 J Clin Aesthet Dermatol. 2021;14(6):25–30.
J Clin Aesthet Dermatol. 2021;14(6):25–30.
by Lisa Fronek, DO; Allyson Brahs, DO; Maheera Farsi, DO; and Richard Miller, DO, FAOCD
Drs. Fronek, Brahs, and Miller are with HCA Healthcare USF Morsani College of Medicine Largo Medical Center Program in Largo, Florida. Dr. Farsi is with the Department of Mohs Micrographic Surgery at the University of Florida in Gainesville, Florida.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Trichilemmal carcinoma (TC) is a rare cutaneous tumor thought to be derived from the follicular outer root sheath (ORS). It often manifests as a nondescript skin-colored or pink papule on the hair-bearing, sun-exposed anatomic sites of elderly patients. Trichilemmal carcinoma shows many histologic features reminiscent of follicular ORS—notably, its glycogen-rich clear cells, trichilemmal keratinization, and similar immunostaining profile. Historically, it has been described as following a relatively indolent clinical course, but cases of recurrence, local aggression, and distant metastases have recently been elucidated. Here, we report the case of a 66-year-old male patient who presented with an asymptomatic, erythematous plaque on his neck; biopsy confirmed a diagnosis of TC. The patient deferred Mohs micrographic surgery in favor of wide local excision and was treated successfully with 3-mm margins. Salient histopathologic features, treatment modalities, and management recommendations are discussed.
Keywords: Trichilemmal carcinoma, clear cell, outer root sheath, trichilemmal keratinization, adnexal tumor
Trichilemmal carcinomas (TCs) are rare, malignant, adnexal neoplasms that differentiate from the outer root sheath (ORS) of the hair follicle.1,2 These tumors predominantly occur in elderly patients on sun-exposed areas, specifically on the head and neck, with the face being the most common location.1,3 The mean age of diagnosis is 70 years, with a slight male predominance.3,4 These lesions are commonly identified as papular, nodular, and sometimes exophytic. They generally arise de-novo but might also derivate from an underlying proliferating trichilemmal cyst with a loss of p53, a seborrheic keratosis, a nevus sebaceous, or a scar.5–8 They can be locally aggressive and can exhibit telangiectasias and ulceration due to local destruction.
This entity was first identified and documented by Headington9 in 1976, who described an epidermal-based tumor composed of atypical clear cells derived from adnexal keratinocytes. Known risk factors include ultraviolet (UV) and ionizing radiation, a history of local trauma or burns, immunosuppression, and inherited conditions like xeroderma pigmentosa and Cowden disease.3 While the clinical differential diagnosis commonly includes basal cell carcinoma (BCC), squamous cell carcinoma (SCC), and keratoacanthoma, the histopathological differential also includes trichilemmoma, trichoepithelioma, clear cell SCC, clear cell porocarcinoma, and clear cell hidradenocarcinoma. The main differentiating histological feature in a TC is the evidence of trichilemmal keratinization, where the tumor exhibits an absence of granular layer between the stratum spinosum and stratum corneum.1 The standard of treatment for TC is wide local excision (WLE) with tumor free margins; however, there has been increasing evidence regarding the efficacy of Mohs micrographic surgery (MMS).
Case Presentation
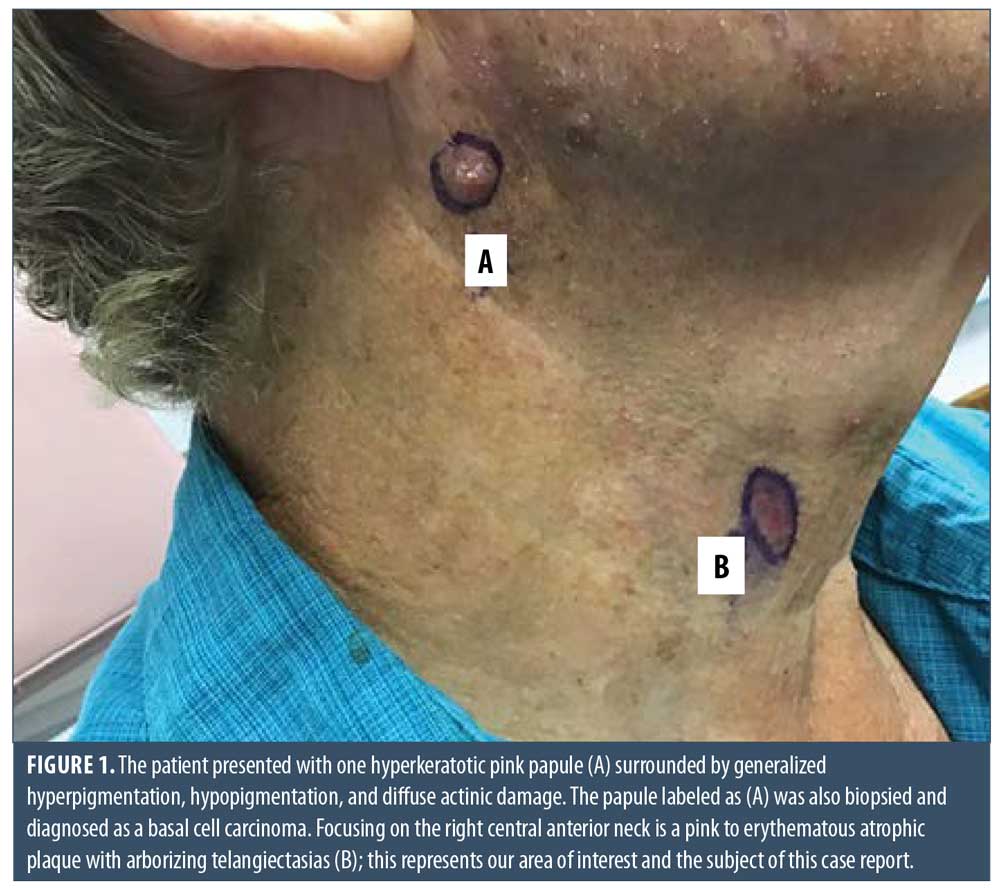
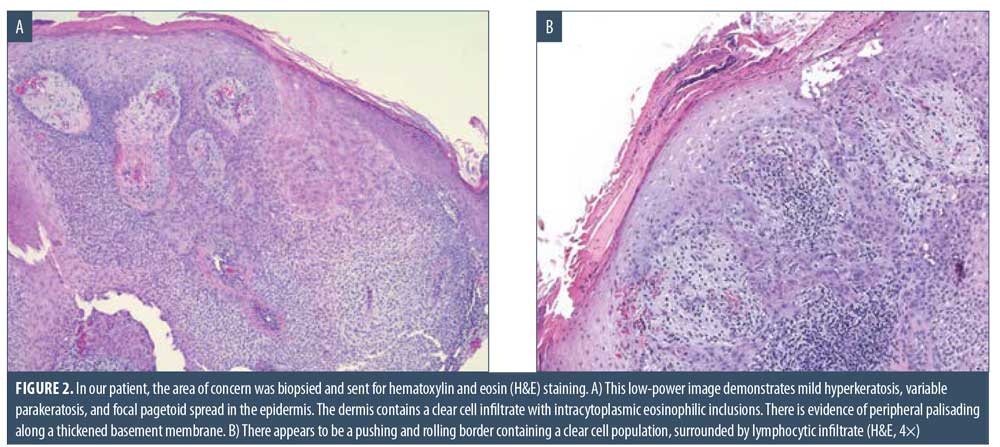
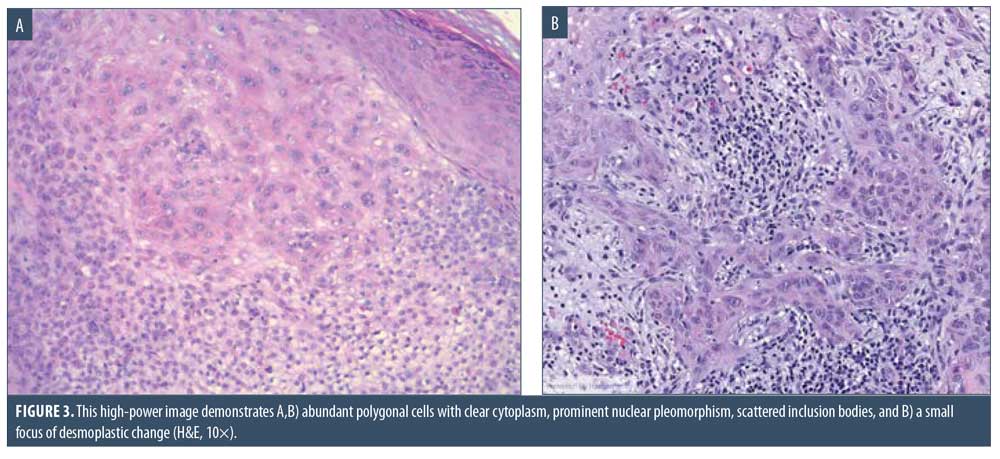
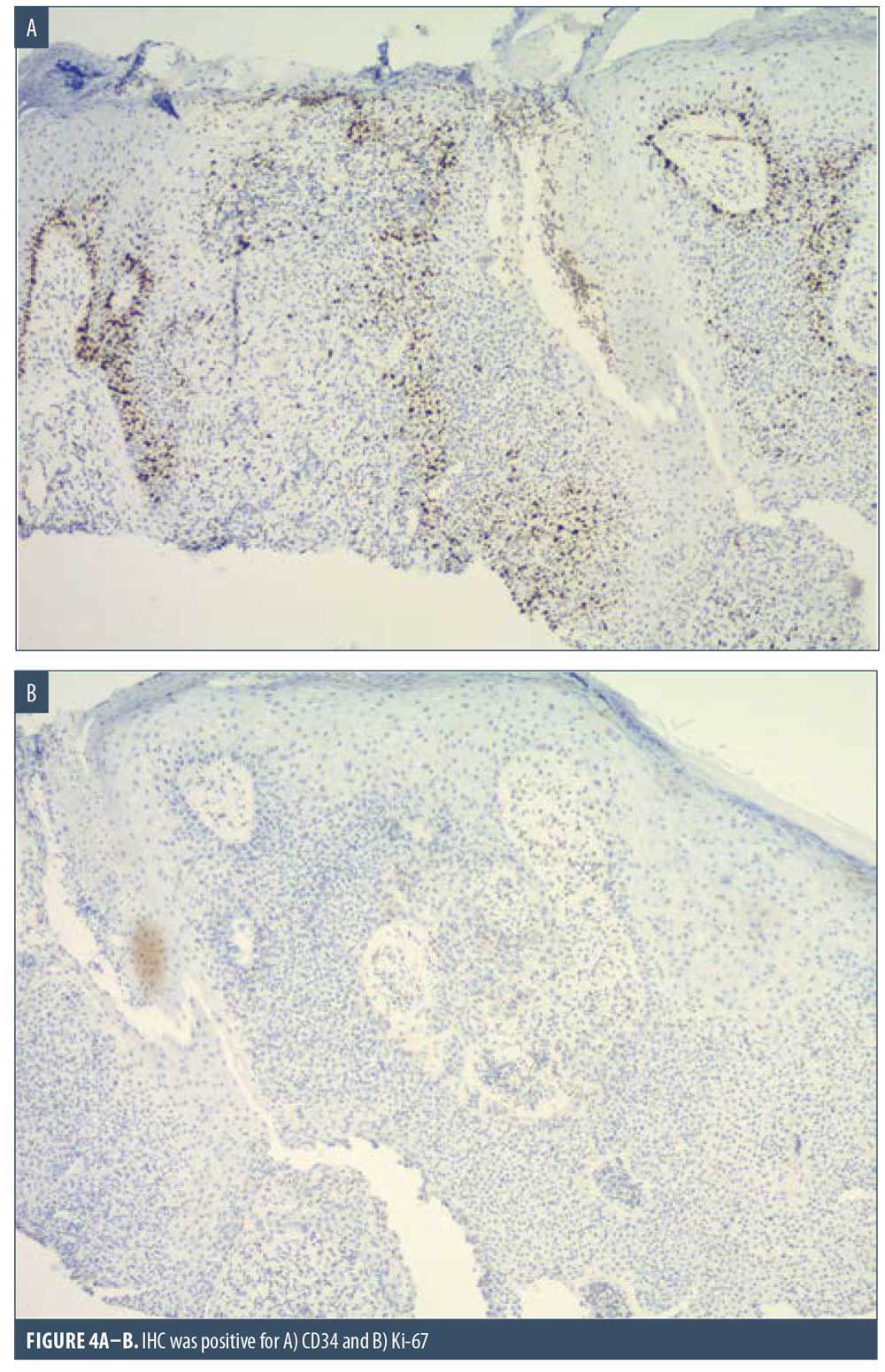
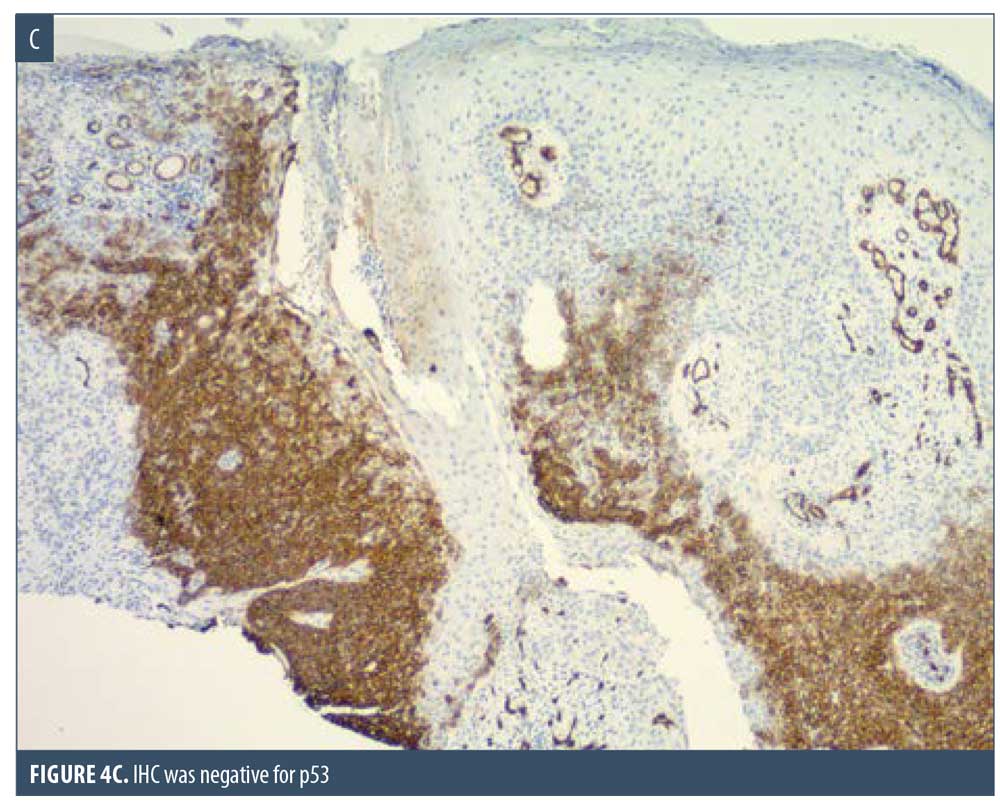
Here, we present the case of a 66-year-old Caucasian male patient who visited our clinic for a routine full body skin examination and was diagnosed with a biopsy-proven TC. The patient had a history of actinic keratoses treated with topical 5-fluorouracil (5-FU) and BCCs status-post excision and MMS. During the physical exam, a pink to erythematous, pearly plaque with arborizing telangiectasias and fine scaling was located on the right central anterior neck; no ulceration or discharge was noted (Figure 1). Tangential shave biopsy of the lesion was performed and the histopathologic report revealed an adnexal neoplasm with trichilemmal differentiation, desmoplastic component, and atypia with margins involved (Figures 2A, 2B, 3A, and 3B). Immunohistochemical staining (IHC) was positive for CD34, and tumor cells displayed uptake of the Ki-67 proliferation marker; staining for p53 was negative (Figures 4A–4C). The differential diagnoses, as described by the pathologist, included the favored diagnosis of a well-differentiated TC versus desmoplastic trichilemmoma with atypia. The pathologist suggested complete removal of the lesion with further evaluation. The patient favored WLE over MMS and was treated successfully with 3-mm margins. The final pathology report identified no residual adnexal neoplasm and an incidental superficial BCC with free margins.
Discussion
TC is a rare, adnexal tumor with evidence of follicular ORS or trichilemmal differentiation. It is considered the malignant analogue of the trichilemmoma.10,11 This tumor typically affects hair-bearing, sun-exposed sites of elderly individuals, though it can occur in non-sun-exposed areas in an immunocompromised host.11,12 By 2014, 103 cases of TC had been published, with 82 cases providing anatomic distribution data, half of which occurred on the face.3 Actinic damage is thought to play an important role in the development of this malignancy and solar elastosis is frequently found surrounding the tumor.13,14 The clinical presentation is variable and can be described as an asymptomatic, flesh-colored to reddish brown papule or nodule, often with an accelerated growth phase.11,15,16 This commonly exophytic lesion can display overlying hyperkeratosis or ulceration. In our case, it mimicked a BCC by manifesting as a smooth-surfaced, pearly pink plaque with arborizing telangiectasias, a well-known dermoscopic finding in BCC that is also seen in adnexal tumors.17 Due to its nonspecific clinical appearance, it can also be mistaken for SCC, keratoacanthoma, proliferating trichilemmal tumor, or amelanotic melanoma.3 Aside from one case in which multiple TCs were found on the chest of a patient with a history of abundant exposure to chest-directed ionizing radiation, this tumor is generally observed as a solitary lesion.18 Following its initial description by Headington in 1976, TC made a resurgence in the early 1990s when multiple case series were published further characterizing the clinicopathological features.12,13,19,20 These case series described TC to exhibit a relatively indolent clinical course; however, on occasion, recurrence, local aggression, perineural invasion, and distant metastases have been observed.3,15,21–24
Due to its ability to resemble many different clinical entities, the diagnosis of TC relies on histological evaluation, accompanied by IHC if necessary. Microscopically, TC features a solid, lobular, or trabecular growth pattern often centered around a pilosebaceous unit.20 The extent of this neoplasm can remain confined to the intraepithelial space or spread to the reticular dermis.25,26 The tumor cells are clear, polygonal, and glycogen-rich (periodic acid-Schiff positive [PAS], diastase-sensitive2,3,10) reminiscent of the clear cells of the normal ORS.27 It exhibits peripheral palisading of basaloid cells abutting a sometimes thickened hyalinized basement membrane. Upon closer inspection, cytologic atypia, nuclear pleomorphism, prominent nucleoli, and a high mitotic index can be seen.26,28 The atypical keratinocytes sometimes show a tendency for pagetoid spread.25 When present, trichilemmal keratinization is a significant feature aiding in the histologic diagnosis. Analogous to the trichilemmal keratinization seen by the outer sheath at the isthmus of the hair follicle, the lack of a granular layer leads to abrupt nonlamellar keratinization. CD34 stains the external layer of the ORS without labelling the rest of the follicle, so it can be used to suggest outer sheath differentiation.10,29 Ki-67 and p53 labelling can be used to support the malignant nature of a suspected TC.2,5,28 Though it appears to be cytologically malignant under the microscope, TC typically demonstrates nonaggressive clinical behavior.
Histologic mimics include other tumors of trichogenic origin, such as the trichilemmoma, desmoplastic trichilemmoma, infundibuloma, proliferating trichilemmal tumor, and trichoepithelioma. Desmoplastic trichilemmoma shares similar histologic features with TC and displays a central zone of irregular dense sclerotic stroma that can resemble invasive carcinoma,30 yet trichilemmoma and its desmoplastic variant both lack the marked pleomorphism, cytologic atypia, and mitotic figures.3,11,25 Infundibulomas, also referred to as tumors of the follicular infundibulum (TFI), can be distinguished by their strands of eosinophilic, pale keratinocytes with interlacing connections to the epidermis and hair follicles resulting in a reticular appearance.31 A proliferating trichilemmal tumor exhibits very similar features as TC, but the presence of well-circumscribed borders, areas of calcification, foreign-body reaction, and distribution to the scalp can be useful diagnostic discriminants.9,27,28,32 Trichoepithelioma shows evidence for differentiation toward the ORS and therefore has many overlapping features but distinctively displays lobules of basaloid cells with keratin horn cysts.26,27
It is also important to differentiate TC from many cutaneous tumors that can display clear cell changes, notably clear cell squamous cell carcinoma (CCSCC), BCC, sebaceous carcinoma, clear cell porocarcinoma, clear cell hidradenocarcinoma, balloon cell melanoma, and metastatic renal cell carcinoma. Differentiating between TC and CCSCC can be the most challenging, and some even question whether a distinction truly exists.10 Features suggestive of TC over CCSCC include foci of trichilemmal keratinization,21 cytokeratin 7 and 17 positivity,33 epithelial membrane antigen (EMA) negativity,33 lobular proliferation,18,21,28,34 peripheral palisading, a hyalinized basement membrane, a pushing as opposed to infiltrative growth pattern, and glycogen-rich cells.10,26,35 BCCs can contain minor areas of clear cell change, but it remains identifiable by its background changes consistent with a traditional BCC36 and positive staining for Ber-EP4.1 Sebaceous carcinoma is generally located around the eyelids and demonstrates foamy PAS-negative clear cells with centrally located, indented nuclei.21,36 Clear cell porocarcinoma and hidradenocarcinoma display ductal differentiation as evidenced by positive CEA and EMA stains.37 Additionally, there is absence of peripheral palisading, and they can show intracytoplasmic lumen formation.26 If melanoma is suspected, S100 or HMB-45 staining can be performed.26 Lastly, the diagnosis of cutaneous metastatic renal cell carcinoma is supplemented by IHC analysis with markers, such as EMA, CEA, renal cell carcinoma marker, and CD10.38,39
WLE with histological demonstration of clear margins has traditionally been the treatment of choice.2–4,20 Anecdotal reports equate the risk of recurrence and lymph node metastasis to that of SCC,40 and, therefore, TC is treated accordingly.3,16 Surgical margins generally emulate the 4- to 6-mm standard margins for SCC,16 though a few authors suggest utilizing safety margins greater than 1cm.15,41
MMS has been increasingly employed for numerous rare adnexal tumors, including TC.42 By 2014, at least seven cases of TC had been treated with MMS,3 and another seven were reported in a single-center series at the Mayo Clinic in 2015.4 In the Mayo Clinic study, four of the seven cases were successfully cleared in the first stage using margins that ranged from less than 1mm to around 5mm.4 Of the collective 14 patients treated with MMS, one had recurrence after multiple excisions and MMS, required a second MMS, and remained tumor-free 24 months following the second MMS.21 In comparison, of 35 cases of TC treated with WLE, recurrence occurred in three patients with an average follow-up of 33 months.3 In another study of 26 patients with TC treated with WLE showing tumor-free margins, recurrence occurred in two patients.41 Since TC predominately affects cosmetically-sensitive regions—namely, the head and neck—and typically follows a clinically indolent course, margins greater than 1cm can excessively deplete healthy tissue. This is a therapeutic gap that MMS can potentially fill, and the recurrence rate appears to compare favorably with that of WLE, though conclusions are limited by publication bias, the retrospective nature of these studies, and the small sample size.
Other treatment modalities have been used for TC. In a patient who declined surgical intervention, imiquimod 5% was applied three times a week for eight weeks and there was no evidence of recurrence 16 months later.43 In patients with profound tumors on the head and neck, radical resection with neck dissection and postexcision radiotherapy has been performed.44
Metastatic disease seldom occurs but carries a poor prognosis when it does. A few chemotherapeutic regimens have been used for metastatic TC. Cisplatin in combination with either cyclophosphamide, 5-FU, or vindesine and bleomycin have slowed the progression of metastatic disease but have not been curative.23,41,45 While recurrence is rare, patients should be examined for such sequela with periodic surveillance.
Conclusion
In summary, TC is a rare adnexal tumor that grossly and microscopically mimics many entities. A biopsy is essential for diagnosis and often requires supplemental immunostaining. It should be distinguished from other tumors of follicular origin and from other cutaneous malignancies. Complete excision with tumor-free margins is the typical treatment modality, though MMS has some advantages, including comprehensive visualization of the margins and greater preservation of healthy tissue. As we struggle for uniformity in treatment recommendations, the therapeutic modality and surgical margins should be decided on an individual basis considering the patient’s preference and the lesion’s clinicopathologic features. For instance, in the present case, given the cosmetically sensitive location, mildly atypical histologic features, and the patient’s preference to avoid MMS, we adopted 3-mm margins, which successfully produced tumor-free margins. Further characterization of the true nature and behavior of TC would contribute to a standard treatment recommendation. Additionally, a study comparing MMS to WLE for the treatment of TC would be of great utility, yet such an endeavor is limited by the rarity of the disease.
References
- Chai MK, Tenzel P, Iacob C, et al. Eyelid trichilemmal carcinoma. Saudi J Ophthalmol. 2017;31(3):183–185.
- Kim YH, Lee YK, Choi KW, et al. A case of trichilemmal carcinoma treated with Mohs micrographic surgery. Ann Dermatol. 2008;20(3):157–161.
- Hamman MS, Brian Jiang SI. Management of trichilemmal carcinoma: an update and comprehensive review of the literature. Dermatol Surg. 2014;40(7):711–717.
- Tolkachjov SN, Hocker TL, Camilleri MJ, Baum CL. Mohs micrographic surgery in the treatment of trichilemmal carcinoma: the Mayo Clinic experience. J Am Acad Dermatol. 2015;72(1):195–196.
- Takata M, Rehman I, Rees JL. A trichilemmal carcinoma arising from a proliferating trichilemmal cyst: the loss of the wild-type p53 is a critical event in malignant transformation. Hum Pathol. 1998;29(2): 193–195.
- Oyama N, Kaneko F. Trichilemmal carcinoma arising in seborrheic keratosis: a case report and published work review. J Dermatol. 2008;35(12):782–785.
- Chun SH, Kim BY, Park JH, et al. Simultaneous presentation of trichilemmal carcinoma and syringocystadenoma papilliferum within a nevus sebaceous. Ann Dermatol. 2018;30(3):368–370.
- Ko T, Tada H, Hatoko M, et al. Trichilemmal carcinoma developing in a burn scar: a report of two cases. J Dermatol. 1996;23(7):463–468.
- Headington JT. Tumors of the hair follicle. A review. Am J Pathol. 1976;85(2):479–514.
- Dalton SR, LeBoit PE. Squamous cell carcinoma with clear cells: how often is there evidence of tricholemmal differentiation?. Am J Dermatopathol. 2008;30(4):333–339.
- Garrett AB, Azmi FH, Ogburia KS. Trichilemmal carcinoma: a rare cutaneous malignancy: a report of two cases. Dermatol Surg. 2004;30(1):113–115.
- Reis JP, Tellechea O, Cunha MF, Baptista AP. Trichilemmal carcinoma: review of 8 cases. J Cutan Pathol. 1993;20(1):44–49.
- Swanson PE, Marrogi AJ, Williams DJ, et al. Tricholemmal carcinoma: clinicopathologic study of 10 cases. J Cutan Pathol. 1992;19(2):100–109.
- Sajin M, Luchian MC, Hodorogea Prisacaru A, et al. Trichilemmal carcinoma—a rare cutaneous malignancy: report of two cases. Rom J Morphol Embryol. 2014;55(2 Suppl):687–691.
- Xu B, Wang T, Liao Z. Surgical treatment of trichilemmal carcinoma. World J Oncol. 2018;9(5-6):141–144.
- Wilkie MD, Munir N, Roland NJ, Lancaster J. Trichilemmal carcinoma: an unusual presentation of a rare cutaneous lesion. BMJ Case Rep. 2013;2013:bcr2012008369.
- Martín JM, Bella-Navarro R, Jordá E. Vascularización en dermatoscopia [Vascular patterns in dermoscopy]. Actas Dermosifiliogr. 2012;103(5):357–375.
- Chan KO, Lim IJ, Baladas HG, Tan WT. Multiple tumour presentation of trichilemmal carcinoma. Br J Plast Surg. 1999;52(8): 665–667.
- Boscaino A, Terracciano LM, Donofrio V, et al. Tricholemmal carcinoma: a study of seven cases. J Cutan Pathol. 1992;19(2):94–99.
- Wong TY, Suster S. Tricholemmal carcinoma. A clinicopathologic study of 13 cases. Am J Dermatopathol. 1994;16(5):463–473.
- Allee JE, Cotsarelis G, Solky B, Cook JL. Multiply recurrent trichilemmal carcinoma with perineural invasion and cytokeratin 17 positivity. Dermatol Surg. 2003;29(8): 886–889.
- Maya-Rico AM, Jaramillo-Pulgarín C, Londoño-García Á, Peña-Zúñiga B. Locally aggressive trichilemmal carcinoma. An Bras Dermatol. 2018;93(4):579–581.
- Yi HS, Sym SJ, Park J, et al. Recurrent and metastatic trichilemmal carcinoma of the skin over the thigh: a case report. Cancer Res Treat. 2010;42(3):176–179.
- Hiramatsu K, Sasaki K, Matsuda M, et al. A case of trichilemmal carcinoma with distant metastases in a kidney transplantation patient. Transplant Proc. 2015;47(1):155–157.
- Billingsley EM, Davidowski TA, Maloney ME. Trichilemmal carcinoma. J Am Acad Dermatol. 1997;36(1):107–109.
- Calonje E, Brenn T, Lazar AJ, Billings SD. Chapter 31: Tumors of the hair follicle. In: McKee’s Pathology of the Skin: With Clinical Correlations. 5th ed. Philadelphia, PA: Elsevier; 2019: 1545–1588.
- Tellechea O, Cardoso JC, Reis JP, et al. Benign follicular tumors. An Bras Dermatol. 2015;90(6):780–798.
- Lee JH, Shin YW, Oh YH, Lee YJ. Trichilemmal carcinoma of the upper eyelid: a case report. Korean J Ophthalmol. 2009;23(4):301–305.
- Poblet E, Jimenez-Acosta F, Rocamora A. QBEND/10 (anti-CD34 antibody) in external root sheath cells and follicular tumors. J Cutan Pathol. 1994;21(3):224–228.
- Schweiger E, Spann CT, Weinberg JM, Ross B. A case of desmoplastic trichilemmoma of the lip treated with Mohs surgery. Dermatol Surg. 2004;30(7):1062–1064.
- Alomari A, Subtil A, Owen CE, McNiff JM. Solitary and multiple tumors of follicular infundibulum: a review of 168 cases with emphasis on staining patterns and clinical variants. J Cutan Pathol. 2013;40(6):532–537.
- Satyaprakash AK, Sheehan DJ, Sangüeza OP. Proliferating trichilemmal tumors: a review of the literature. Dermatol Surg. 2007;33(9):1102–1108.
- Ricci C, Dika E, Di Nanni DD, et al. Could EMA and cytokeratin 7 be useful in distinguishing tricholemmal carcinoma from clear-cell squamous cell carcinoma? A case series from our department and a brief review of the literature. Acta Histochem. 2019;121(6): 765–767.
- Lai TF, Huilgol SC, James CL, Selva D. Trichilemmal carcinoma of the upper eyelid. Acta Ophthalmol Scand. 2003;81(5):536–538.
- Kuo T. Clear cell carcinoma of the skin. A variant of the squamous cell carcinoma that simulates sebaceous carcinoma. Am J Surg Pathol. 1980;4(6):573–583.
- Song MG, Min HG, Jung SY, et al. Trichilemmal carcinoma with a cutaneous horn. Br J Dermatol. 2000;143(3):646–647.
- Hong YJ, Oh JE, Choi YW, et al. A case of clear cell eccrine porocarcinoma. Ann Dermatol. 2010;22(3):330–332.
- Ferhatoglu MF, Senol K, Filiz AI. Skin metastasis of renal cell carcinoma: a case report. Cureus. 2018;10(11):e3614.
- Perna AG, Ostler DA, Ivan D, et al. Renal cell carcinoma marker (RCC-Ma) is specific for cutaneous metastasis of renal cell carcinoma. J Cutan Pathol. 2007;34(5):381–385.
- Van Zele D, Arrese JE, Heymans O, et al. Invasive tricholemmal carcinoma of the nose. Dermatology. 2002;204(4):315–317.
- Zhuang SM, Zhang GH, Chen WK, et al. Survival study and clinicopathological evaluation of trichilemmal carcinoma. Mol Clin Oncol. 2013;1(3):499–502.
- Tolkachjov SN. Adnexal carcinomas treated with Mohs micrographic surgery: a comprehensive review. Dermatol Surg. 2017;43(10):1199–1207.
- Jo JH, Ko HC, Jang HS, et al. Infiltrative trichilemmal carcinoma treated with 5% imiquimod cream. Dermatol Surg. 2005;31(8 Pt 1):973–976.
- Feng Z, Zhu HG, Wang LZ, et al. Tricholemmal carcinoma of the head and neck region: a report of 15 cases. Oncol Lett. 2014;7(2): 423–426.
- Roismann M, Freitas RR, Ribeiro LC, et al. Trichilemmal carcinoma: case report. An Bras Dermatol. 2011;86(5):991–994.

