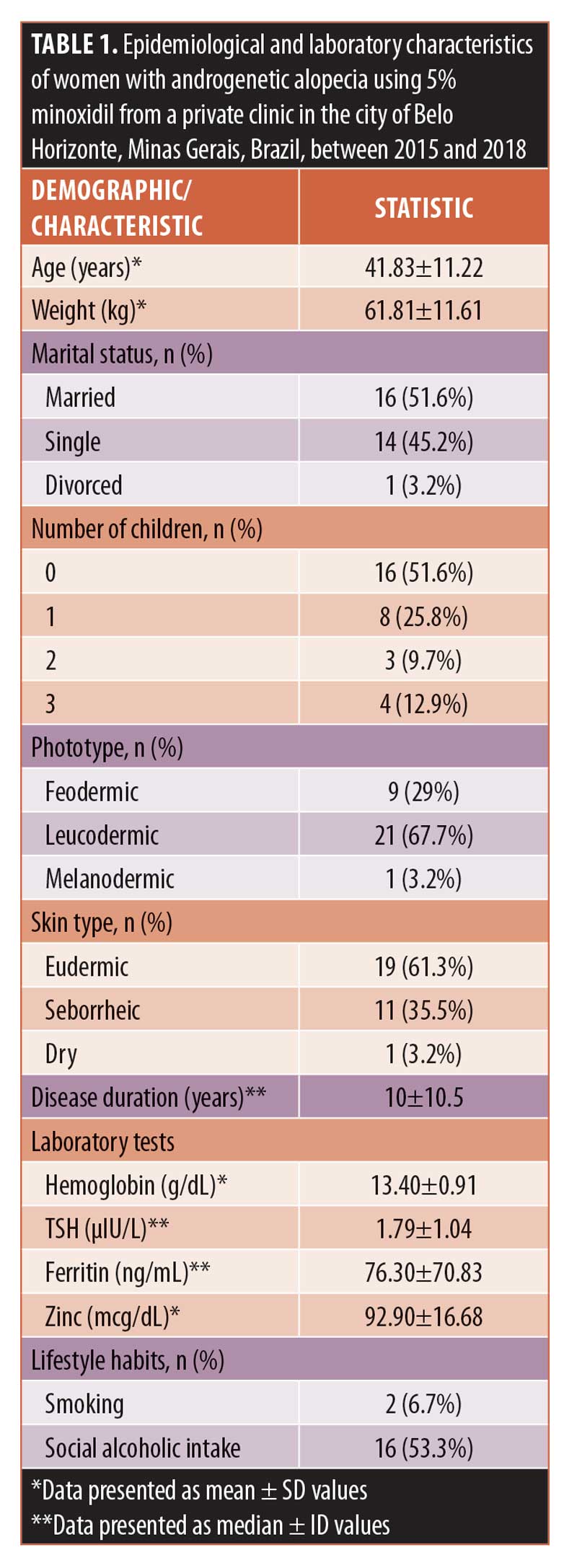
Satisfaction with treatment and comfort was reported by 83.9 percent (n=26). Among 54.8 percent (n=17), hair loss influenced their social life, while, for 87.1 percent (n=27), it influenced the style of their hair cut/hairstyle. For 6.5 percent (n=2), their problem influenced their partner’s social life. In 51.6 percent (n=16), the perception of hair loss was slightly increased, 74.2 percent (n=23) perceived a frontoparietal location, 61.3 percent (n=19) were considered to have very low capillary density, and 32.3 percent (n=10) had trichodynia. The traction test was negative in all patients (Table 2).
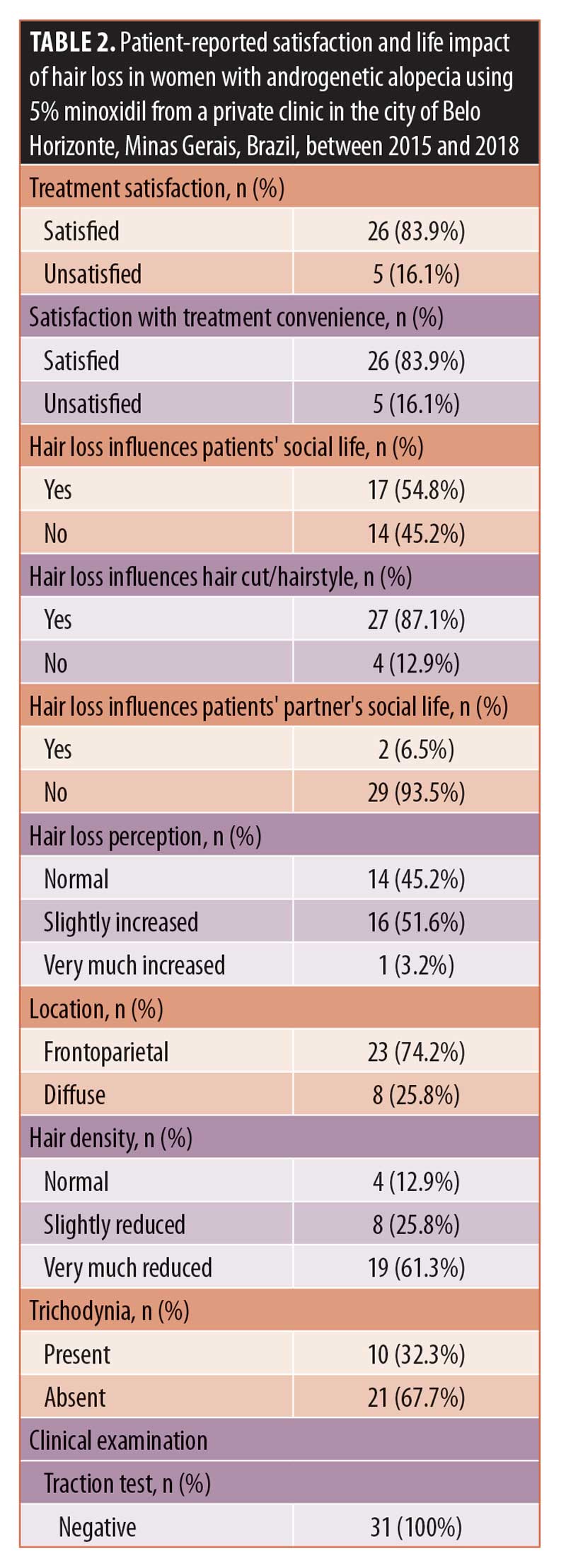
On tricoscopy (Table 3), miniaturization occurred in all cases. There was a reduction of the average hair thickness in the frontal/occipital area in 83.9 percent (n=26) of the participants, while there more than 10 percent of velus hairs existed in the frontal area in 83.9 percent (n=26). The number of hair shafts per follicular unit was reduced in 67.7 percent (n=21), while there was a frontal/occipital increase in the ratio of the number of follicular units with one hair in 74.2 percent (n=23). Empty follicles were not found in 38.7 percent (n=12) of cases, and a peripilar brownish halo was seen in large numbers in 58.1 percent (n=18). Pigmentation of the scalp was observed in 45.2 percent (n=14), while, in 61.3 percent (n=19) of patients, Ludwig’s baldness was mild. The median quality of life score was 4±3.5.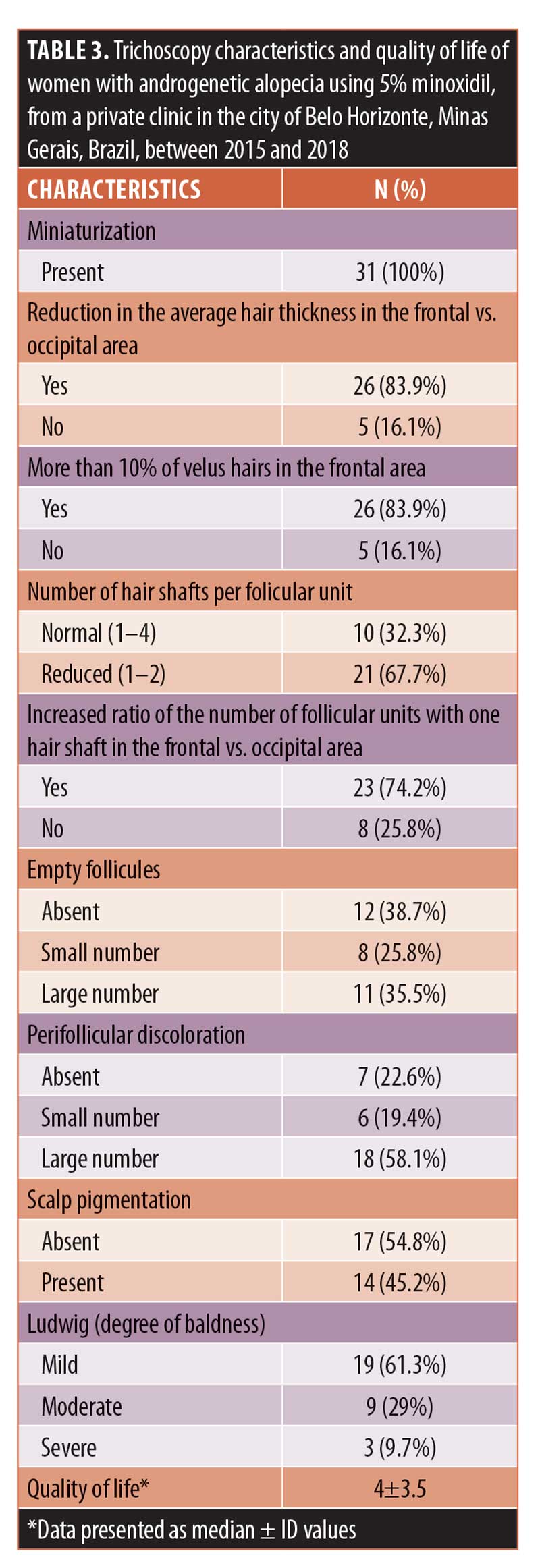
Patient satisfaction and quality of life were not associated with age, the degree of alopecia, or the amount of reduction in the number of hair shafts per follicular unit.
Discussion
Favorable patient opinions regarding their treatment for AGA is extremely important, as AGA is a chronic dermatosis that requires daily treatment adherence. Satisfaction with treatment is crucial to ensure good adherence to it and to attain therapeutic results.
Subjective satisfaction is determined by several psychological factors, such as treatment motivation. Patients whose motives are internal, such as improved self-appearance, have more satisfactory results than patients with external motivations, such as pleasing others.3 A patient’s initial expectations upon starting treatment is also an important influence on their satisfaction with the results of their treatment; if a patient’s expectation goes beyond what is achievable with medical treatment, it will likely lead to disappointment. Unrealistic expectations can lead to poor treatment adherence and premature treatment discontinuation.3 For most patients, the reversal or stabilization of hair loss can be a stimulus for the desired psychological changes.3
According to Hasanzadeh et al,14 satisfaction with the efficacy of minoxidil treatment in men with AGA was high at Week 24 (75%), as was the satisfaction found in our study (83.9%) in women. Lulic et al15 found 43.8 percent of male patients were satisfied with their treatment with minoxidil. The benefits of using minoxidil in women with AGA in this study were also seen in men in a clinical trial setting, with global improvements, and in the quality of life, hair styling, and hair growth.16 Regarding the use of medication, 91.6 percent of male patients reported being very satisfied.14 In our study, we recorded that 83.9 percent of our participants were satisfied.
Physicians should spend sufficient time discussing therapeutic strategies with patients and allowing them to be involved in choosing treatment, as this generates greater satisfaction and adherence to treatment. According to Willianson et al,13 40 percent of patients with alopecia were dissatisfied with their medical appointment. This might reflect a low estimate of the effects of hair loss by health professionals, who seem unaware of feelings of vulnerability in their patients. It was found that 18.5 percent of patients felt that their doctor did not offer treatment and did not ask for exams.13 It is important that health care professionals be trained to give appropriate support.
According to Lulic et al15 three treatment characteristics considered most important by patients were hair restoration, the onset of action, and the cost of treatment. Potential side effects were considered less important than promoting hair growth or retarding the progression of alopecia.15
Hair loss impairs the social lives of patients. In our study, 54.8 percent of patients reported that alopecia interferes with their social life. The daily impact seems to be greatest in women, who feel pressure from society and themselves to be physically attractive and not deviate from the “normal” female appearance.1,17,18,19 In our study, 87.1 percent of the patients stated that alopecia influences their hair cut and hairstyle. In male patients, a daily impact was reported by 43.5 percent.15
Although the traction test was negative in all patients in our study, perceptions of hair loss by patients were considered normal by 45.2 percent and slightly increased by 51.6 percent. It is suggested that anxious women disproportionately perceive their degree of hair loss and should be asked about depression and marital problems, as this makes them overly sensitive to hair loss that would not normally concern them to the point of seeking medical advice. It is suggested that they might be using their hair in a symbolic way to get help with underlying issues.20 In our study, 74.2 percent of patients noticed hair loss in the frontoparietal region, and 25.8 percent believed that it occurred throughout the scalp. Capillary density was considered very low by 61.3 percent of patients and normal by only 12.9 percent. Trichodynia was present in 32.3 percent of patients. Trichodynia might be secondary to the emotional stress related to hair loss.21
Dermoscopy in AGA is characterized by a variability in the hair shaft diameter of greater than 20 percent.22 In our study, hair shaft miniaturization was present in 100 percent of cases.
Radowska et al23 reported a lower average thickness of the hair shaft in the frontal region (0.047±0.007mm) as compared with 0.052±0.008mm in the occipital region. A greater percentage of thin hair and a simultaneous decline in the amount of thick hair were also observed, especially in the frontal area, in 83.9 percent of our participants.
A higher average percentage of fine hairs (less than 0.03mm in diameter) was observed in patients with FAGA in the frontal region (20.9%±12%) in the study by Radowska et al.23 In our study, 83.9 percent of patients presented with more than 10 percent of velus hairs in the frontal region.
The number of hair shafts per follicular unit was reduced to one or two in 67.7 percent of the patients in our study. Yazdabadi et al24 showed that women with AGA had 2.4 terminal hairs per follicular unit compared to 3.38 per unit in the control group (p=0.0001).24 This reduction in terminal hairs per follicular unit explains the reduction in hair density in women with AGA. Secondary and tertiary follicles undergo miniaturization first, which explains the diffuse pattern of hair loss and predominance of single-hair shaft follicular units. The mechanism seems to be related to the interaction between stem cells in the bulge and the arrector pili muscle.24 The average percentage of follicular units with one hair shaft in the frontal area was 65.2 percent ± 19.9 percent in the study by Radowska et al23 and was present in 74.2 percent of the patients in our study.
The increase in the number of empty follicles, revealed during trichoscopy as yellow dots, was observed in both our study and that by Radowska et al.23 There was an increase in the number of perifollicular discolorations in 77.5 percent of the patients in our study and in 32.4 percent ± 4.7 percent of the follicles in the study by Radowska et al.23 Scalp pigmentation was noted in 45.2 percent of patients in our study. This characteristic is usually found in sun-exposed areas of AGA patients.22 In the present study, 61.3 percent had mild AGA, while, in the study by Zhuang et al,11 this proportion was 45.6 percent.
Generally, patients with FAGA seeking treatment are anxious, which impacts their quality of life.3,19 Prior to the initiation of treatment, DLQI scores in patients with hair loss ranged from 8.3±5.6 points13 to 9.62±5.92 points,11 with values above 10 points indicating a severe impact on the quality of life. Our study revealed DLQI scores of 4±3.5 points in patients already using minoxidil, while Zhuang et al11 reported DLQI scores of 4.45±3.36 points. Thus, the use of minoxidil improves the quality of life in patients with androgenic alopecia. There were no differences in DLQI scores between responders and nonresponders to minoxidil treatment.11 Subjective satisfaction and quality of life do not correspond to objective assessment after treatment.
In our study, we established a six-month period to evaluate the benefits of consultation and initiation of treatment. Biondo et al25 did not report a benefit while using oral antiandrogen therapy for two months and suggested the possibility of using the medication for longer periods and a role for adjuvant psychological intervention in the treatment of hair loss.
In our study, there was no correlation between age and satisfaction with treatment, similar to the findings by Zhuang et al.11 However, while Zhuang et al observed a relationship between disease severity and DLQI score, in the present study, no correlation was found between the degree of alopecia and satisfaction with treatment, probably because their analysis was performed before the start of treatment and our analysis was conducted in the patients already undergoing treatment.
To our knowledge, this is the first study to correlate treatment satisfaction and dermatoscopic criteria. Longer disease duration usually presents clinically with a reduction in the number of hair shafts per follicular unit. However, in the present study, we found no correlation between a reduction in the number of hair shafts and satisfaction with treatment; Zhuang et al also did not report correlation between satisfaction with treatment and disease duration.11
Conclusion
We conclude the following satisfaction and quality of life of women with AGA on 5% topical minoxidil are high, although hair loss influences the social and daily routines of these individuals. The epidemiological characteristics of women with FAGA described in the literature are not very different from those without it. Physical examination and trichoscopy findings among women with FAGA are quite distinct and characteristic compared to those without it. The lack of association between a reduction in the number of hair shafts, disease severity, and age with treatment satisfaction and quality of life, as measured by the DQLI, reveals that patients’ psychological profile might be more important than clinical data.The results of hemoglobin, ferritin, TSH, and zinc tests in patients with FAGA were within reference values. Overall patient satisfaction and quality of life among women in this real-world investigation were similar to those seen when minoxidil was used in the clinical trial setting. The dermatology quality of life in patients using minoxidil shows us that clinical treatment can have psychological benefits. We believe that unrealistic expectations seem to be the main factor leading to treatment failure and, therefore, these expectations should be thoroughly discussed among physicians and patients.
References
- Dolte KS, Girman CJ, Hartmaier S, et al. Development of a health-related quality of life questionnaire for women with androgenetic alopecia. Clin Exp Dermatol. 2000;25(8): 637–642.
- Gan DC, Sinclair RD. Prevalence of male and female pattern hair loss in Maryborough. J Investig Dermatol Symp Proc. 2005;10(3):184–189.
- Cash TF. The psychosocial consequences of androgenetic alopecia: a review of the research literature. Br J Dermatol. 1999;141(3):398–405.
- Rebora A, Guarrera M. Kenogen. A new phase of the hair cycle? Dermatology. 2002;205(2):108–110.
- Van Zuuren EJ, Fedorowicz Z, Carter B, et al. Interventions for female pattern hair loss. Cochrane Database Syst Rev. 2012(5):Cd007628.
- Park SY, Na SY, Kim JH, et al. Iron plays a certain role in patterned hair loss. J Korean Med Sci. 2013;28(6):934–938.
- Vincent M, Yogiraj K. A descriptive study of alopecia patterns and their relation to thyroid dysfunction. Int J Trichology. 2013;5(1):57–60.
- Famenini S, Slaught C, Duan L, Goh C. Demographics of women with female pattern hair loss and the effectiveness of spironolactone therapy. J Am Acad Dermatol. 2015;73(4):705–706.
- Aiempanakit K, Jandee S, Chiratikarnwong K, et al. Low plasma zinc levels in androgenetic alopecia. Indian J Dermatol Venereol Leprol. 2017;83(6):741.
- Kelly Y, Blanco A, Tosti A. Androgenetic alopecia: an update of treatment options. Drugs. 2016;76(14):1349–1364.
- Zhuang XS, Zheng YY, Xu JJ, Fan WX. Quality of life in women with female pattern hair loss and the impact of topical minoxidil treatment on quality of life in these patients. Exp Ther Med. 2013;6(2):542–546.
- Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150(2):186–194.
- Williamson D, Gonzalez M, Finlay AY. The effect of hair loss on quality of life. J Eur Acad Dermatol Venereol. 2001;15(2):137–139.
- Hasanzadeh H, Nasrollahi SA, Halavati N, et al. Efficacy and safety of 5% minoxidil topical foam in male pattern hair loss treatment and patient satisfaction. Acta Dermatovenerol Alp Pannonica Adriat. 2016;25(3):41–44.
- Lulic Z, Inui S, Sim WY, et al. Understanding patient and physician perceptions of male androgenetic alopecia treatments in Asia-Pacific and Latin America. J Dermatol. 2017;44(8):892–902.
- Olsen EA, Dunlap FE, Funicella T, et al. A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and placebo in the treatment of androgenetic alopecia in men. J Am Acad Dermatol. 2002;47(3):377–385.
- Cash TF, Price VH, Savin RC. Psychological effects of androgenetic alopecia on women: comparisons with balding men and with female control subjects. J Am Acad Dermatol. 1993;29(4):568–575.
- Van der Donk J, Passchier J, Knegt-Junk C, et al. Psychological characteristics of women with androgenetic alopecia: a controlled study. Br J Dermatol. 1991;125(3):248–252.
- Van Der Donk J, Hunfeld JA, Passchier J, et al. Quality of life and maladjustment associated with hair loss in women with alopecia androgenetica. Soc Sci Med. 1994;38(1): 159–163.
- Eckert J. Diffuse hair loss in women: the psychopathology of those who complain. Acta Psychiatr Scand. 1976;53(5):321–327.
- Mubki T, Rudnicka L, Olszewska M, Shapiro J. Evaluation and diagnosis of the hair loss patient: part I. History and clinical examination. J Am Acad Dermatol. 2014;71(3):415.e1–e15.
- Miteva M, Tosti A. Hair and scalp dermatoscopy. J Am Acad Dermatol. 2012;67(5):1040–1048.
- Rakowska A, Slowinska M, Kowalska-Oledzka E, et al. Dermoscopy in female androgenic alopecia: method standardization and diagnostic criteria. Int J Trichology. 2009;1(2):123–130.
- Yazdabadi A, Magee J, Harrison S, Sinclair R. The Ludwig pattern of androgenetic alopecia is due to a hierarchy of androgen sensitivity within follicular units that leads to selective miniaturization and a reduction in the number of terminal hairs per follicular unit. Br J Dermatol. 2008;159(6):1300–1302.
- Biondo S, Sinclair R. Quality of life in Australian women with female pattern hair loss. The Open Dermatology Journal. 2010;4:90–94

