 J Clin Aesthet Dermatol. 2023;16(1):35-40.
J Clin Aesthet Dermatol. 2023;16(1):35-40.
by Nader Pazyar, MD; Motahareh Babazadeh Dezfuly, MD; Maryam Hadibarhaghtalab, MD, MPH; Seyedeh Yasamin Parvar; Seyedeh Nasrin Molavi, MD; Mohammad Ali Mapar, MD; and Maryam Zeinali, MD
Dr. Pazyar is an Assistant Professor with the Department of Dermatology at Imam Khomeini Hospital at Ahvaz Jundishapur University of Medical Sciences in Ahvaz, Iran. Drs. Dezfuly and Molavi are Assistants of Dermatology with the Department of Dermatology at Imam Khomeini Hospital at Ahvaz Jundishapur University of Medical Sciences in Ahvaz, Iran. Dr. Hadibarhaghtalab and Ms. Parvar are with the Molecular Dermatology Research Center at Shiraz University of Medical Sciences in Shiraz, Iran. Ms. Pavar is additionally with the Student Research Committee at Shiraz University of Medical Sciences in Shiraz, Iran. Drs. Mapar and Zeinali are with the Department of Dermatology at Ahvaz Jundishapur University of Medical Sciences in Ahvaz, Iran.
ABSTRACT: Objective. Melasma is an acquired and chronic hyperpigmentation disorder associated with a negative impact on patients’ quality of life. This study compares the efficacy of 100mg/mL intradermal TA with 4% topical HQ on female patients presenting with melasma lesions.
Methods. In this randomized double-blind controlled trial, 48 women with melasma were allocated into two groups, treated with either 100mg/mL intradermal TA or topical 4% HQ. The MASI (Melasma Area and Severity Index) score was assessed by paired t-tests and repeated measured ANOVAs. The Dynamic Physician General Assessment (PGA) was also performed by taking photographs with a digital camera.
Results. The average MASI score for the HQ and TA groups was 7.7 (3.0 SD) and 5.9 (2.5 SD), respectively. In both groups, the MASI decreased significantly after three months of treatment; however, the decrease was not significant between the two groups (P=0.1). All participants developed mild degrees of burning pain in the injection site without serious adverse effects.
Limitations. First, we only used the MASI score to measure melasma degree. Second, this is a single-center study with a small sample size. Third, the before-after photos were not taken with a high-quality camera.
Conclusion. The results of our study showed that both TA and continuous HQ significantly reduced the MASI score of patients without any significant differences and serious side effects. Although many treatment modalities are available for melasma, this condition is still challenging for dermatologists with a high recurrence rate after treatment.
Keywords: Melasma, hydroquinone, tranexamic acid, skincare, dermatology
Melasma is an acquired, chronic, and symmetric hyperpigmentation disorder.1 Melasma lesions are irregular brown patches in three predominant facial patterns: centrofacial, malar, and mandibular.2 The overall prevalence of melasma in the general population was estimated between 1 to 50 percent based on several studies.2 Ultraviolet sunlight exposure and female hormonal activity are the two most important risk factors for developing melasma.3 Positive family history is another risk factor reported in 55 to 64 percent of cases.4, 5
According to a study by Amatya et al,6 melasma is associated with a significant impact on quality of life compared to other dermatologic diseases. Adverse effects of melasma on quality of life include cosmetic disfiguration and decreased self-esteem and self-confidence. Feeling inferior to others is another negative effect of melasma.7,8
Different options are available for melasma treatment, including topical agents, oral medicine, microinjections, mesotherapy, lasers ablation, and combination therapies.9-11 Topical hydroquinone (HQ) treatment is the standard gold therapy with a high recurrence rate of melasma lesions.8,10 In contrast, tranexamic acid (TA) has recently been introduced as a novel therapy for melasma, which is the only treatment option that can prevent the activation of melanocytes by sunlight and hormonal activity.11,12 TA is widely used in oral, topical, and intradermal solutions with different dosages.13 A recently published meta-analysis showed that the dosage and treatment time is positively associated with the melasma improvement; however, recurrence is still a serious issue in planning the treatment.14 The present study aimed to evaluate the efficacy of 100mg/mL intradermal TA and compare its adverse effects with topical 4% HQ standard treatment on female patients presenting with melasma lesions.
Methods
This prospective, double-blind, randomized controlled trial was conducted according to the CONSORT statement and declarations of Helsinki from July 2020 to June 2021. The sample size was estimated to be 48 patients splitting into two groups by block randomization based on Pazyar et al’s study.15 According to the following formula with a 95 percent confidence interval and 80 percent power (α: 0.02, β: 0.04, S1: 9.4, S2: 7.5, mean 1: 2.2, mean 2: 2.2):N=z1-2+z1-β212+22d2
The study population included women aged 18 to 50 years who were referred to the dermatology clinic of Imam Khomeini Hospital in Ahwaz, Iran, to treat symmetrical melasma with Fitzpatrick II−V Skin Types. Exclusion criteria were as follows: pregnant or breastfeeding women, previous history of melasma treatment in the past month, active facial herpes simplex lesions, allergies to the study drugs, any history of coagulation disorders and thrombotic problems, facial warts, patients on anticoagulants and anticonvulsants (e.g., phenytoin), or oral contraceptive therapy in the past year.
After the approval by the ethics committee of the university and the registry of clinical trials, all patients signed informed consent and were given the necessary information regarding the controlled trial. Patients’ data, including age, duration of Melasma and its patterns, family history of melasma, Fitzpatrick Skin Type, and history of previous treatments, were obtained. Predisposing factors consisted of ultra-violate light, pregnancy, oral contraceptive pills, etc. Randomization was done by flipping a coin and each side of the participant’s face was randomLy treated with TA and hydroquinone to reduce the selection bias.
Wood’s lamp examination was performed at the beginning of the study to determine the melasma types, including dermal, epidermal, and mixed. In the first group, 60 minutes after applying local anesthetic agents (Lidocaine+Prilocaine) and dressing on their face, 100mg/mL of TA (Tranexipm®; Caspian Tamin Company, Iran), which is available in vials of 500mg per 5mL solutions was injected intradermally on the melasma lesions using 1cc insulin syringe. Intradermal injection of TA was done every two weeks. One-centimeter distances were measured with a ruler and marked with an easy-cleaning marker to avoid cosmetic disfigurement. Each spot was injected at an angle of 5 to 15 degrees with 0.1mL solution to form a wheal-like area on the skin. Then, spots were cleaned 30 minutes after the procedure using an alcohol pad. Ice compress was used after the procedure.
In another group, HQ 4% was applied topically every night. Both groups were recommended to prevent excessive sun exposure and use non pigmented sunscreen. Patients underwent therapy for three months and were followed up for another three months post-treatment every four weeks at Weeks 4, 8, 12, and 24. Four patients in the HQ group and five in the TA group did not present for the final follow-up.
Assessments were performed during each visit using the Melasma Area and Severity Index (MASI) score. The patients’ face was divided into four areas: forehead (30%), right malar (30%), left malar (30%), and chin (10%). The melasma severity was assessed by three variables: the percentages of total area involved (A), darkness (D), and the homogeneity of hyperpigmentation (H). The forehead on each side accounted for 15 percent, 30 percent on the cheek, and 5 percent on the chin. The MASI score was calculated according to the below formula, and the score for each side of the face ranged from 0 to 24. MASI=0.15(A)(D+H) + 0.3(A)(D+H) + 0.05(A)(D+H).
The Dynamic Physician General Assessment (Dynamic PGA) was also performed by taking photographs with a digital camera (Samsung Galaxy S8) at the beginning and the last visit. According to Dynamic PGA score, 0%−25% improvement was reported as poor improvement, 26%−50% as fair improvement, 51%−75% as good improvement, and 76%−100% improvement in lightening was reported as an excellent improvement.
Statistical analyses were done using the IBM Statistical Package for Social Sciences (SPSS, version 21; SPSS Inc., Chicago, IL, USA), and the significance level of the P-value was set at 0.05. The Mean, standard deviation (SD), and frequencies were used to describe the baseline characteristics of the study population after checking the normal distribution. Paired t-tests and repeated measured ANOVAs were also used to assess the MASI score. Dermatology residents did all the assessments. The analyzers were blinded.
Results
Fifty-six female patients with melasma were enrolled in the study (Figure 1). Eight participants were excluded from the study: Two patients left the trial due to using other treatment modalities, one patient became pregnant, three participants had trouble with transportation, and the other two patients developed ecchymosis and ochronosis. Therefore, a total number of 48 participants were allocated into two groups.
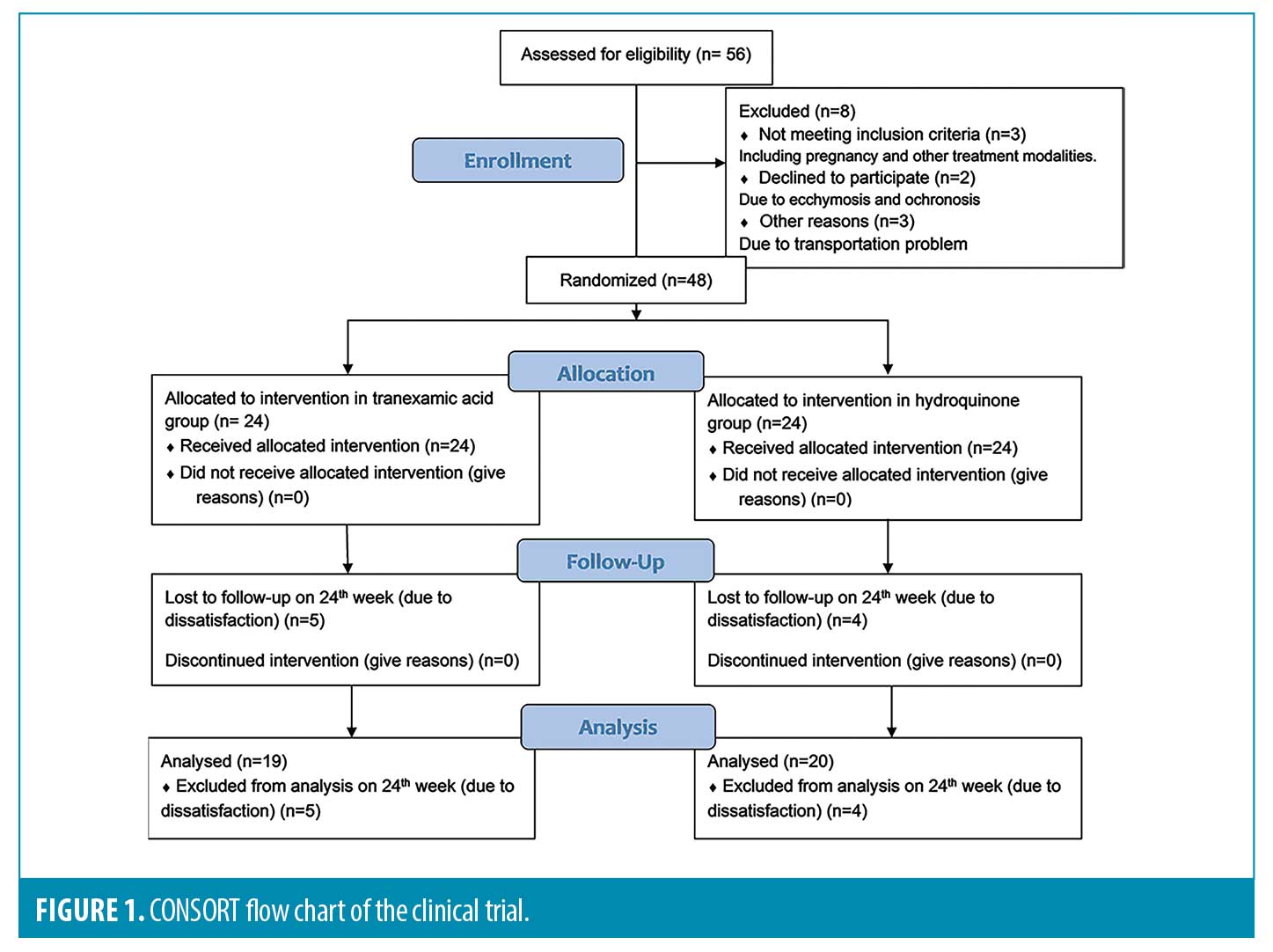
The patients’ mean age was 35.6 (5.7 SD) years (range, 24-48), and the duration of melasma varied from six months to 10 years (mean 4.2, SD 3.1). Twenty-eight patients had a positive family history of melasma, and 22 had a history of previous melasma treatment. According to the Fitzpatrick Skin Type, 46 participants had Type III and IV, and the other two patients had Type II and V. The pattern of melasma was malar in 33 patients, centrofacial in 13, and mandibular in two patients. The type of melasma was dermal in three patients, epidermal in 37 patients, and mixed pattern in eight patients. Pregnancy was the most frequent precipitating factor in 15 patients, followed by UV exposure and oral contraceptives in 13 and 10 patients, respectively. Baseline characteristics were adjusted between the two groups, as shown in Table 1.
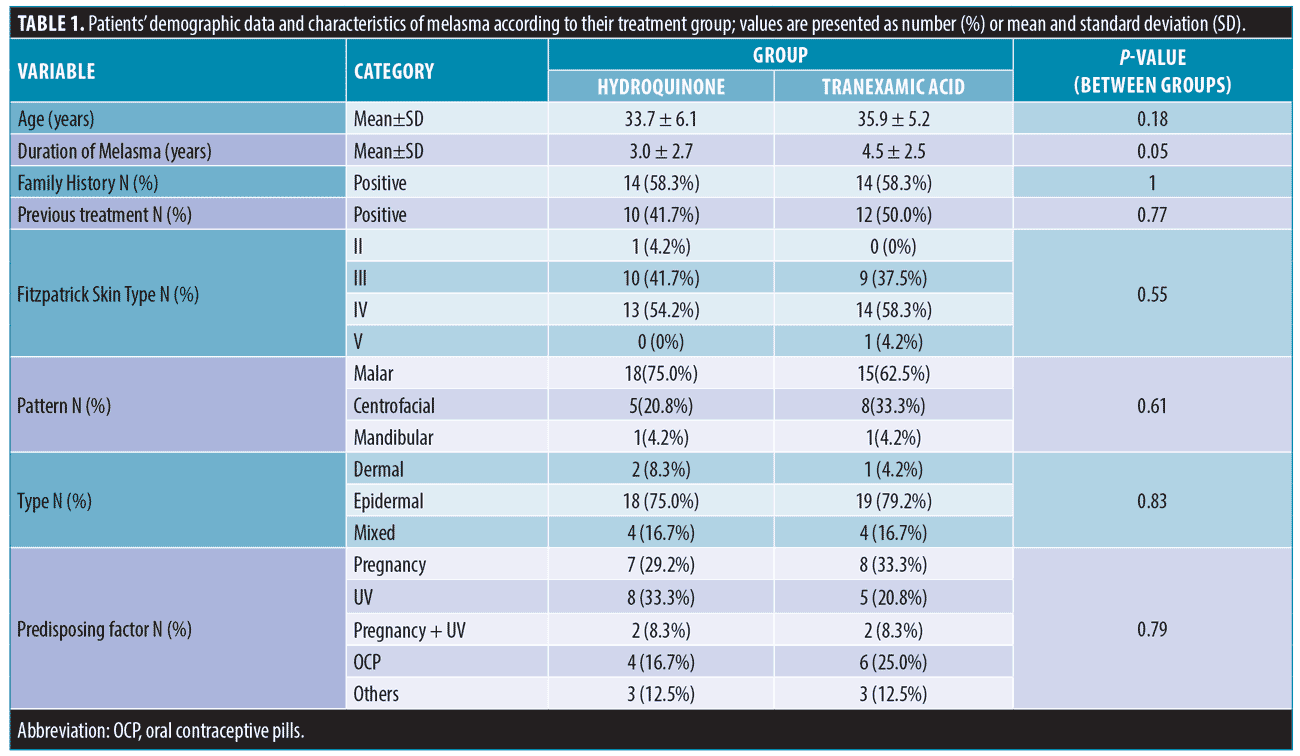
According to Table 2, the onset average MASI score for the HQ group was 7.7 (95% CI, 3.0 SD) and 4.8 (95% CI, 2.9 SD) after 24 weeks (P=0.001). The initial MASI score for the TA group was 6.1 (95% CI, 2.5 SD) and 3.5 (95% CI, 2.0 SD) at the end of the study (P<0.001). The MASI score decreased significantly in all follow-ups compared to the previous one in both groups (all P-values were <0.001 except for 12-24th weeks follow up, which was 0.007). It is also worth mentioning that the average MASI score of the last follow up in the 24th week was not statistically significant between the two groups (P=0.1), whereas the post-treatment MASI score in the 12th week was statistically significant between the two groups (P=0.02)
The average MASI reduction for the HQ group was 62 percent, and for the TA group, 57 percent. The decrease in the MASI score in the 4th week compared to the start of the study and in the 24th week compared to the 12th was statistically significant between the two groups (P<0.0001). It was also worth mentioning that the average MASI score reduction on the last follow-up compared to the baseline was not statistically significant between the two groups (P=0.64).
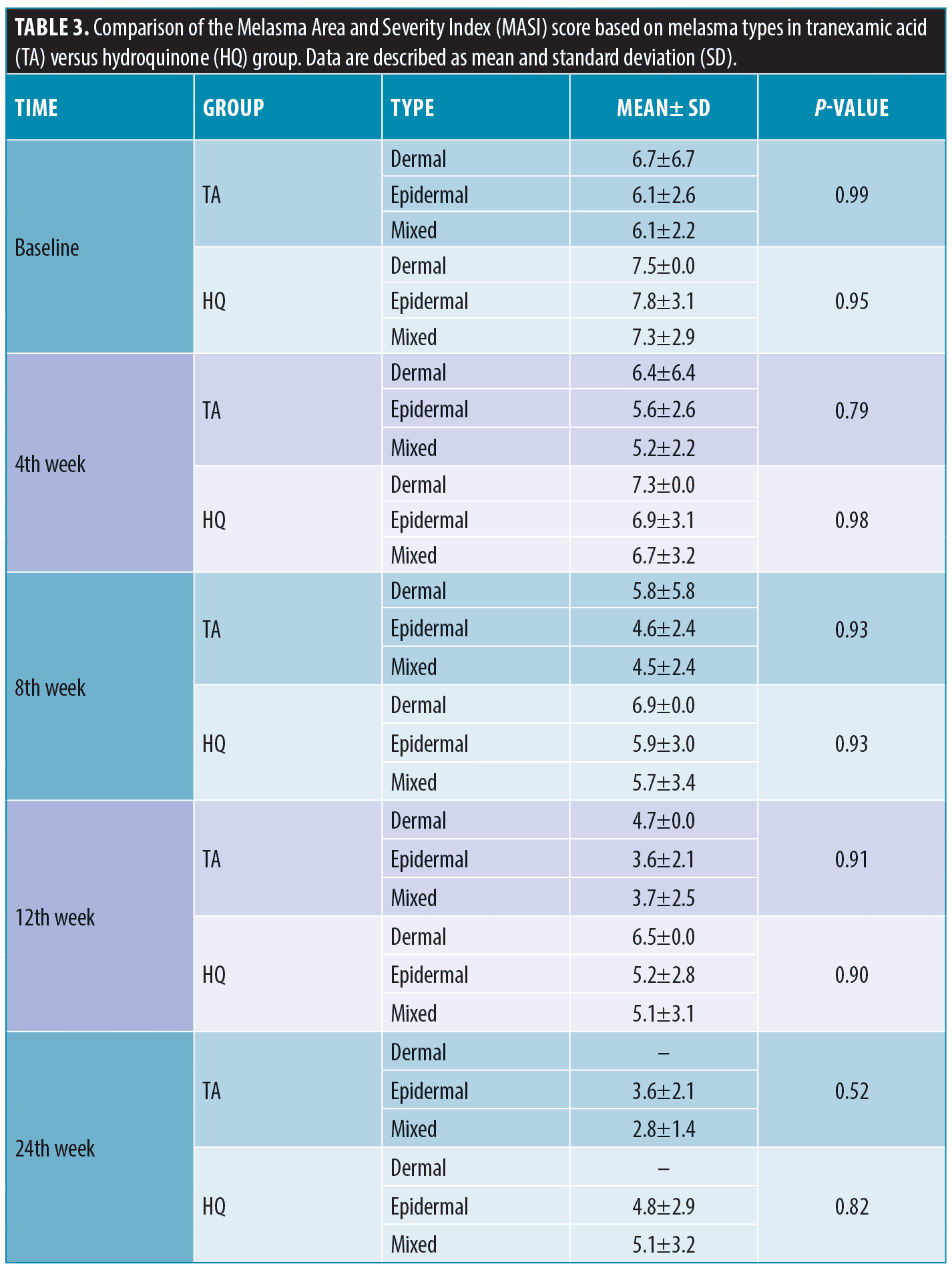
A comparison of the effectiveness of treatments on the MASI score based on the melasma types showed no significant differences in all visits (P>0.05). As presented in Table 3, the mean MASI score in patients injected with TA decreased over time from 6.1 (2.6 SD) in the baseline to 3.6 (2.1 SD) in the 24th week in the epidermal melasma type. It dropped from 6.1 (2.2 SD) in the baseline to 2.8 (1.4 SD) in the 24th week in the mixed pattern. This decreasing pattern was also observed in the HQ group. The mean MASI score decreased from 7.8 (3.1 SD) in the baseline to 4.8 (2.9 SD) in the 24th week in the epidermal melasma type and decreased from 7.3 (2.9 SD) in the baseline to 5.1(3.2 SD) in 24th week in the mixed pattern. Figures 2 and 3 show melasma lesions in four patients treated with TA or HQ before and after six months.
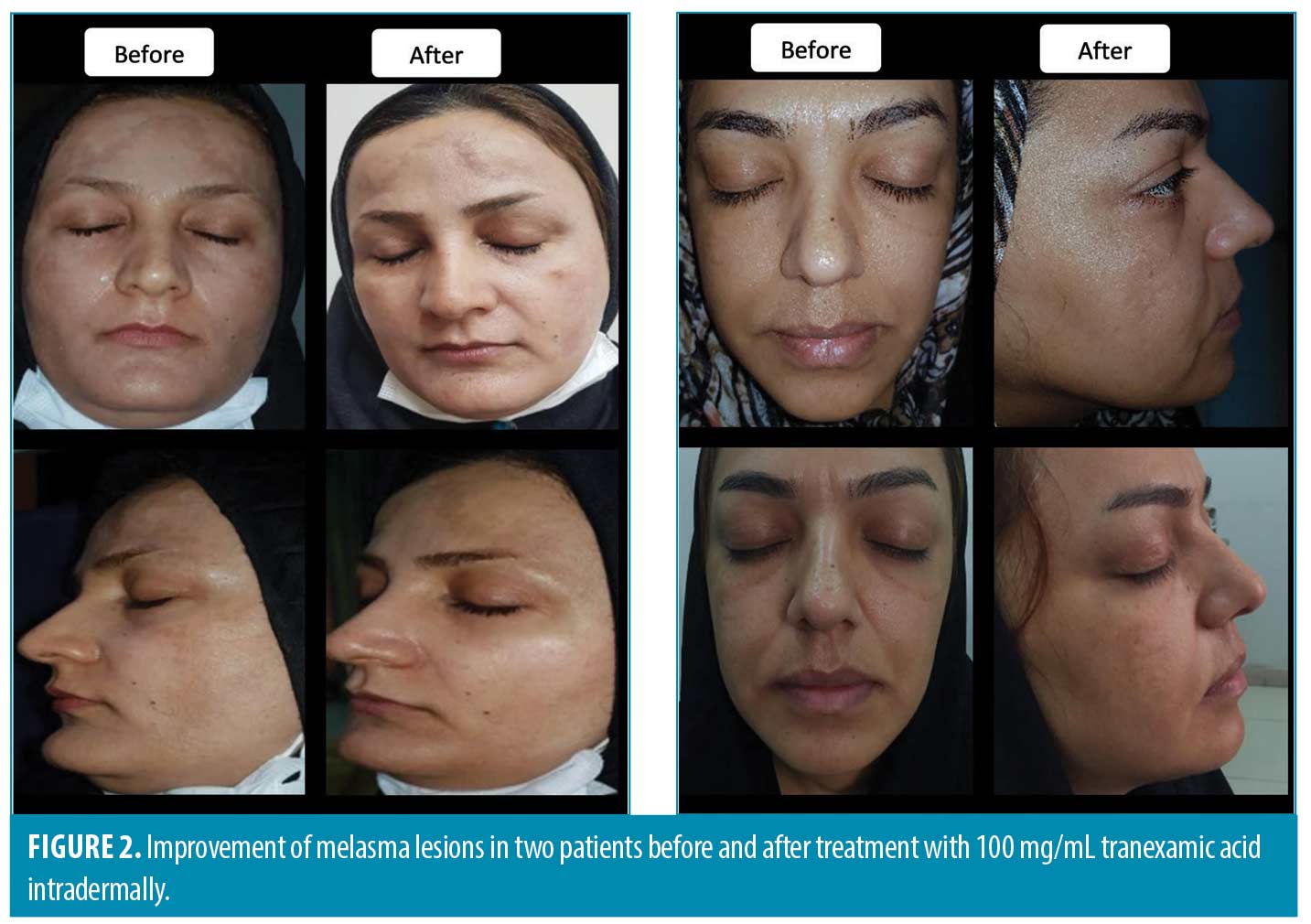
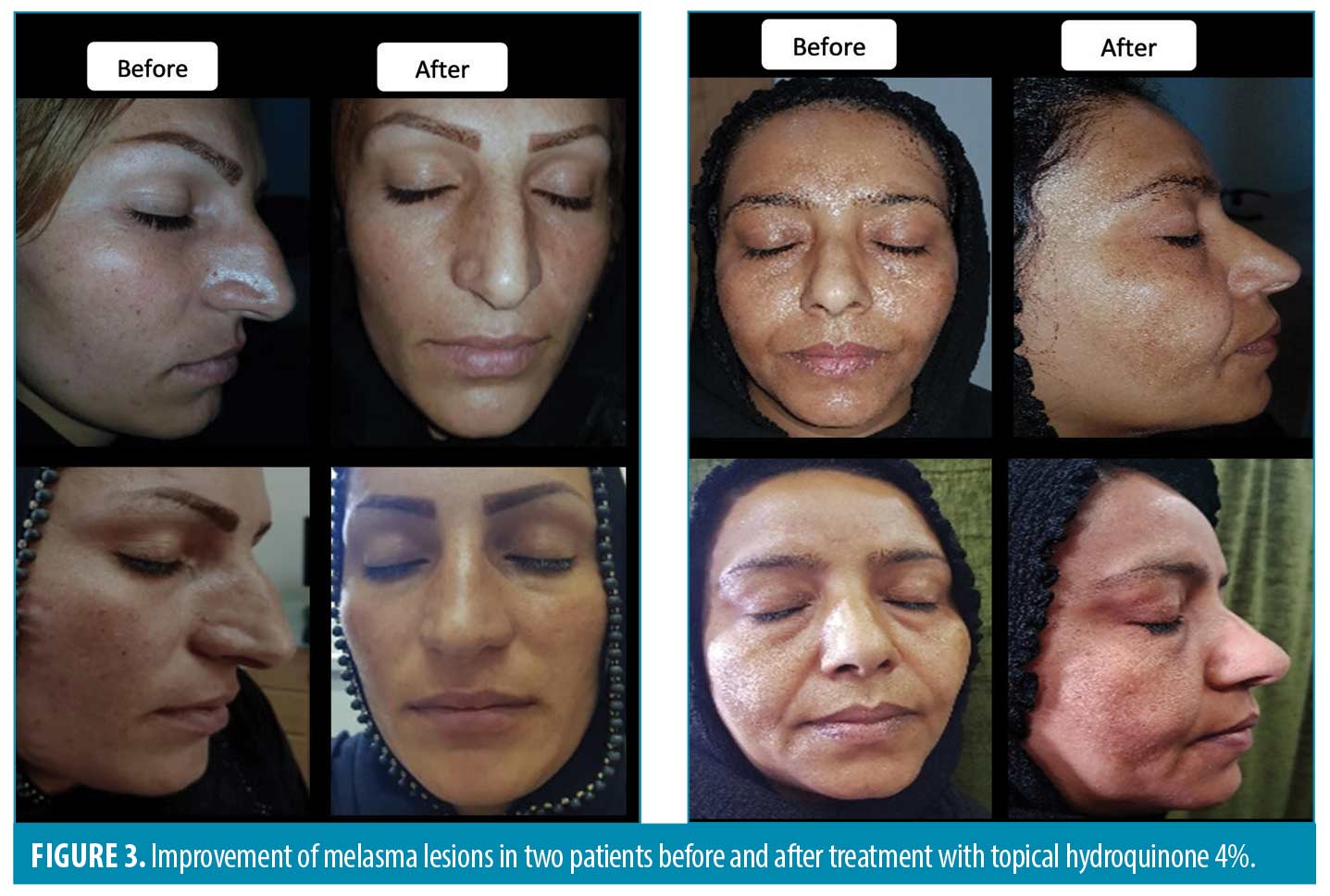

The PGA rated the HQ group as excellent in three patients, good in eight, poor in six, and fair in seven. The TA-treated group was rated outstanding in three, good in twelve, poor in five, and fair in four patients (Figure 4). The results did not show a statistically significant difference between the two groups (P=0.63).
Despite admitting to topical anesthesia, all participants developed mild degrees of burning pain at the injection site. No other serious adverse effects were reported.
Discussion
This study compared two treatment modalities in female patients diagnosed with melasma, including topical 4% HQ and 100mg/mL intradermal TA. Our results show that both TA and topical hydroquinone significantly reduced the MASI score overtime. Still, HQ was substantially more effective in reducing the MASI score in the 24th-week follow-up compared to the 12th week. These results suggest that the long-term administration of HQ could be helpful in treating melasma lesions. The results also indicated a higher complete satisfaction rate in the TA group than in the HQ group. It is also worth mentioning that the decrease in the MASI score in the HQ group was significantly higher than the TA group.
For many years, HQ was the gold standard treatment modality for treating melasma. In recent articles, TA was indicated as an alternative in treating and hypopigmentation of melasma lesions. TA is a hemostatic agent and inhibits ultraviolet-induced pigmentation.13,16 Intradermal injection of TA seems to be an effective and safe treatment option for melasma and can significantly reduce the MASI score in previous studies.17-19 Saki et al20 compared the efficacy of 2% HQ and 20mg/mL TA on melasma patients. Both drugs decreased the melanin index, which was not statistically significant between the two groups. Based on another study conducted in the southwest of Iran, the closest study to ours on 50 female patients, the right side of the patient’s face was injected with 4mg/mL TA, and 10mg/mL TA plus topical 4% HQ was used on the left side. Topical 4% HQ had equal efficacy on the 12th week compared to 10mg/mL TA which was more effective than the lower dose of TA. These results showed that increasing TA dosage can significantly increase the effectiveness of treatment.15
Despite local anesthesia, all studied patients in the TA group experienced mild burning pain at the injection site, which was also reported in the Pazyar et al15 study. Another study conducted in 2022 reported that burning pain was significantly higher in the group treated with TA plus ascorbic acid than TA alone.17 TA’s other side effects include nausea, vomiting, diarrhea, color vision disturbances, and orthostatic imbalances.12,21 Skin irritation on the 4% HQ cream application side was the significant adverse effect that has been reported.22 Other commonly reported adverse effects of HQ include irritant contact dermatitis, exogenous ochronosis, corneal degeneration, nail discoloration, erythema, and burning.23,24
Despite multiple treatment modalities for melasma, the treatment strategy is challenging due to its chronicity and relapsing state.25 We suggest a combination therapy of HQ and other bleaching agents that have been suggested to be more effective than monotherapies. The combination of HQ and kojic acid or triple-combination of corticosteroid cream has been well established in the literature.26 The synergistic process of the triple-combination agents achieves significantly higher depigmentation in melasma agents than in HQ monotherapy in eight weeks without any considerable adverse effect.27,28 In addition, a combination of oral TA and HQ cream significantly enhanced the effectiveness of HQ alone and had a more significant decrease in the MASI score.21
The usual effective dose of HQ for melasma is 2%, while we used 4% HQ as a control group. Contrary to the previous studies that injected a lower dose of TA, the advantage of the present study was using intradermal TA in a higher dose than previous studies, as mentioned above. A survey by Mafune et al29 showed that higher therapeutic doses of TA may be more effective in treating melasma lesions. However, other studies reported that long-term treatment should have a better effect than increasing the dosage.12,30
At last, there are several limitations to this study. First, we only used the MASI score to measure melasma degree, whereas other modalities, including Mexameter and Visioface photography, can be used to analyze skin color more comprehensively. Second, greater generality would have been achieved if the sample size had been larger. This is a single-center study with a small sample size, and some patients in both groups did not show up for the final follow-up due to transportation issues. Third, the before-after photos were not taken with a high-quality camera. So, the treatments’ improvement cannot be appropriately seen in the pictures.
Conclusion
The results of our study showed that both TA and continuous HQ significantly reduced the MASI score of patients with melasma during 12 weeks of treatment without any significant differences and serious side effects. Although several treatment modalities are used for melasma, it is a challenging condition for dermatologists with a high recurrence rate after treatment. Therefore, further comparative studies with more cases are needed on the effectiveness of different dosages of these two modalities.
Acknowledgments
This article is based on a thesis by Dr. Motahareh Babazadeh Dezfuly, a dermatology resident. The authors would like to thank the Clinical Research Development Unit, Imam Khomeini Hospital, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran, for their cooperation.
References
- Navarrete-Solís J, Castanedo-Cázares JP, Torres-Álvarez B, et al. A double-blind, randomized clinical trial of niacinamide 4% versus hydroquinone 4% in the treatment of melasma. Dermatology Research and Practice. 2011;2011:379173.
- Ogbechie-Godec OA, Elbuluk N. Melasma: An up-to-date comprehensive review. Dermatology and therapy. 2017;7(3):305–318.
- Ali R, Aman S, Nadeem M, et al. Quality of life in patients of melasma. Journal of Pakistan Association of Dermatologists. 2013;23(2):143–148.
- Moin A, Jabery Z, Fallah N. Prevalence and awareness of melasma during pregnancy. International Journal of Dermatology. 2006;45(3):285–288.
- Hernández‐Barrera R, Torres‐Alvarez B, Castanedo‐Cazares J, et al. Solar elastosis and presence of mast cells as key features in the pathogenesis of melasma. Clinical and Experimental Dermatology: Continuing Professional Development (CPD). 2008;33(3):305–308.
- Amatya B, Jha AK, Shrestha S. Frequency of different types of facial melanoses referring to the department of dermatology and venereology, nepal medical college and teaching hospital in 2019, and assessment of their effect on health-related quality of life. BMC Dermatology. 2020;20(1):1–7.
- Ikino JK, Nunes DH, Silva VPMd, et al. Melasma and assessment of the quality of life in brazilian women. Anais Brasileiros de Dermatologia. 2015;90:196–200.
- Jiang J, Akinseye O, Tovar-Garza A, et al. The effect of melasma on self-esteem: A pilot study. International Journal of Women’s Dermatology. 2018;4(1):38–42.
- Ahramiyanpour N, Saki N, Akbari Z, et al. Efficacy of topical cysteamine hydrochloride in treating melasma: A systematic review. Journal of Cosmetic Dermatology. 2021;20(11):3593–3602.
- Bronzina E, Clement A, Marie B, et al. Efficacy and tolerability on melasma of a topical cosmetic product acting on melanocytes, fibroblasts and endothelial cells: A randomized comparative trial against 4% hydroquinone. Journal of the European Academy of Dermatology and Venereology. 2020;34(4):897–903.
- Kaleem S, Ghafoor R, Khan S. Comparison of efficacy of tranexamic acid mesotherapy versus 0.9% normal saline for melasma; a split face study in a tertiary care hospital of karachi. Pakistan Journal of Medical Sciences. 2020;36(5):930.
- Khurana VK, Misri RR, Agarwal S, et al. A randomized, open-label, comparative study of oral tranexamic acid and tranexamic acid microinjections in patients with melasma. Indian J Dermatol Venereol Leprol. 2019;85(1):39–43.
- Lee JH, Park JG, Lim SH, et al. Localized intradermal microinjection of tranexamic acid for treatment of melasma in asian patients: A preliminary clinical trial. Dermatologic Surgery. 2006;32(5):626–631.
- Neagu N, Conforti C, Agozzino M, et al. Melasma treatment: A systematic review. Journal of Dermatological Treatment. 2021(just-accepted):1–39.
- Pazyar N, Yaghoobi R, Zeynalie M, et al. Comparison of the efficacy of intradermal injected tranexamic acid vs hydroquinone cream in the treatment of melasma. Clinical, Cosmetic and Investigational Dermatology. 2019;12:115.
- Maeda K, Naganuma M. Topical trans-4-aminomethylcyclohexanecarboxylic acid prevents ultraviolet radiation-induced pigmentation. Journal of Photochemistry and Photobiology B: Biology. 1998;47(2-3):136–141.
- Pazyar N, Molavi SN, Hosseinpour P, et al. Efficacy of intradermal injection of tranexamic acid and ascorbic acid versus tranexamic acid and placebo in the treatment of melasma: A split‐face comparative trial. Health Science Reports. 2022;5(2):e537.
- Sharma R, Mahajan V, Mehta K, et al. Therapeutic efficacy and safety of oral tranexamic acid and that of tranexamic acid local infiltration with microinjections in patients with melasma: A comparative study. Clinical and Experimental Dermatology. 2017;42(7):728–734.
- Shetty VH, Shetty M. Comparative study of localised intradermal microinjection of tranexamic acid and oral tranexamic acid for the treatment of melasma. Int J Res Dermatol. 2018;4(3):363–367.
- Saki N, Darayesh M, Heiran A. Comparing the efficacy of topical hydroquinone 2% versus intradermal tranexamic acid microinjections in treating melasma: A split-face controlled trial. Journal of Dermatological Treatment. 2018;29(4):405–410.
- Lajevardi V, Ghayoumi A, Abedini R, et al. Comparison of the therapeutic efficacy and safety of combined oral tranexamic acid and topical hydroquinone 4% treatment vs. Topical hydroquinone 4% alone in melasma: A parallel‐group, assessor‐and analyst‐blinded, randomized controlled trial with a short‐term follow‐up. Journal of Cosmetic Dermatology. 2017;16(2):235–242.
- Banihashemi M, Zabolinejad N, Jaafari MR, et al. Comparison of therapeutic effects of liposomal tranexamic acid and conventional hydroquinone on melasma. Journal of Cosmetic Dermatology. 2015;14(3):174–177.
- Bandyopadhyay D. Topical treatment of melasma. Indian Journal of Dermatology. 2009;54(4):303.
- Jow T, Hantash BM. Hydroquinone-induced depigmentation: Case report and review of the literature. Dermatitis. 2014;25(1):e1–e5.
- Kagha K, Fabi S, Goldman MP. Melasma’s impact on quality of life. Journal of Drugs in Dermatology: JDD. 2020;19(2):184–187.
- Godse KV. Triple combination of hydroquinone, tretinoin and mometasone furoate with glycolic acid peels in melasma. Indian Journal of Dermatology. 2009;54(1):92.
- Draelos ZD. Skin lightening preparations and the hydroquinone controversy. Dermatologic Therapy. 2007;20(5):308–313.
- Sehgal VN, Verma P, Srivastava G, et al. Melasma: Treatment strategy. Journal of Cosmetic and Laser Therapy. 2011;13(6):265–279.
- Mafune E, Morimoto Y, Iizuka Y. Tranexamic acid and melasma. Farumashia. 2008;44:437–442.
- Tse TW, Hui E. Tranexamic acid: An important adjuvant in the treatment of melasma. Journal of Cosmetic Dermatology. 2013;12(1):57–66.

