
J Clin Aesthet Dermatol. 2021;14(11):E61–E63.
by Stamatios Gregoriou, PhD; Vasiliki Markantoni, MD; Anna Campanati, PhD; Emanuela Martina, PhD; Annamaria Offidani, PhD; Anargyros Kouris, PhD; Eftychia Platsidaki, MD; Haralambos Bokotas, MD; Alexandros Stratigos, phD; Dimitrios Rigopoulos, phD; and George Kontochristopoulos, phD
Drs. Gregoriou, Markantoni, Kouris, Platsidaki, Stratigos, and Rigopoulos are with the Faculty of Medicine, 1st Department of Dermatology-Venereology at Andreas Sygros Hospital, National and Kapodistrian University in Athens, Greece. Drs. Campanati, Martina, and Offidani are with the Dermatological Clinic, Department of Clinical and Molecular Sciences, Polytechnic Marche University in Ancona, Italy. Bokotas and Kontochristopoulos are with the Department of Dermatology-Venereology, Andreas Sygros Hospital in Athens, Greece.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. Topical glycopyrrolate is a well-established therapeutic option for focal hyperhidrosis; however, there are no data on its efficacy in the treatment of bromhidrosis.
Objectives. The objective of this open-label, non-randomized study was to investigate the efficacy and safety of a galenic formulation of 2% glycopyrronium bromide cream, in the treatment of bromhidrosis.
Methods. Nineteen patients with bromhidrosis were prescribed a 2% glycopyrronium bromide cream, to apply in both axillae, every night, for 12 weeks. Malodor was assessed on a four-point scale. Scores for the Dermatology Life Quality Index (DLQI), Hyperhidrosis Disease Severity Scale (HDSS), and the Hospital Anxiety and Depression Scale (HADS) were recorded at baseline and after 12 weeks.
Results. Statistically significant improvements in malodor and HDSS, DLQI, and HADS scores, respectively, were observed after treatment. One patient reported irritation at the site of application and another reported mydriasis, which resolved spontaneously. All but one of the patients declared that they were either completely (52/6%) or partially (42.1%) satisfied regarding the treatment.
Conclusion. Our results indicate that 2% glycopyrronium bromide cream is effective and safe for 12 weeks of application in patients with axillary bromhidrosis.
Key words: Hyperhidrosis, Hospital Anxiety and Depression Scale, quality of life, treatment, topical glycopyrrolate
Bromhidrosis, also known as osmidrosis, is a chronic condition in which excessive body odor significantly affects patients both professionally and socially. Its incidence is unclear and probably under-reported. It usually affects the axillae, genital skin, mammary area, and soles of the feet. Apocrine secretion and decomposition of sweat by bacteria are major factors in the pathogenesis of bromhidrosis.1
Therapeutic modalities for bromhidrosis include topical antibacterial agents, antiperspirants, botulinum toxin injections, lasers, microwaves, and invasive treatments, including liposuction curettage and upper thoracic sympathectomy.2–6 Topical anticholinergics are an emerging therapeutic class in the treatment of focal hyperhidrosis. Glycopyrronium tosylate 3.75% cloth was approved by the United States Food and Drug Administration (FDA) for the treatment of axillary hyperhidrosis in adults and children aged nine years or older in 2018.7 Solfipironium bromide 5% gel received approval in Japan; Phase II trials presented good efficacy and safety, and Phase III trials are to follow in United States.8 Umeclimidium has been evaluated in a Phase IIa trial with promising efficacy.9 The objective of this open-label, non-randomized study was to investigate the clinical efficacy and safety of topical 2% glycopyrronium bromide in the treatment of bromhidrosis.
Methods
Nineteen consecutive patients who presented to the hyperhidrosis outpatient clinic of Andreas Sygros Hospital in Athens, Greece, and the Dermatology Clinic of Polytechnic Marche University in Ancona, Italy, with bromhidrosis were included in the study. Eligible patients included those (1) aged at least 18 years and (2) with a diagnosis of bromhidrosis, defined as malodor confirmed by both the patient and investigator. Exclusion criteria included (1) metabolic disorders that could be associated with bromhidrosis, (2) concomitant secondary or generalized hyperhidrosis, (3) a history of any prior surgical procedures or use of medical devices for hyperhidrosis, and (4) treatment with botulinum toxin during the last 12 months or treatment with anticholinergic agents during the last four weeks. The study protocol was approved by the ethics committees at both hospitals, and informed consent was obtained from all participants.
Patients were prescribed a galenic formulation of 2% glycopyrronium bromide cream to apply topically in both axillae every night for 12 weeks. They were instructed to continue using the same bath cleanser and to maintain their routine activities and daily schedule. The degree of malodor was self-assessed subjectively by patients as “none,” “mild,” “moderate,” and “severe.” The Dermatology Life Quality Index (DLQI) was used to assess quality of life. The clinical efficacy was graded by the patients using a scale of excellent (75%–100%), good (50%–75%), fair (25%–50%), and poor (0%–25%) improvement. Due to the fact that 16 of the 19 included patients presented with hyperhidrosis, scores on the Hyperhidrosis Disease Severity Scale (HDSS) were also recorded.10 Depression and anxiety were evaluated using the Hospital Anxiety and Depression Scale (HADS).11 Patient satisfaction was evaluated as totally satisfied, partially satisfied, or unsatisfied with the treatment used. Adverse events were recorded. Patients were followed up with for 12 weeks after the end of treatment.
Statistical analysis using the Mann–Whitney U test was used to evaluate mean, median, and standard deviation values for all variables reported. To assess differences in DLQI scores between the two time points (baseline and after treatment), parametric paired t-tests were carried out. For the variables HADS total score, HADS anxiety subscore, and HADS depression subscore, the Mann–Whitney U test was used. The exact sign test was carried out to assess differences between time points in HDSS scores. All analyses were carried out using the Statistical Package for the Social Sciences version 11.0 software program (IBM Corporation, Armonk, New York).
Results
Nineteen patients (6 male, 13 female) met the study inclusion criteria and were enrolled in this study. Results are presented in Table 1. All patients had bromhidrosis located in the axillae, while three patients had it in both axillae and soles of the feet. The mean duration of bromhidrosis was three years. All (94.7%) but one of the included patients reported no family history of bromhidrosis. Seventeen of the patients (89.5%) had tried topical over-the-counter products with limited efficacy in the past.
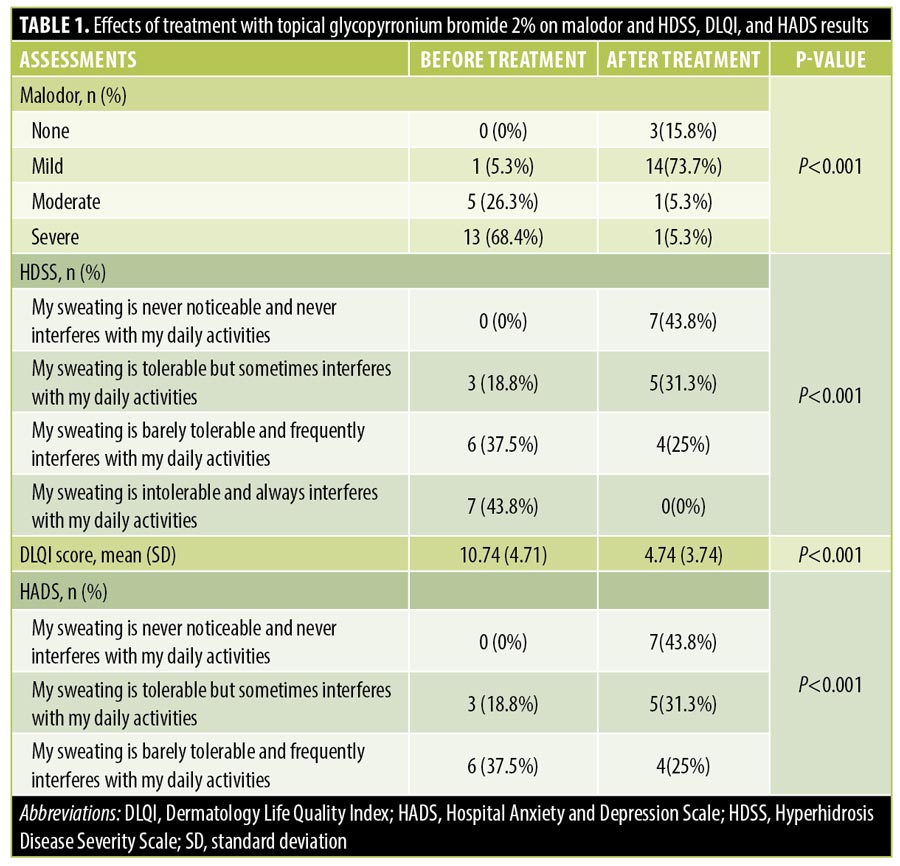
The subjectively assessed degree of malodor was improved after the first month of treatment, and was improved further after the second month. There was a statistically significant improvement in DLQI score before and after treatment (P<0.001). A statistically significant improvement in all HADS scores was also evident after treatment. All but one of the patients declared that they were either completely (52.6%) or partially (42.1%) satisfied with the results of treatment. The patient who was unsatisfied also had concomitant hyperhidrosis that reduced the HDSS score of four points recorded before treatment to a score of three points after treatment. Clinical efficacy was rated by most of the patients as excellent (47.4%) or good (42.1%). Two patients rated the clinical efficacy as poor; both had concomitant hyperhidrosis that reduced HDSS scores after treatment by one point (from 4 to 3 points and 3 to 2 points, respectively). Two of the three patients who reported bromhidrosis without concomitant hyperhidrosis reported excellent efficacy and complete satisfaction, and one patient reported good efficacy and partial satisfaction.
All patients reported control of bromhidrosis, even after stopping treatment, for a mean duration of three weeks.
Adverse events were documented in two patients. One patient reported topical irritation at the site of application, presenting as mild erythema and desquamation, after the second month of treatment; however, it receded after the application of low-potency topical corticosteroids. Another patient reported mydriasis, which resolved after recounselling the patient on the need of meticulous hand-washing after cream application in order to avoid contamination of the periocular skin.
All 16 patients with concomitant hyperhidrosis experienced improvement in their hyperhidrosis. Improvement in HDSS scores after treatment was statistically significant (P<0.001).
Discussion
The role of hyperhidrosis in the pathogenesis of bromhidrosis is unclear. Hyperhidrosis may increase the dispersion of sweat and contribute further to bromhidrosis by creating a moist environment. On the other hand, eccrine hyperhidrosis may trigger a reduction in odor because the eccrine sweat dilutes the more odoriferous apocrine sweat.12
Systemic anticholinergic agents decrease hyperhidrosis but are often associated with adverse events due to systemic absorption.13 Topical anticholinergics have been employed in the treatment of hyperhidrosis; however, data on their efficacy for bromhidrosis control are scarce. In the population studied, use of topical glycopyrronium bromide 2% resulted in significant improvement of bromhidrosis after 12 weeks. Even though only three out of the 19 patients had bromhidrosis without concomitant hyperhidrosis, the efficacy of glycopyrronium bromide 2% cream was evident in both groups. We also observed a statistically significant improvement in HDSS scores after treatment, suggesting that its mode of action in bromhidrosis is through control of sweat secretions.
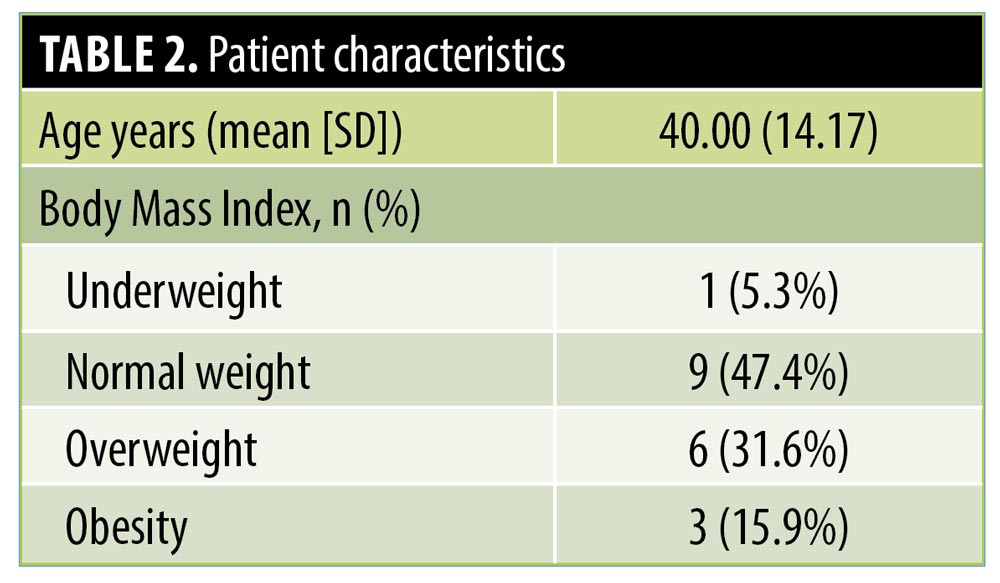
The mean body mass index of the study participants was 25.14 kg/m2, suggesting that obesity might not be a significant aggravating factor of bromhidrosis (Table 2). Although hyperhidrosis has been linked to obesity,14 the evidence supporting the association is limited.15 To the best of our knowledge, there is no reference that correlates body weight with bromhidrosis. We believe that a possible association should be further explored in future studies.
The patients in our study did not report dry mouth, blurred vision, or any other anticholinergic adverse event, suggesting that systemic absorption of glycopyrronium bromide 2% was minimal.
Limitations. Limitations of the current study include the lack of a control group and the subjective assessment of efficacy by the patients. A control group for hyperhidrosis/bromhidrosis intervention studies is difficult to manage and maintain in the long run.16 Bromhidrosis is difficult to evalute objectively, as the same odor might be considered offensive or not by different observers. The intensity of the offensive odor might also vary within 24 hours, suggesting that a patient who can assess their odor continuously might be a more suitable evaluator compared to an investigator who will assess it at predetermined time points during a site visit. Although the assessment of bromhidrosis by an investigator has been employed in the literature, the other option of evaluating the smell of a worn t-shirt17 to discern dissipation of the odor over time, means that our method may be more suitable for short term-comparisons. Both of these limitations mean that our results cannot be compared with results of randomized, placebo-controlled, bromhidrosis intervention studies.
Conclusion
In our study, glycopyrronium bromide 2% cream was effective in improving bromhidrosis in the population studied. The mode of action of glycopyronium bromide in bromhidrosis could be through the achievement of anhidrosis in osmidrotic areas. Randomized, placebo-controlled studies of glycopyrronium bromide 2% cream are needed to support our findings.
References
- Mao GY, Yang SL, Zheng JH. Cause of axillary bromidrosis. Plast Reconstr Surg. 2009;123(2):81e–82e.
- Jung SK, Jang HW, Kim HJ, et al. A prospective, long-term follow-up study of 1,444 nm Nd: YAG laser: a new modality for treating axillary bromhidrosis. Ann Dermatol. 2014;26(2): 184–188.
- Wang T, Dong J, He J. Long term safety and efficacy of botulinum toxin A treatment in adolescent patients with axillary bromhidrosis. Aesthetic Plast Surg. 2018;42(2):560–564.
- Van TN, Manh TN, Minh PPT, et al. The Effectiveness of local surgicaltechnique in treatment of axillary bromhidrosis. Open Access Maced J Med Sci. 2019;7(2):187–191.
- He J, Wang T, Zhang Y, Dong J. Surgical treatment of axillary bromhidrosis by combining suction-curettage with subdermal undermining through a miniature incision. J Plast Reconstr Aesthet Surg. 2018;71(6): 913–918.
- Sánchez-Carpintero I, Martín-Gorgojo A, Ruiz-Rodríguez R. Microwave treatment for axillary hyperhidrosis and bromhidrosis. Actas Dermosifiliogr. 2017;108(5):418–422.
- Glaser DA, Hebert AA, Nast A, et al. Topical glycopyrronium tosylate for the treatment of primary axillary hyperhidrosis: Results from the ATMOS-1 and ATMOS-2 phase 3 randomized controlled trials. J Am Acad Dermatol. 2019;80(1):128–138.
- Paik J. Sofpironium bromide: first approval. Drugs. 2020;80(18):1981–1986.
- Nasir A, Bissonnette R, Maari C, et al. A phase 2a randomized controlled study to evaluate the pharmacokinetic, safety, tolerability and clinical effect of topically applied Umeclidinium in subjects with primary axillary hyperhidrosis. J Eur Acad Dermatol Venereol. 2018;32(1):145–151.
- Solish N, Bertucci V, Dansereau A, et al. A comprehensive approach to the recognition, diagnosis, and severity-based treatment of focal hyperhidrosis: recommendations of the Canadian Hyperhidrosis Advisory Committee. Derm Surg. 2007;33(8):908–923.
- Bjelland I, Dahl AA, Haug TT, et al. The validity of the hospital anxiety and depression scale. An updated literature review. J Psychosom Res. 2002;52(2):69–77.
- Mao GY, Yang SL, Zheng JH. Etiology and management of axillary bromidrosis: a brief review. Int J Dermatol. 2008;47(10): 1063–1068.
- Gregoriou S, Sidiropoulou P, Kontochristopoulos G, Rigopoulos D. Management strategies of palmar hyperhidrosis: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:733–744.
- Liu Y, Bahar R, Kalia S, et al. Hyperhidrosis prevalence and demographical characteristics in dermatology outpatients in Shangai and Vancouver. PloS One. 2016;11(4):e0153719.
- Astman N, Friedberg I, Wikstrom JD, et al. The association between obesity and hyperhidrosis: a nation-wide, cross-sectional study of 2,77 million Israeli adolescents. J Am Acad Dermatol. 2019;81(2): 624–627.
- Hebert AA, Glaser DA, Green L, et al. Glycopyrronium tosylate in pediatric primary axillary hyperhidrosis: Post hoc analysis of efficacy and safety findings by age from two phase three randomized controlled trials. Pediatr Dermatol. 2019;36(1):89–99.
- Gregoriou S, Rigopoulos D, Chiolou Z, et al. Treatment of bromhidrosis with a glycine-soja sterocomplex topical product. J Cosmet Dermatol. 2011;10(1): 74–77.

