 J Clin Aesthet Dermatol. 2022;15(2):18–22.
J Clin Aesthet Dermatol. 2022;15(2):18–22.
by BS Chandrashekar, MBBS, MD, DNB and Soragavi Soumya, MBBS, MD, FRGUHS
Dr. Chandrashekar and Dr. Soragavi Soumya are with the Department of Dermatology and the CUTIS Academy of Cutaneous Sciences in Vijayanagar, Bangalore, India.
FUNDING: This study was funded by Menarini India Pvt Ltd.
DISCLOSURES: The authors report no conflicts of interest relavant to the content of this article.
ABSTRACT: Objective. We sought to determine the efficacy and safety of a topical under-eye serum (Melalumin™; Menarini India Pvt Ltd.) in patients with periorbital hyperpigmentation (POH).
Methods. In this prospective, open-label single-arm study, 90 patients aged 18 to 55 years with Grade I to IV pigmentary POH, were given the under-eye serum for three months. Follow-up visits were scheduled at one, two and three months from baseline. Effectiveness was evaluated by two independent dermatologists using a skin colorimeter (Dermacatch) and dermoscopy (FotoFinder Systems, Inc., Medical Imaging Systems; Columbia, Maryland), as well as global photographs and patient-reported satisfaction ratizngs (excellent, very good, good, not satisfied). Adverse events were recorded. The colorimeter values were evaluated using the paired T test and the single-mean T test was used for dermoscopy and global clinical photographs.
Results. Of the 90 patients included, 85 completed the study. Significant reductions in colorimeter values were noted in both melanin (from 708 to 621) and erythema (from 450 to 417) over three months (p<0.05). Mean improvement in dermoscopic assessment was 48.41 percent; Most (n=73/85; 85.88%) patients achieved >25-percent improvement; over one-third (n=31/85; 36.47%) showed >50-percent improvement. Global photographs improved by 49.47 percent; most (n=75/85; 88.24%) patients showed >25-percent improvement, over one-third (n=38/85; 44.71%) showed >50-percent improvement. Patient satisfaction levels were high (Excellent: 16 [18.82%]; Very good: 38 [44.71%]); Good: 26 [30.59%]; Not satisfied: 5 [5.88%]). No adverse events were noted.
Conclusion. This study demonstrates safety and effectiveness of the studied under-eye serum in patients with pigmentary POH. In addition to clinical improvements noted by the investigators, significant improvements were also noted in colorimeter values, dermoscopy results, and global photographs. Patients exhibited high satisfaction levels with treatment outcomes. No safety concerns were noted.
Keywords. Hyperpigmentation, eye, topical, serum
Periorbital hyperpigmentation (POH), one of the most commonly encountered conditions in dermatology practice, is rapidly emerging as a significant cosmetic concern.1-3 As a result of variable constitutive pigmentation around the world, individuals with darker skin tones, such as those living in India, are more susceptible to hyperpigmentation disorders.4-6 Around 50 percent of Indian women have moderate-to-severe dark circles that increase with age.5
In view of the increasing importance ascribed to physical appearance in today’s world, POH exerts a significant psychological impact on a person’s quality of life, especially among young women, who might be perceived as sad, tired, or stressed due to dark circles. Hence, most people are willing to incur a substantial cost burden to conceal and manage POH.7-9
Though management of POH begins merely with identification of specific etiologic factors,9 treatment is not simple.7 As dermatitis, allergy, systemic disorders, sleep disturbances, or nutritional deficiencies can result in POH, a complete medical history and physical examination is encouraged prior to treating the aesthetic component.10 Among several systemic therapies available for POH, most either lack adequate evidence,1,11 or are associated with adverse effects.1,12 Hence, topical agents that improve blood circulation and/or reduce melanin in the periorbital region are most suitable and convenient as first-line therapy.1, 12, 13 Hydroquinone 4% the most widely prescribed topical anti-pigmentation agent worldwide, is now unavailable in Europe and Asia due to possible carcinogenicity.14 This complicated scenario calls for safe, convenient treatment options, whose effectiveness is demonstrated in well-conducted studies. Therefore, this open-label, single-arm, prospective study was conducted to assess the safety and effectiveness of a topical under-eye serum containing aldavine, tyrostat and lanachrys, in Indian patients with POH.
Methods
This was an open-label, single-arm, prospective study, conducted between March 2018 to August 2018 in accordance with the principles of the Declaration of Helsinki, International Conference on Harmonization Good Clinical Practice (ICH-GCP) guidelines and Indian regulatory guidelines (Indian Council of Medical Research and Indian GCP guidelines). The study protocol was approved by the institutional ethics committee (IEC). All patients provided written consent in their patient authorization form prior to participating in the study.
Patient selection. Eligibility criteria. Adults <55 years of age, diagnosed with mild-to-severe (Grade I–IV) pigmentary POH without complications, who had not participated in similar investigations in the preceding four weeks and were willing to comply with the study protocol and refrain from existing active therapy for at least 10 days prior to study serum application were included. Subjects with any systemic disorder or active skin disease other than hyperpigmentation, overt bacterial/viral infection, superficial viral skin infections (e.g., herpes simplex or varicella; active/<6 months history), those perviously on systemic treatment for atopic dermatitis or topical/transdermal treatments on/near the intended site (<14 days prior to first application of study medication), those allergic to any of the study formulation ingredients, those with scars/moles/tattoos/body piercings/sunburn in test areas, pregnant women, lactating women, and fertile women not on adequate contraceptive measures were excluded.
Study procedures and data collection Application of under-eye serum. All subjects were instructed to apply the under-eye serum twice daily for three months. They were allowed to use only their usual cleansing products under the eyes; their usual day and night creams were allowed on the face, but not under the eyes. They were advised to avoid excessive exposure to sunlight.
Study visits. The study participants completed four visits: at baseline (Visit 1), one month (Visit 2), two months (Visit 3), and three months (Visit 4).
Study endpoints. Two independent dermatologists assessed the following primary efficacy endpoints:
Skin colorimeter readings. Change in skin color/complexion around the periorbital region (melanin quantification) was measured using a colorimeter (Dermacatch).
Dermoscopic assessment. Mean percentage improvement in POH lesions via dermoscopy was graded into four classes, as follows:
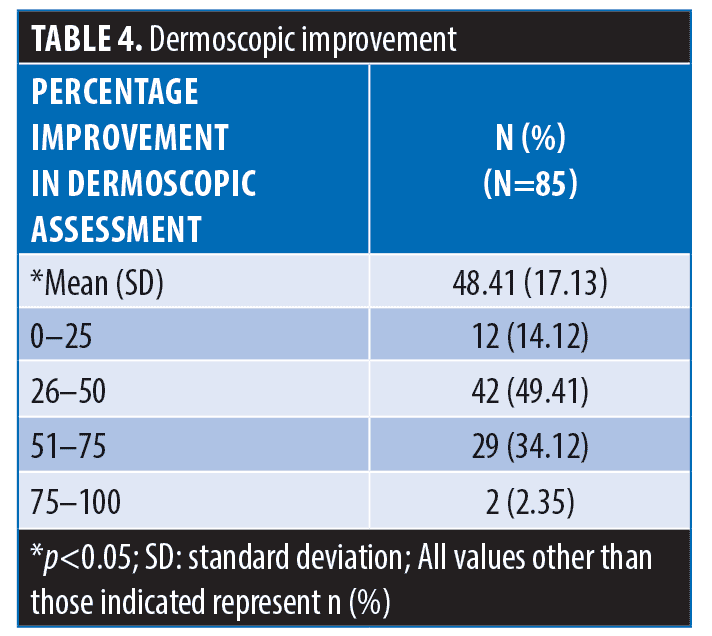
Grade 1=0%–25% reduction; Grade 2=26%–50% reduction; Grade 3=51%–75% reduction; Grade 4=76%–100% reduction.
Global (clinical) photographs assessment. Mean percentage improvement in global pictures was graded into four classes, as follows: Grade 1=0%–25% reduction; Grade 2=26%–50% reduction; Grade 3=51%–75% reduction; Grade 4=76%–100% reduction.
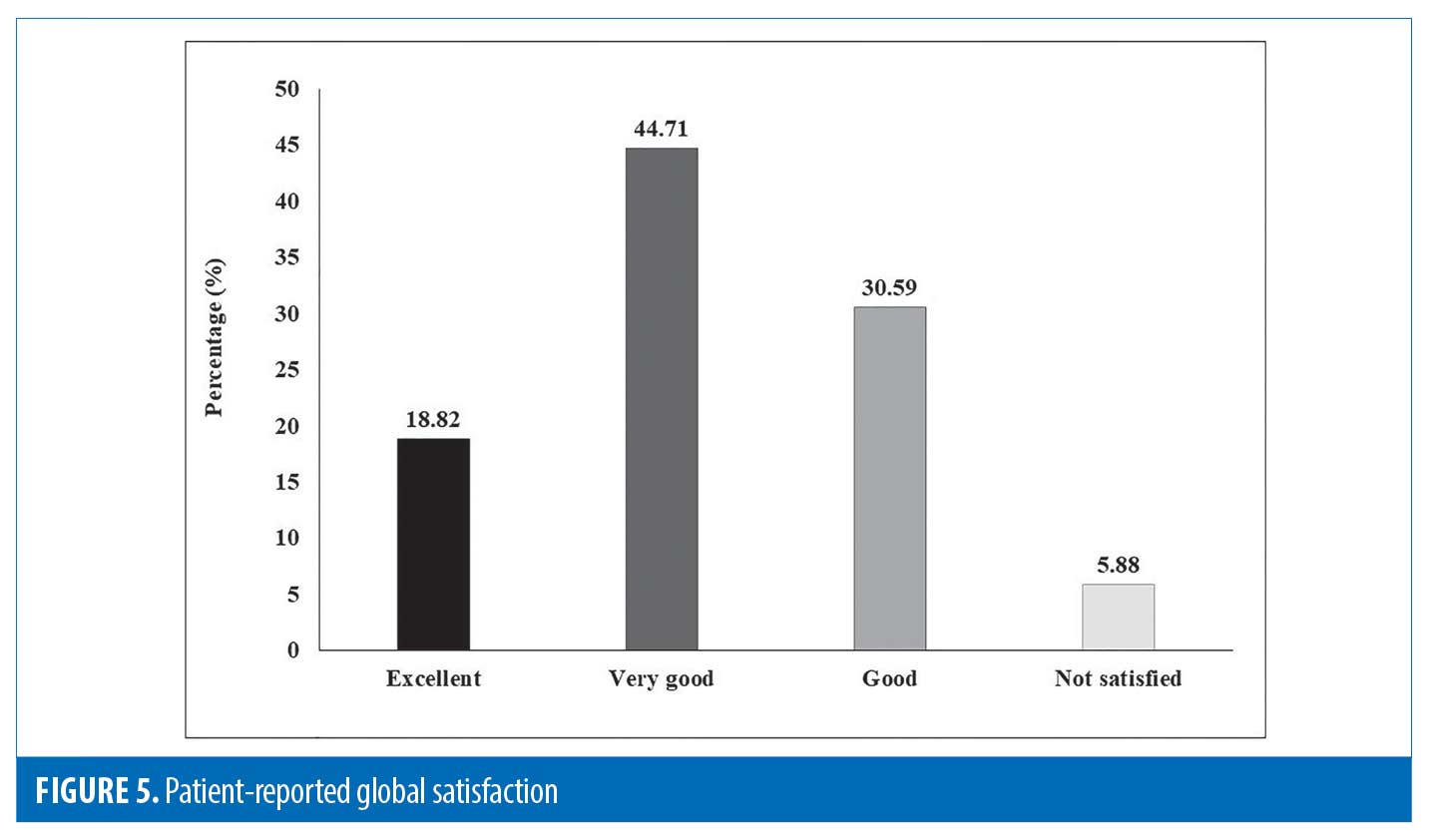
Patient’s global satisfaction assessment. Patient satisfaction was graded into four classes, as 1) excellent; 2) very good; 3) good; and 4) not satisfied.
Colorimeter readings, dermoscopic assessments and global photographs were taken at all four visits; patient satisfaction was assessed at the final Visit 4 (three months).
Two independent dermatologists also assessed secondary endpoints. Safety was assessed by history and clinical examination. Adverse events, if any, were noted at each visit.
Dermoscopic evaluation method. Dermoscopy was conducted using polarized dermoscope (Fotofinder medicam 800 HD; 20X magnification). Before taking the overview images, we ensured that the room was bright and there were no large light variations. We always checked if additional light was required to prevent shadows on the patient’s images. We also avoided taking pictures of the front window to prevent overexposed images. We used the medicam’s ring flood light for taking overview images. We also switched on the LED light (the video camera is equipped with an integrated LED-floodlight illumination, which allows capturing of excellent overview images up to a distance of 70cm) before starting a record. A distance holder was used, along with the integrated LED-floodlight illumination, for images with a distance from 2cm to 5cm. In order to ensure that the same site was examined each time, we used the “Marker” function on the dermoscopy device’s user interface, which allows the user to add arrows to indicate lesion locations, and to assign these to micro images later. A marker indicates the position in an overview image, where a dermoscopic image of a lesion should be taken.
Statistical analysis. Sample size calculation. The sample size was determined using the following formula:
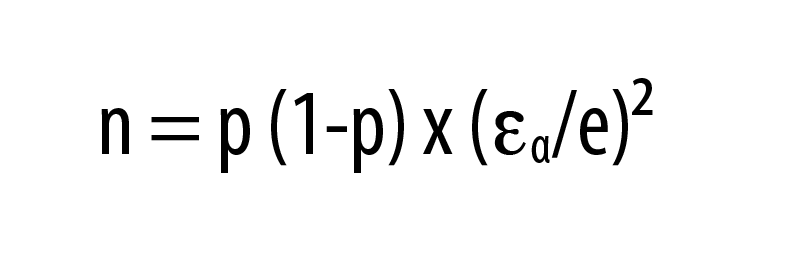
Here, n represents the required sample size, p represents the estimated proportion of subjects with pigmentary POH among those attending skin outpatient departments in India, ??=1.96 for ?=5%, and e represents the margin of error. Assuming that 30 percent of subjects attending skin outpatient departments in India had pigmentary POH,15 at a 5% level of significance and a 10% margin of error, a minimum of 81 subjects was required. Considering a drop-out rate of 10% we decided to enroll 90 patients.
Statistical methods. All recorded data were summarized using descriptive analyses. Mean, standard deviation (SD), median and range (min-max) were used to describe continuous variables. Categorical values were presented using frequency and percentage with a 95% confidence interval. The paired-T test was used to evaluate the significance of improvements in colorimeter values, while the single-mean T test was used to evaluate the significance of percentage improvements in dermoscopy and global clinical photographs (a mean improvement of 30% in dermoscopy values and global clinical photographs was assumed to be significant).
Results
Among 90 enrolled patients, 85 completed the study. The mean age of patients was 29.68±7.40 years; majority were women (72/85; 84.71%). Most patients (76/85; 89.41%) were in the age group of 20–40 years (Table 1).
Global photographs assessment. At three months, global photographs showed an overall mean percentage improvement of 49.47% (p<0.05) (Figures 1 and 2).
The majority (75/85; 88.24%) of patients showed >25-percent improvement in global photographs. Over one-third (38/85; 44.71%) showed >50-percent improvement in global photographs (Table 2).
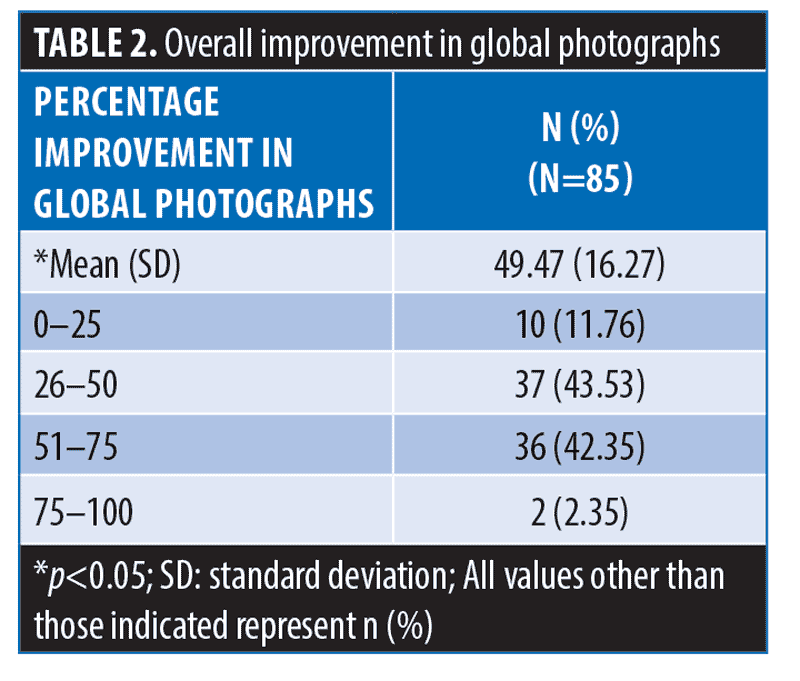
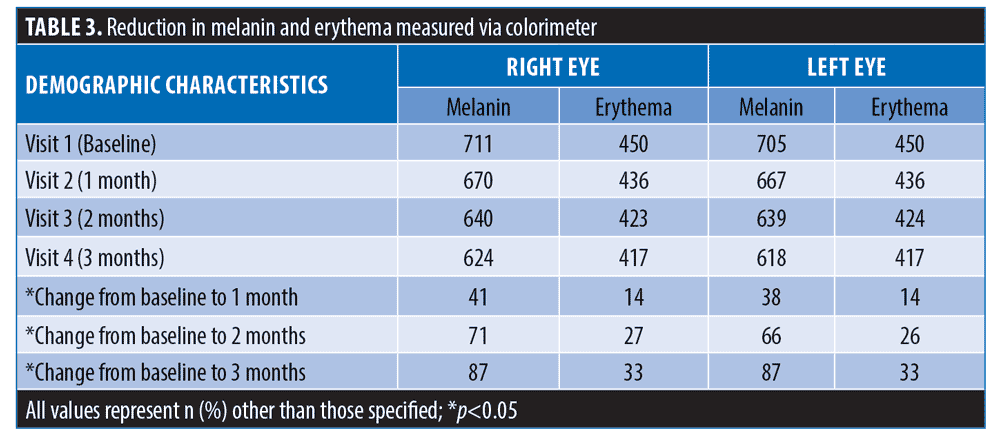
Colorimeter values. At three months, melanin values reduced from 708 to 621 (mean reduction: 12.29%; p<0.05). Similarly, erythema values reduced from 450 to 417 (mean reduction: 7.33%; p<0.05) (Table 3).
Dermoscopic results. At three months, mean improvement in dermoscopic assessment was 48.41% (p<0.05) (Figures 3 and 4).
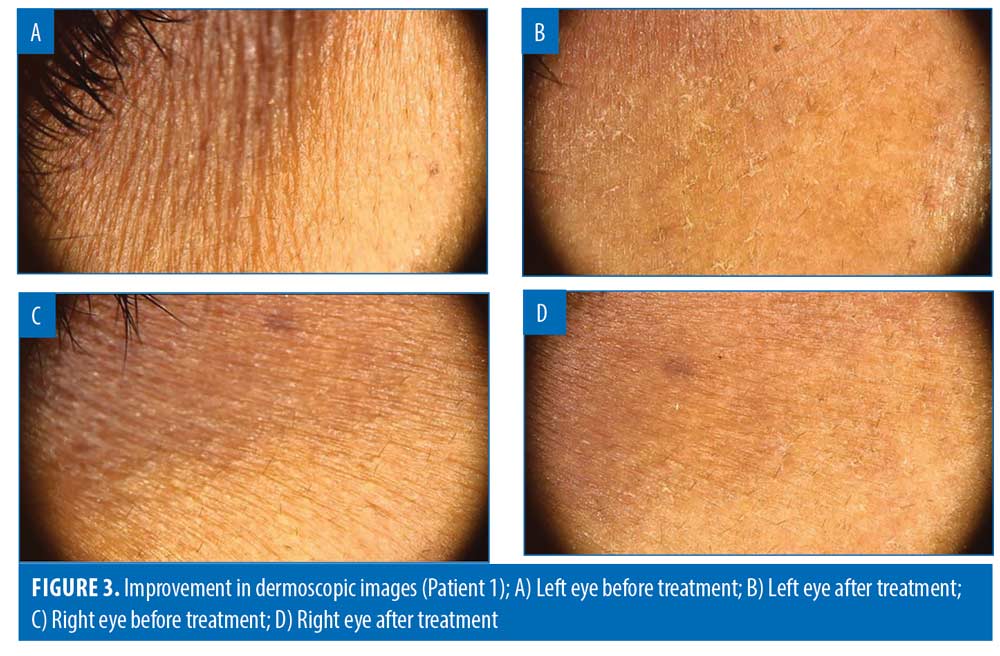
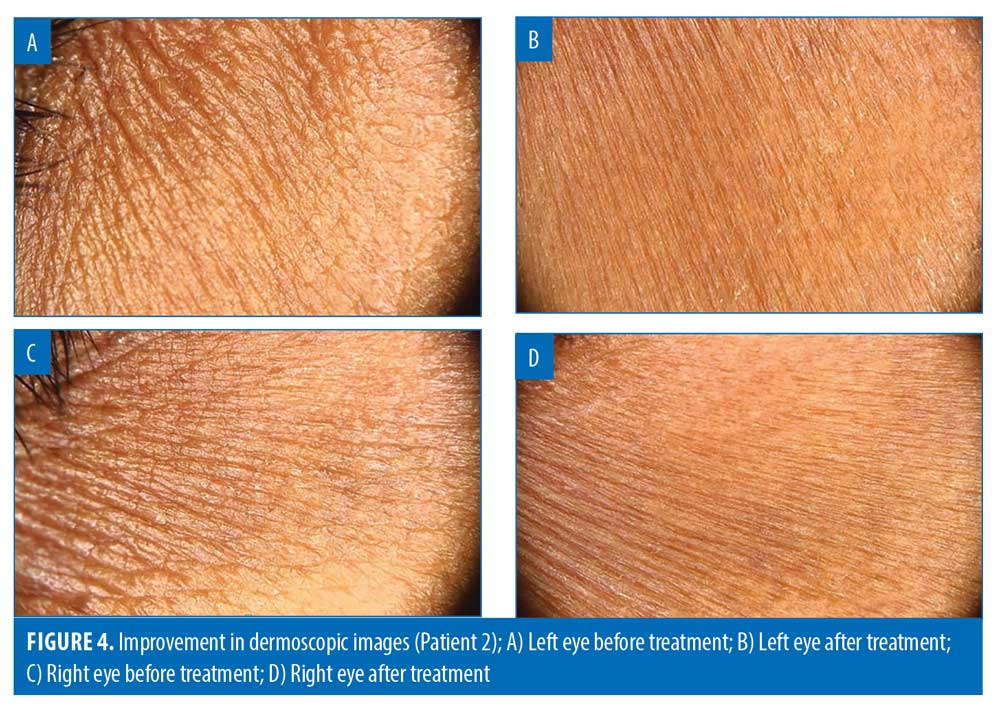
The majority of the participants (73/85; 85.88%) achieved >25-percent improvement in dermoscopic assessment. Over one-third of the patients (31/85; 36.47%) showed >50-percent improvement in dermoscopic assessment (Table 4).
Patient’s global satisfaction. Of 85 patients, 16 (18.82%) patients rated their satisfaction with treatment as “excellent,” 38 (44.71%) patients rated it as “very good,” 26 (30.59%) patients rated it as “good,” and five (5.88%) patients rated it as “not satisfied.” (Figure 5)
Safety. No adverse events were noted.
Discussion
This open-label, single-arm, prospective study showed that in adults with mild-to-severe (Grade I to Grade IV) pigmentary POH using the investigated under-eye serum for three months, significant improvements were observed in all effectiveness endpoints, including colorimeter values, dermoscopy assessment, global photographs. Most patients were satisfied with treatment. No adverse events were noted.
In our study, a majority (84.71%) of the patients with pigmentary POH were women and most (89.41%) were in the age group of 20 to 40 years. Similar findings were observed in another observational study on patients with POH between December 2010 and June 2012 in, where nearly 80.49% POH patients were women and most (40.2%) patients belonged to the age group of 21 to 30 years.15 Few studies have shown that POH incidence is almost equal between men and women, while, others suggest greater preponderance in women than men.15–17
The deposition of melanin pigment and haemosiderotic pigment are the principal factors in the pathogenesis of periorbital dark circles. Deposition of the melanin pigment is observed when excessive and cumulative exposure to the sun increases the production of melanin. Hemosiderotic deposition, on the other hand, results when intense vascularisation in the palpebral region causes dermal blood extravasation.18,19 Based on pathogenic factors and clinical appearance, POH is classified into the following four types:20, 21 1) pigmented (appears as an infraorbital brown hue); 2) vascular (presents as an infraorbital blue, pink, or purple hue with or without periorbital puffiness); 3) structural (appears as structural shadows of skin color formed by facial anatomic surface contours, and may be associated with infraorbital palpebral bags, blepharoptosis, and loss of fat with bony prominence); and 4) mixed (combines two or three of the above appearances). It is further classified into four subtypes—pigmented-vascular, pigmented-structural, vascular-structural, and a combination of the three.
The above classification can be useful to guide the selection of the appropriate therapeutic modalities, as different types of POH respond to different types of treatment.21
Clinical examination (including manual stretching of the lower eyelid skin) can help to differentiate between true pigmentation and shadowing effect, that may occur due to tear trough. On manual stretching of the lower eyelids, true pigmentation retains its appearance, a shadowing effect improves or resolves entirely, while in case of thin eyelid skin or hypervascularity of lower eyelid, an increase in violaceous discoloration is noted.21, 22
Dermoscopy is a non-invasive diagnostic technique for the evaluation of POH, that better visualises subtle clinical patterns of skin lesions and subsurface structures, not normally visible to an unaided eye. Hence, it can be used to determine if the origin of pigment is due to melanin or due to underlying vasculature i.e. it helps to differentiate the type of POH (vascular, pigmented, mixed).1,8,20,23,24 Likewise, the chromameter is able to characterize skin color and to quantify small skin color changes. A study observed moderate to high significant linear correlations between the chromameter color parameters and the erythema/melanin indices.25 Hence, we used these two diagnostic tests to evaluate the effect of therapy in patients with pigmentary POH.
The eye serum used in our study contains aldavine 5X (1%), tyrostat 09 (2%) and lanachrys 2B (1%), which, according to the manufacturers of these ingredients, stimulate the production of important structural proteins, promote collagen synthesis, maintain microcapillary integrity upon ultraviolet exposure, increase skin elasticity and resilience, and reduce the appearance of dark circles.26–28 According to the manufacturers of aldavine 5X, it mainly targets the vascular endothelial growth factor (VEGF) cytokine, modulates PGE2 activity and inhibits MMP-2 activity. These biochemical mediators associated with microcapillary dilatation and hyperpermeability are up-regulated in the skin with aging, ultraviolet exposure and environmental stress. It is suggested that aldavine 5X inhibits these mediators, potentially promoting dermis matrix integrity and preventing fluid leakage and vasodilatation.26
This open-label single-arm prospective study, conducted to evaluate the use of topical under-eye serum MelaluminTM in POH patients in India, provides valuable data regarding its effectiveness and safety. In view of the limited number of studies exploring POH management options in Indian patients, the study provides crucial insights and lays the foundation for the conduct of randomized controlled trials to better explore effectiveness and safety outcomes in larger population groups. The study met the required sample size, thus confirming the validity of the findings. However, in the absence of a placebo-controlled arm, the study was subject to the inherent shortcomings of observational studies including bias, confounding factors etc. Moreover, the effectiveness of the study drug was not compared to other treatment modalities like chemical peels, lasers, dermal fillers, due to lack of studies exploring other treatments in Indian patients with POH. Despite suggesting the effectiveness of the under-eye serum in Indian patients, the study did not analyze the effectiveness of treatment based on patient profile (demographics, causes, disease severity i.e., mild, moderate, severe), which could have helped determine specific patient profiles where treatment may be most suitable or effective. Also, no follow-up was carried out post three months to ascertain the long-term prognosis or refractoriness of the condition.
Conclusion
Our study demonstrates the safety and effectiveness of this under-eye serum in Indian patients with pigmentary POH. In addition to clinical improvements observed by the dermatologists, patients also exhibited high satisfaction levels with the treatment outcomes. As expected, being a topical application, no safety concerns were noted. However, further large-scale studies in the Indian population may be required to confirm these findings and to elucidate key treatment determinants, such as ideal patient profile and duration of therapy, etc that can optimize treatment outcomes.
References
- Agrawal S. Periorbital Hyperpigmentation: Overcoming the Challenges in the Management. Nepal Journal of Dermatology, Venereology & Leprology. 2018;16(1):2-11.
- Andrei F, Grujic D, Lazar C, Dragomirescu A. Periorbital hyperpigmentation, a dermatologic condition having a strong geographic and ethnic determinism. 2018.
- Sheth PB, Shah HA, Dave JN. Periorbital hyperpigmentation: a study of its prevalence, common causative factors and its association with personal habits and other disorders. Indian J Dermatol. 2014;59(2):151-157.
- Jablonski NG, Chaplin G. The evolution of human skin coloration. Journal of human evolution. 2000;39(1):57-106.
- Nouveau S, Agrawal D, Kohli M, et al. Skin hyperpigmentation in Indian population: insights and best practice. Indian journal of dermatology. 2016;61(5):487.
- Taylor SC, Cook-Bolden F, Rahman Z, Strachan D. Acne vulgaris in skin of color. Journal of the American Academy of Dermatology. 2002;46(2 Suppl Understanding):S98-106.
- Daroach M, Kumaran MS. Periorbital hyperpigmentation? An overview of the enigmatous condition. Pigment International. 2018;5(1):1.
- Gaón NQ, Romero W. Dermoscopy in periorbital hyperpigmentation: An aid in the clinical type diagnosis 2014. 171-172 p.
- Vrcek I, Ozgur O, Nakra T. Infraorbital Dark Circles: A Review of the Pathogenesis, Evaluation and Treatment. J Cutan Aesthet Surg. 2016;9(2):65-72.
- Roberts W. Periorbital Hyperpigmentation: Review of Etiology, Medical Evaluation, and Aesthetic Treatment. Journal of drugs in dermatology : JDD. 2014;13:472-482.
- Elson ML, Nacht, S. . Treatment of periorbital hyperpigmentation with topical vitamin K/vitamin A. Cosmet Dermatol. Cosmet Dermatol. 1999;1232-34.
- Taskin B. Periocular pigmentation: Overcoming the difficulties. Journal of Pigmentary Disorders. 2015;2(1): 1–3.
- Alsaad SM, Mikhail M. Periocular hyperpigmentation: a review of etiology and current treatment options. J Drugs Dermatol. 2013;12(2):154-157.
- Saad A. News in the Treatment of Periorbital Hyperpigmentation. Journal of Dermatology and Therapies. 2017;1(1):19-23.
- Chatterjee M, Suwal B, Malik A, Vasudevan B. A study of epidemiological, etiological, and clinicopathological factors in periocular hyperpigmentation. Pigment International. 2018;5(1):34.
- Aguilera Díaz L. Pathology and genetics of bipalpebral hyperpigmentation1971. 397-410 p.
- Yaar M, Gilchrest BA. Skin aging: postulated mechanisms and consequent changes in structure and function. Clinics in geriatric medicine. 2001;17(4):617-630, v.
- Al-Shami SH. Treatment of periorbital hyperpigmentation using platelet-rich plasma injections. Am J Dermatol Venereol. 2014;3(5):87-94.
- Souza DM, Ludtke C, Souza E, et al. Periorbital hyperchromia. Surg Cosmet Dermatol. 2011;3(3):233-239.
- Huang YL, Chang SL, Ma L, et al. Clinical analysis and classification of dark eye circle. International journal of dermatology. 2014;53(2):164-170.
- Sarkar R, Ranjan R, Garg S, et al. Periorbital Hyperpigmentation: A Comprehensive Review. J Clin Aesthet Dermatol. 2016;9(1):49-55.
- Friedmann DP, Goldman MP. Dark circles: etiology and management options. Clinics in plastic surgery. 2015;42(1):33-50.
- Ahuja S, Deshmukh A, Khushalani S. A study of dermatoscopic pattern of periorbital hypermelanosis. Pigment International. 2017;4(1):29-34.
- Jage M, Mahajan S. Clinical and dermoscopic evaluation of periorbital hyperpigmentation. Indian Journal of Dermatopathology and Diagnostic Dermatology. 2018;5(1):42.
- Clarys P, Alewaeters K, Lambrecht R, Barel A. Skin color measurements: comparison between three instruments: the Chromameter®, the DermaSpectrometer® and the Mexameter®. Skin research and technology. 2000;6(4):230-238.
- Aldavine™ 5X. Algae Polysaccharides Complex. Lucas Meyer Cosmetics Technical file. Available at https://www.lucasmeyercosmetics.com/sites/lucasmeyer-corp-v2/files/products/pdf/114.aldavine-2015.pdf. Accessed on Feb 14 2022.
- Lanachrys® 2B. Chrysantellum indicum extract. Lucas Meyer Cosmetics Technical file. Available at https://www.lucasmeyercosmetics.com/en/products. Accessed on Feb 14 2022.
- Tyrostat™. Rumex extract. Lucas Meyer Cosmetics Technical file. Available at https://www.lucasmeyercosmetics.com/en/products. Accessed on Feb 14 2022.

