 J Clin Aesthet Dermatol. 2022;15(6):10–21.
J Clin Aesthet Dermatol. 2022;15(6):10–21.
by Je-Young Park, MD; Jeng-Feng Chen, MD; Hosung Choi, MD; Wilson W. S. Ho, MBChB, FRCS; Ni Nyoman Indra Lesthari, MD; Joyce Teng Ee Lim, MBBS, FRCPI, FAMS (Dermatology); Ting Song Lim, MD; Stephen Lowe, MBChB, MPH; Beverly Ong-Amoranto, MD; Vasanop Vachiramon, MD; Rungsima Wanitphakdeedecha, MD; Martina Kerscher, MD, PhD
Dr. Park is with the Apkoo-Jung Oracle Dermatology Clinic in Seoul, South Korea. Dr. Chen is with Beauté J’adore Dermatology and Esthetic Medicine in Taipei, Taiwan. Dr. Choi is with the Piena Clinic in Seoul, South Korea. Dr. Ho is with The Specialists: Lasers, Aesthetic & Plastic Surgery in Hong Kong. Dr. Lesthari is with the Sano Clinic Bali in Bali, Indonesia. Dr. Teng Ee Lim is with Joyce Lim Skin and Laser Clinic in Singapore. Dr. Ting Song Lim is with the Clique Clinic in Kuala Lumpur, Malaysia. Dr. Lowe is with MUSE Clinic in Sydney, Australia. Dr. Ong-Amoranto is with the Department of Dermatology at Asian Hospital and Medical Center in Metro Manila, Philippines. Dr. Vachiramon is with the Division of Dermatology, Faculty of Medicine Ramathibodi Hospital at Mahidol University in Bangkok, Thailand. Dr. Wanitphakdeedecha is with the Department of Dermatology, Faculty of Medicine at Siriraj Hospital, Mahidol University in Bangkok, Thailand. Dr. Kerscher is with the Division of Cosmetic Science and Aesthetics at the University of Hamburg in Hamburg, Germany
FUNDING: Funding for this work was provided by Merz Aesthetics.
DISCLOSURES: Dr. Park serves as a consultant for Merz Aesthetics. Dr. Ong-Amoranto serves as an advisory board member for Merz Philippines Healthcare Inc. Prof. Kerscher serves as an advisory board member and consultant for Merz Aesthetics. All authors have received honoraria from Merz Aesthetics for their contributions at the advisory board meeting and subsequent manuscript preparation.
ABSTRACT: Objective. We sought to examine the current skin quality trends and gaps in clinical practice in the Asia Pacific region and develop a practical guide to improve skin quality.
Methods. Medical practitioners from 11 countries in the Asia Pacific region completed an online survey on current trends in skin quality treatment. A panel of 12 leading experts convened for a virtual meeting to develop a practical guide for skin quality improvement.
Results. A total of 153 practitioners completed the survey. The four most common skin quality issues were uneven skin tone, skin surface unevenness, skin laxity, and sebaceous gland hyperactivity and enlarged pores. Most practitioners reported using a combination of treatment modalities for each skin quality issue. It was also observed that each treatment modality could be used to treat several skin quality issues. A multimodal approach targeting different interrelated issues across the tissue planes was recommended for balanced results. The panel developed a practical guide for the appropriate combinations and sequence of treatments, and created treatment protocols for specific skin quality outcome goals. The guide employed an “inside-out” approach, treating the deeper tissue planes prior to the superficial layers to achieve harmonious results.
Limitations. Future studies are needed to support the recommended treatment protocols for skin quality improvement.
Conclusion. These findings provide valuable insights on current skin quality trends and gaps in clinical practice. The practical guide provides a framework for practitioners to customize their treatment plan according to each patient’s needs.
Keywords: Skin quality, practical guide, consensus, survey, multimodal, “inside-out” approach
To a large extent, facial skin quality affects an individual’s appearance and has a profound influence on perceived attractiveness, youthfulness, and health.1–3 Poor skin quality can have negative consequences, both psychologically and socially, with significant impact on patient quality of life.4,5 The overall perception of skin quality is influenced by multiple attributes or parameters, which are determined by an interplay of underlying biological processes affecting different anatomical layers of the face.6–9 A multimodal approach targeting different tissue planes (as opposed to only treating the skin surface) is required for skin quality improvement.6 Skin quality attributes or parameters are also interrelated and interdependent.6 Likewise, individual treatment modalities can have effects on more than one attribute or parameter. Therefore, a holistic approach selecting a combination of treatments is beneficial towards achieving balanced results. However, practical guidance on selecting and combining treatment modalities to achieve the desired outcome has not been developed so far. There is also a lack of data on clinical practice trends for skin quality improvement in the Asia Pacific region. This article examines the common skin quality issues and current clinical practice trends in the Asia Pacific region. Consensus recommendations and practical treatment algorithms for skin quality improvement are also presented.
Methods
Survey on skin quality trends and panel discussion. An online survey was initially conducted to understand the current skin quality and clinical practice trends in Asia Pacific. Medical practitioners from 11 countries in Asia Pacific participated in this survey between January and February 2021. A panel of 12 experts convened for a virtual meeting in March 2021 to establish a consensus for skin quality attributes and practices. This panel comprised aesthetic physicians, dermatologists, and plastic surgeons from 10 countries, including Hong Kong, Indonesia, Malaysia, the Philippines, Taiwan, Thailand, Singapore, South Korea, Australia, and Germany. The expert from Germany was invited to provide a global perspective on best practices for skin quality improvement. The panel discussed the survey findings and shared their clinical experience in addressing common skin quality issues encountered by patients in the Asia Pacific region. They also voted on statements relating to skin quality attributes and trends, as well as treatment strategies and current practices for skin quality improvement. Voting results were graded as follows: strong consensus (>95% agreement); consensus (>75% to 95% agreement); majority consent (>50% to 75% agreement), and no majority consent (≤50% agreement). The panel developed a practical guide with recommended treatment algorithms for achieving an improvement in overall skin quality, including suggested combinations and sequence of treatments, based on their extensive clinical experience with various treatment modalities and available evidence.
Results
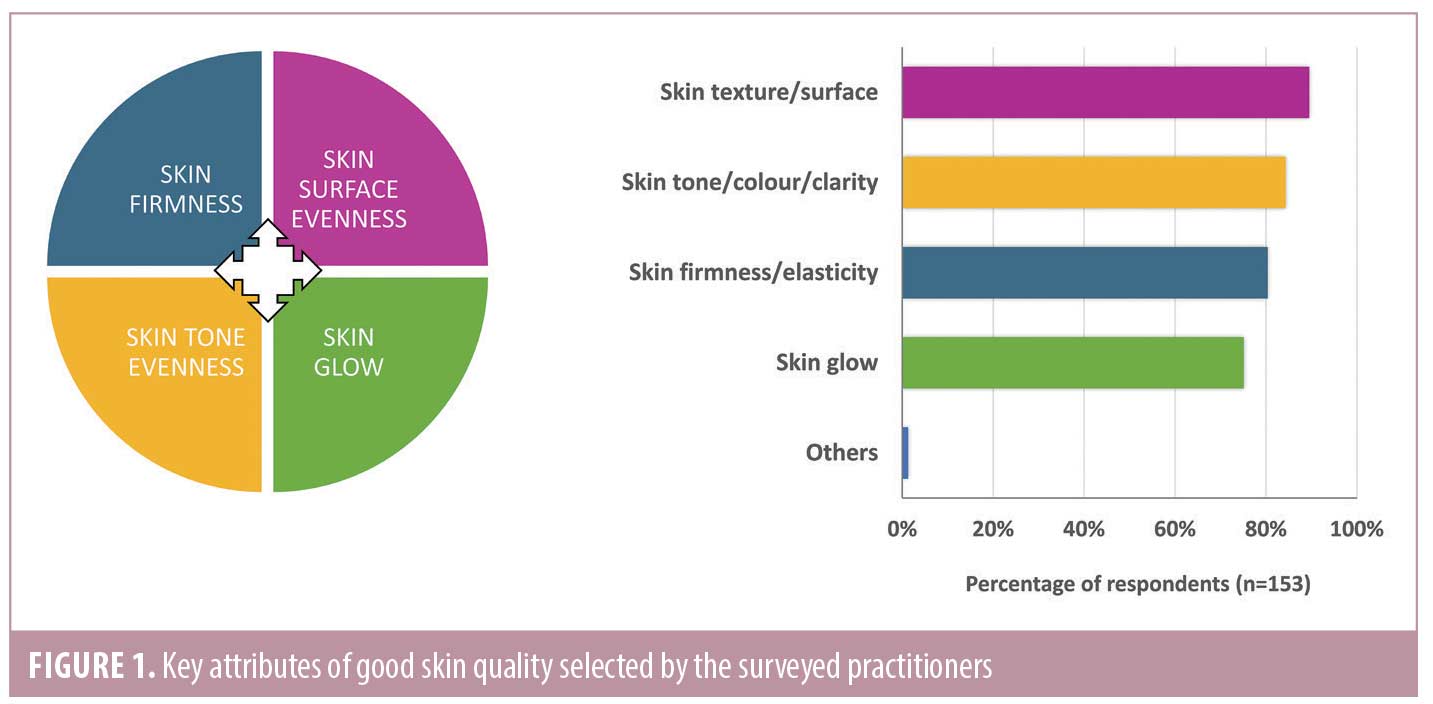
Current skin quality and clinical practice trends in Asia Pacific. A total of 153 medical practitioners in Asia Pacific participated in the survey. Practitioners from Thailand, South Korea, the Philippines, Indonesia, Hong Kong, and Australia constituted around 80% of the participants. The remaining 20% were from Taiwan, India, Malaysia, New Zealand, and Singapore. The surveyed practitioners comprised aesthetic physicians (48%), dermatologists (45%), plastic surgeons (5%), and others (3%). The results of the survey are summarized in Figures 1 and 2, and Table 1. The practitioners recognized four concepts—skin tone evenness, skin surface evenness, skin firmness, and skin glow—as key attributes of good skin quality and attractive skin (Figure 1). The same four skin quality attributes were identified in a recent consensus developed by a panel of experts mostly from the Western countries, which described these as emergent perceptual skin quality categories (EPCs).6
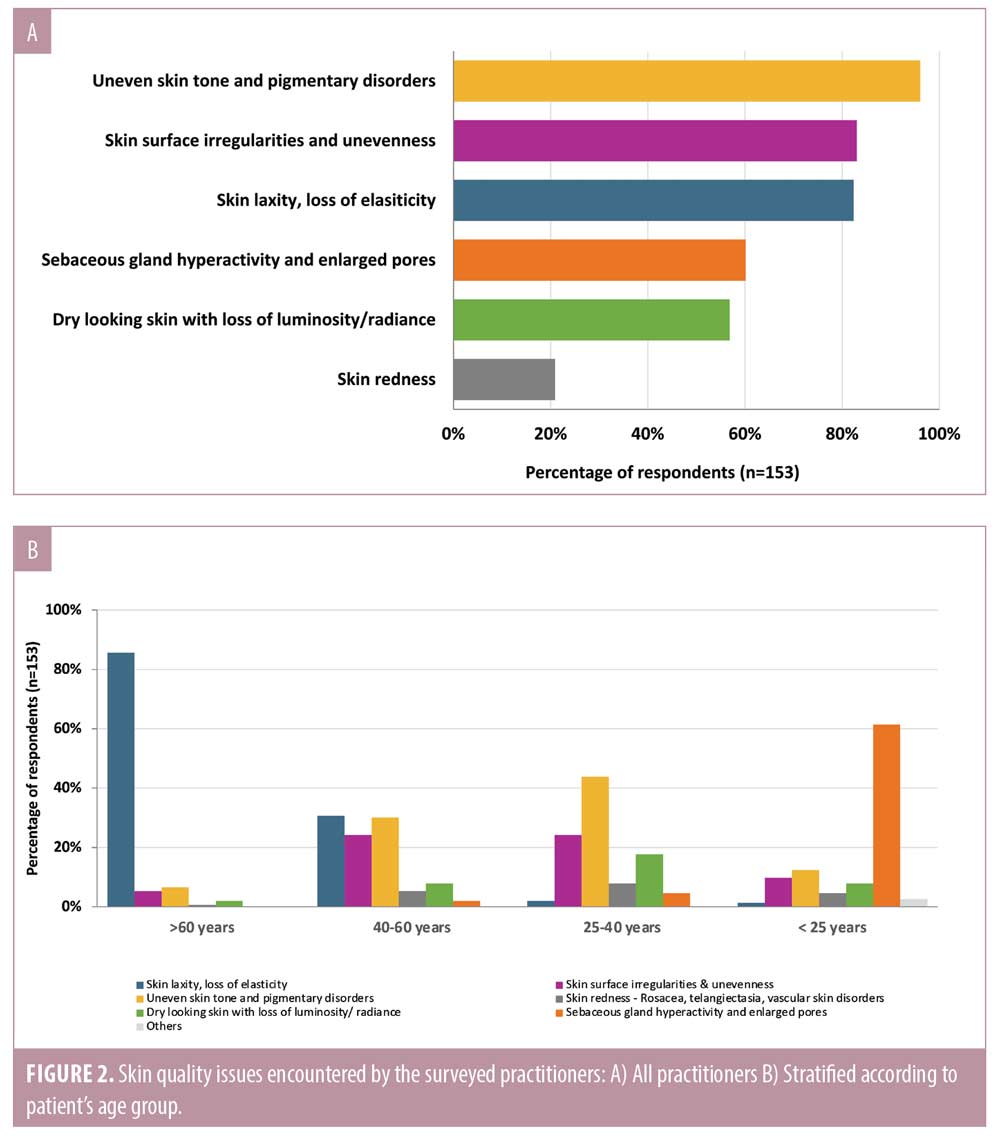
When practitioners were asked about skin quality issues encountered in their clinical practice, they reported that patients were concerned with various skin quality issues. The four most common issues were uneven skin tone, skin surface unevenness, skin laxity, and sebaceous gland hyperactivity and enlarged pores (Figure 2A). However, it is important to note that different skin quality issues were prevalent in different age groups (Figure 2B). Patients younger than 25 years were most disturbed by sebaceous gland hyperactivity and enlarged pores, whereas those older than 60 years were most concerned with skin laxity. Patients in the 25–40 years and 40–60 years age groups were concerned with a range of issues, namely uneven skin tone, skin surface unevenness, dry skin, and/or skin laxity.
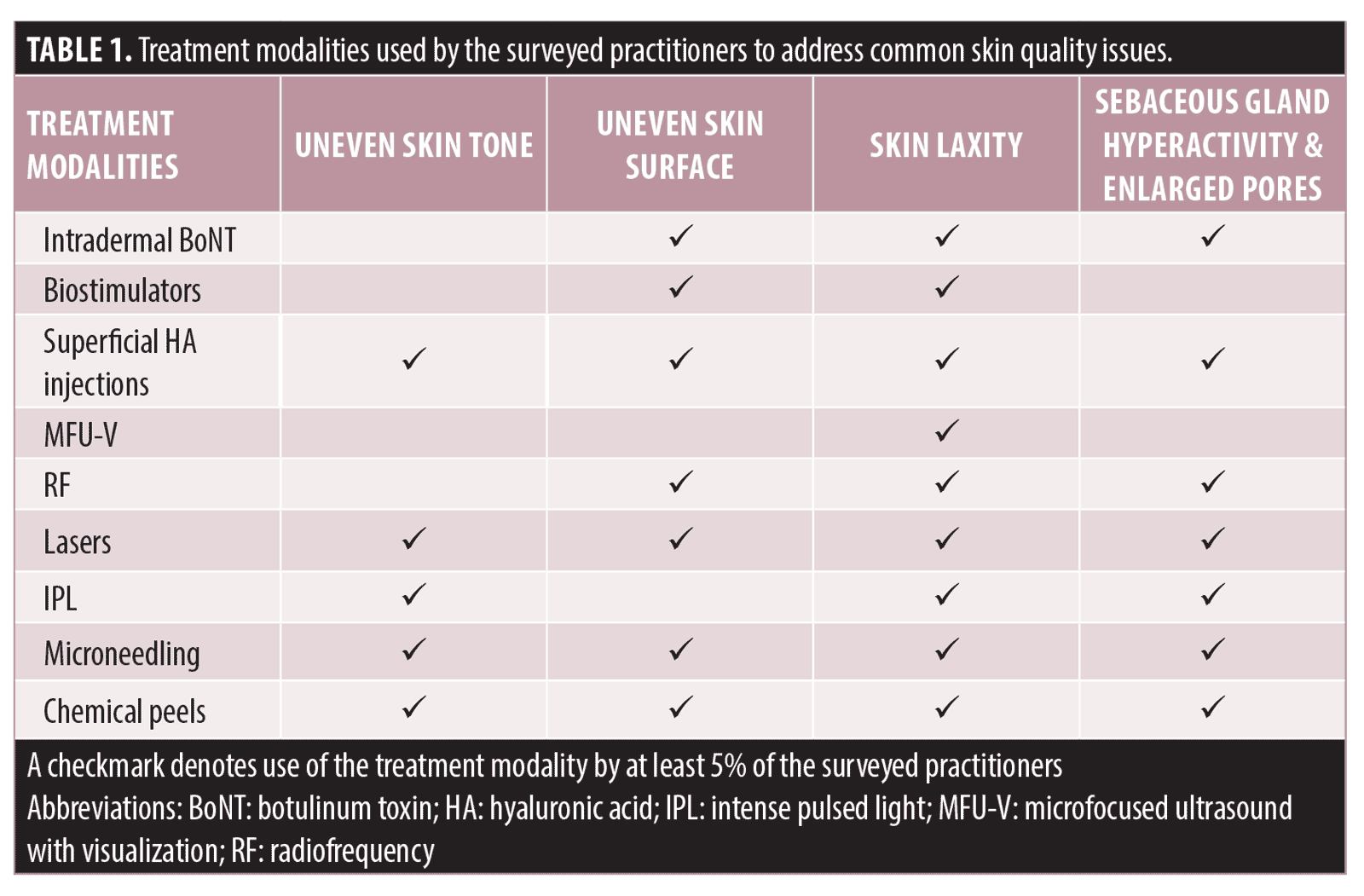
When practitioners were asked about the treatment modalities used in their practice to address common skin quality issues, they reported that more than one treatment modality was often used to address each issue (Table 1). For instance, a combination of lasers, microneedling, chemical peels, and/or injectables were used to address skin surface unevenness in practice. It was also observed that each treatment modality could be used to treat several skin quality issues, often in combination with other modalities. For example, superficial hyaluronic acid (HA) injections alone or in combination with other modalities, were used for managing all four common skin quality issues. On the other hand, practitioners reported relatively low use of certain treatment modalities for some skin quality issues. Less than 5% of the practitioners used microfocused ultrasound with visualization (MFU-V) for improving skin tone or skin surface evenness. Similarly, only 4% used intradermal botulinum toxin (BoNT) and 1% used biostimulators for addressing uneven skin tone.
Consensus on skin quality attributes and practices. A summary of consensus statements relating to skin quality and treatment trends in Asia Pacific is provided in Table 2. Overall, the expert panel expressed high level (≥ 92%) of agreement with all statements. The panel’s opinions were consistent with those of the surveyed practitioners (100% agreement) in terms of key attributes of good skin quality (Figure 1), the four most common skin quality issues encountered in practice (Figure 2A), and the prevalent skin quality issues at different stages in life (Figure 2B). Although the majority of the panel members agreed that the definition and perception of attractive skin are largely similar between different ethnicities (92% agreement), they recognized that Asian patients tend to desire lighter and brighter skin tone compared with Caucasian patients. This is consistent with the survey findings that uneven skin tone and pigmentary disorders were the most common skin quality issues encountered by practitioners in the Asia Pacific region (Figure 2A). The panel also added that skin tone is regarded of greater importance to patients residing in countries with higher sunlight exposure than those living in countries of lower exposure.
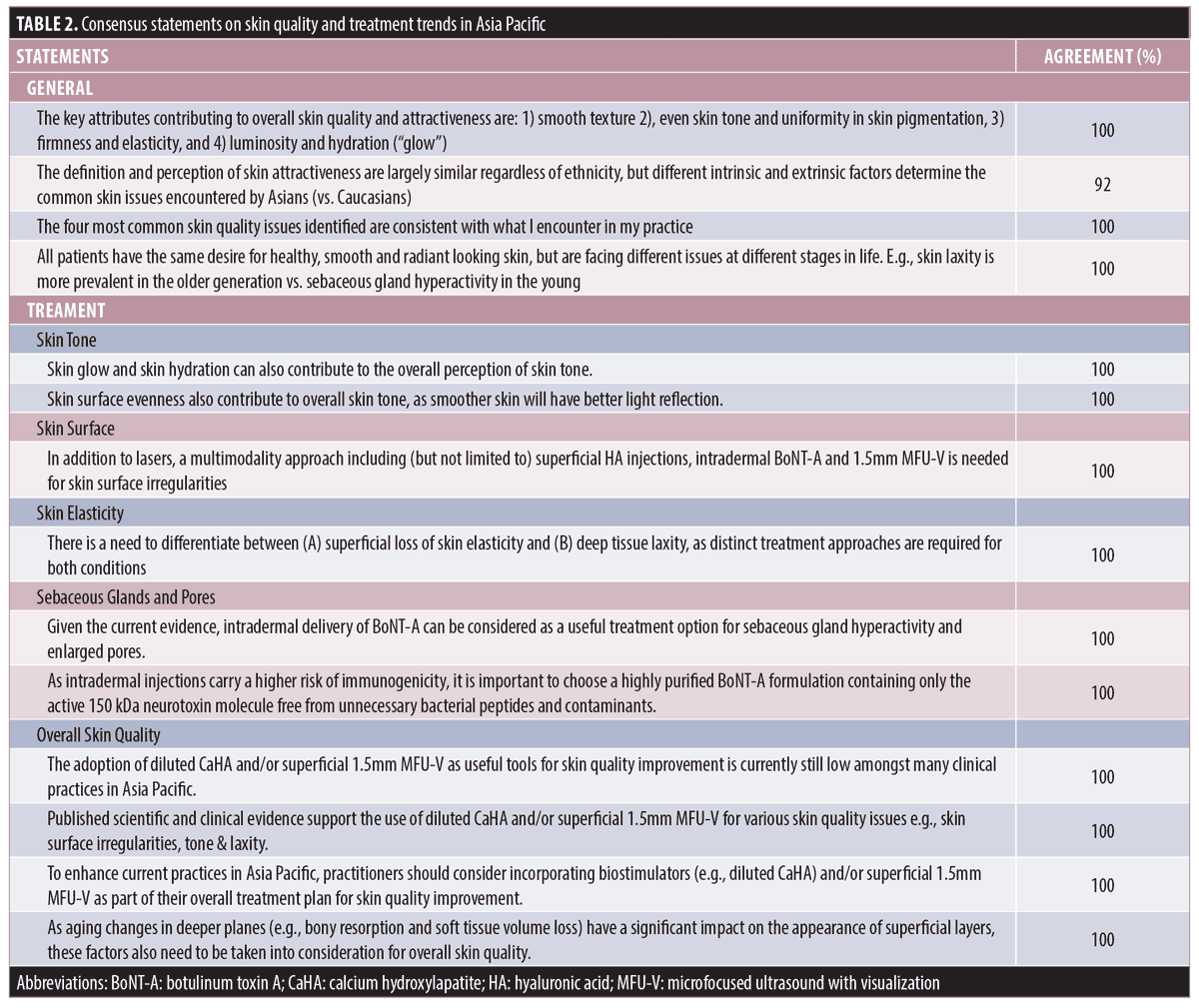
The panel recognized that the overall perception of good skin quality is influenced by multiple attributes, which are interrelated and can affect each other.6 For example, skin glow can affect the overall perception of skin tone, and skin surface evenness can give better light reflection, which can influence the overall skin tone as well. Hence, there was a strong consensus to adopt a holistic approach considering related skin quality issues in the treatment plan to achieve harmonious results (100% agreement). This is in line with the guidance provided in a recent skin quality consensus, which recommended achieving a balance of skin quality attributes for youthful and attractive skin.6 In addition, the panel acknowledged that morphological and structural deficits in deeper tissue planes also affect the appearance of the skin surface.6–9 Hence, it is agreed that practitioners should consider the different tissue layers that might be involved and distinguish treatments between the deep and superficial layers (100% agreement). For example, it is important to differentiate between skin surface unevenness due to alterations in the superficial layer and deeper planes (e.g. bony resorption, soft tissue volume loss, etc.), and between superficial loss of skin elasticity and deep tissue laxity. Distinct treatment approaches are required for addressing age-related changes in different tissue planes. To achieve harmonious and balanced outcomes, the panel recommended a multimodal strategy to address interrelated issues across different tissue planes, and adopting an “inside-out” approach, treating the deeper tissue planes prior to the superficial layers.
Published evidence supports the use of MFU-V, biostimulators (e.g. diluted or hyperdiluted calcium hydroxylapatite [CaHA]), superficial HA injections, and intradermal BoNT as part of treatment plans to address various skin quality issues.10–18 However, the adoption of these modalities, particularly MFU-V and biostimulators for managing certain skin quality issues was noted to be low in clinical practices in Asia Pacific (Tables 1 and 2) despite the published evidence. This could be due to low awareness of these modalities or lack of knowledge and expertise to use or combine them in Asian patients. It could also be related to the fact that the proposed “inside-out” treatment approach has not been frequently used in daily practice as most patients, or even practitioners, commonly view and tackle each issue in isolation without considering the possible interplay of underlying causes. The panel acknowledged that although some of these treatment modalities may not be the first-line treatment for addressing certain skin quality issues, they are complementary and supplementary to current treatment for enhancing aesthetic outcomes. For instance, topical lightening agents and lasers are the first-line treatment options for skin tone/pigmentary disorders, but there is emerging evidence on the use of superficial HA injections and intradermal BoNT for improving skin tone.11,19 Hence, there was strong consensus to include these treatment modalities as part of the overall treatment plan to enhance the current practices for skin quality improvement (100% agreement). The panel recognized the need to provide more specific guidance on appropriate combination and sequence of these treatment modalities in patients with varying skin quality issues, and to create treatment protocols as a guide for practitioners to properly administer each treatment modality to meet specific treatment goals.
Discussion
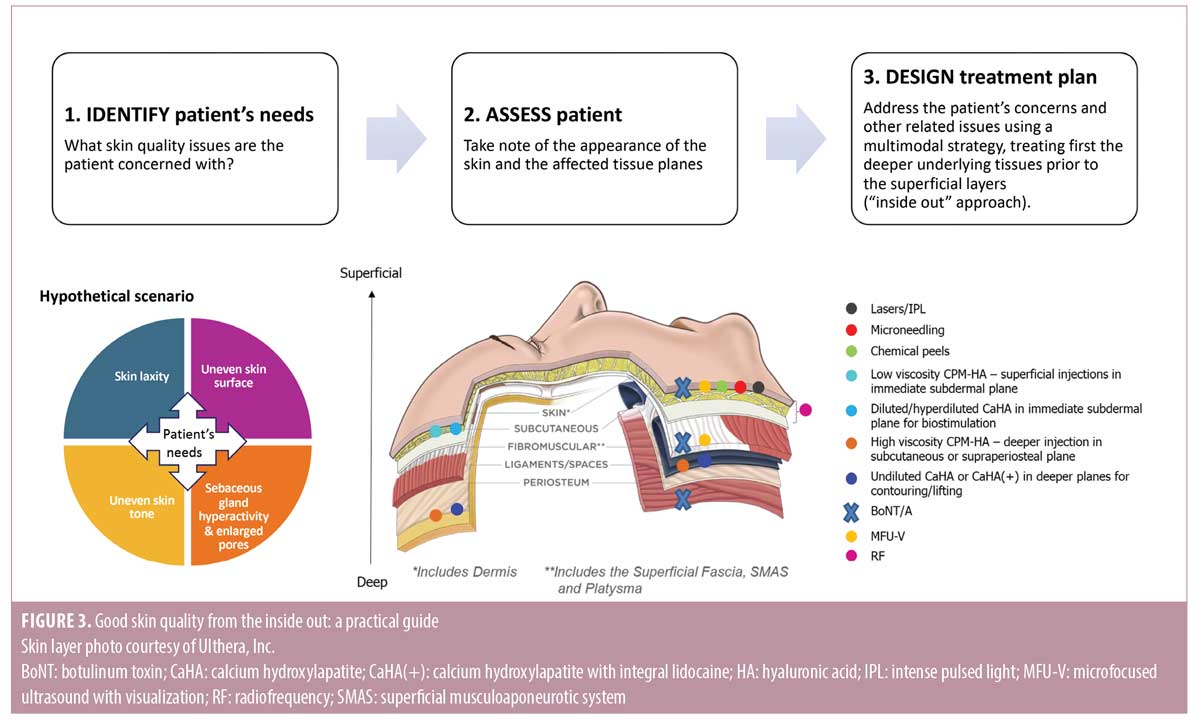
Good skin quality from the inside out: a practical guide. This practical guide integrates current evidence, as well as the panel’s collective clinical experience with various treatment modalities to help practitioners tailor their treatment plans to meet the needs of each patient. Besides including recommendations on the use of treatment modalities for approved indications, the guide also provides practical advice for off-label applications based on published guidelines/consensus, emerging evidence, and clinical expertise of the panel. The recommendations represent a holistic and multimodal approach that uses a combination of MFU-V, diluted/hyperdiluted CaHA, undiluted CaHA, HA fillers, and BoNT, as part of the treatment plan (including other modalities such as lasers, radiofrequency [RF], intense pulsed light [IPL], chemical peels, microneedling, etc.) for enhancing facial skin quality (Figure 3). Treatment protocols detailing how MFU-V, diluted/hyperdiluted CaHA, HA fillers, and/or intradermal BoNT can be administered to meet specific treatment goals are presented in Table 3. As with any treatment, practitioners should be familiar with the complete local product information, including approved indications, precautions, contraindications, and adverse events before administering the treatment.
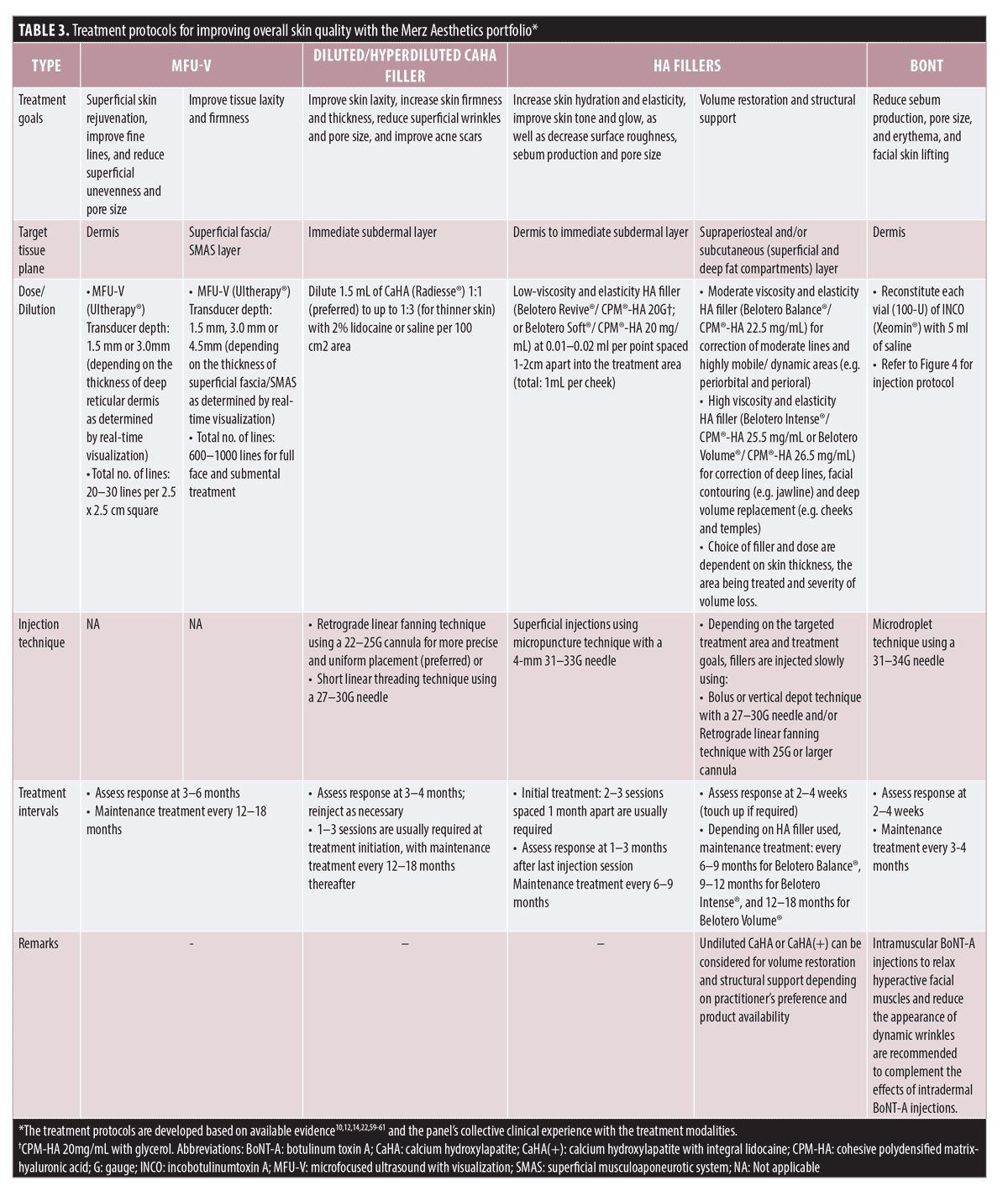
The guide entails a three-step approach—Identify, Assess, and Design (Figure 3). The first step is to identify skin quality issues that concern the patient and to understand his/her preferences and treatment goals. A detailed assessment of the face then follows, taking note of the degree of age-related changes and the affected tissue planes (bone, ligaments, fibromuscular/superficial musculoaponeurotic system [SMAS], subcutaneous [deep and superficial fat compartments], or skin surface [dermis and epidermis]), as well as the appearance of the skin (such as uneven tone, surface unevenness, skin laxity, oily skin, enlarged pores, dry skin, etc.). Facial assessment forms the basis for designing an individualized treatment plan. The overall perception of skin quality is influenced by multiple interrelated attributes, and these attributes can in turn affect one another.6 Hence, when designing a treatment plan to address the patient’s concerns, other related skin quality issues should also be considered for overall skin quality improvement. Skin quality can also be affected by biological processes in multiple tissue planes.6–9 It is therefore important to consider not only the skin surface but also other tissue planes that may be involved, and distinguish treatments between the deep and superficial layers. The panel recommended a strategy that combines treatment modalities targeting different manifestations across the involved tissue planes, and employing an “inside out” approach, treating first the deeper underlying tissues followed by superficial layers (Figure 3 and Table 3). Depending on the patient’s availability and preferences, individual modalities can be administered sequentially on the same day or on separate visits. For stepwise treatment over several sessions, visits should be spaced 1 to 4 weeks apart for resolution of local adverse effects and to assess the treatment response.(20) The sequence of modalities administered depends on several factors such as the patient’s main presenting complaint and skin condition. For instance, some experts prefer to perform MFU-V to strengthen the soft tissue envelope before injecting fillers in patients with severe skin laxity, while some prefer to “prime” the skin and fibroblasts by injecting diluted/hyperdiluted CaHA before MFU-V. However, if patients are unable to commit to multiple treatment visits due to their schedules or preferences, combined treatments on the same day may be performed. Published review on combined same-day treatments with energy-based interventions and injectable treatments (BoNT and fillers) reported no increase in adverse effects, such as spread of neurotoxin, displacement of filler material, or other untoward effects.21 For same-day treatment, MFU-V is recommended before injectable treatments; BoNT and fillers can be administered in either sequence.20
The panel provided suggestions on how MFU-V, diluted/hyperdiluted CaHA, HA fillers, and/or intradermal BoNT can be administered to meet specific treatment goals (Table 3). The following modalities were used to construct examples for the treatment protocols: MFU-V (Ultherapy®; Merz North America, Inc. Raleigh, N.C.), diluted or hyperdiluted CaHA (Radiesse®; Merz North America, Inc), Cohesive Polydensified Matrix® (CPM®)-HA fillers (Belotero Revive®, Belotero Soft®, Belotero Balance®, Belotero Intense®, and Belotero Volume®; Anteis S.A., Geneva, Switzerland), and a BoNT formulation without complexing proteins (incobotulinumtoxin A [INCO]) (Xeomin®; Merz Pharmaceuticals GmbH, Hessen, Germany). Treatment protocols included detailed information on the target tissue planes, doses, injection techniques, injection protocol and treatment intervals. Properties and mechanisms of action for each of these treatment modalities are provided in Supplementary Table 1.
Treatment protocol for using MFU-V. MFU-V is regarded as the gold standard treatment for nonsurgical lifting and tightening of lax skin.16,22 It has been reported to be effective for superficial skin rejuvenation and lifting tissues, as well as reducing skin laxity, fine lines and pore size.23–32 MFU-V provides real-time visualization of distinct tissue layers beneath the skin surface, including the dermal and subdermal layers (superficial fascia, SMAS, and platysma), which allows precise delivery of microfocused ultrasound energy to the intended collagen-rich tissue planes (Supplementary Table 1).16,22,33,34 MFU-V induces the creation of thermal coagulation points in the targeted tissue planes and stimulates the natural wound healing process to trigger the production of new collagen and elastin.33–35 This results in tissue remodeling, increased viscoelasticity, tissue lifting, as well as thickening and tightening of the skin.16,22,33,34
A group of experts recently developed a consensus on the use of MFU-V treatment in Asian patients, that provides recommendations for tailoring MFU-V treatment for optimal aesthetic outcomes.22 The following protocol was established based on the recommendations of the Asian consensus.22 The panel recognized that skin thickness can vary between patients and treatment area, and that the choice of MFU-V transducer is dependent on the patient’s concerns and his/her individual skin anatomy. Practitioners should perform real-time visualization to determine the exact depths of dermal and superficial fascia layers, and select the appropriate transducers to ensure precise delivery of ultrasound energy to the target tissues. To address fine lines, enlarged pore size, and superficial unevenness and laxity, the panel suggested using either a 1.5mm or 3.0mm transducer to target the dermis, delivering 20 to 30 treatment lines per 2.5cm x 2.5cm square. To improve tissue laxity and firmess, the panel suggested using a 1.5mm, 3.0mm, or 4.5 mm transducer to target the superficial fascia or SMAS layer, delivering 600 to 1000 lines for full face and submental treatment. Depending on patient’s concerns, more than one transducer depth can be used per treatment session. For instance, MFU-V can also improve skin texture indirectly through tightening of the deeper underlying tissues. The exact treatment depths and the number of lines depend on the patient’s concerns and condition; practitioners should tailor their treatment protocol according to the individual patient’s needs. The panel recommended assessing the results within 3 to 6 months of the initial treatment, followed by maintenance treatment every 12 to 18 months.
Treatment protocol for using diluted or hyperdiluted CaHA. CaHA comprises microspheres of CaHA suspended in an aqueous carboxymethylcellulose gel matrix.36 In recent years, diluted or hyperdiluted CaHA injections have been used to induce dermal regeneration without creating unnecessary volume gain.10,13,14,37-44 Superficial injections of diluted or hyperdiluted CaHA, as monotherapy or part of a combination approach, have been shown to address a number of skin quality issues leading to improvements in skin quality in the face and body.13,37–44 After injection, the CaHA microspheres act as a scaffold for new tissue formation by stimulating neocollagenesis, neoelastogenesis, dermal cell proliferation, and angiogenesis for long-lasting aesthetic improvements (Supplementary Table 1).37,45,46 CaHA has also been found to restore fibroblast contractility in vitro and induce production of proteoglycans in human skin, indicating its unique beneficial effects on fibroblast activity and extracellular matrix (ECM) remodeling.47,48 Consensus guidelines on the use of diluted or hyperdiluted CaHA for biostimulation in the face and body have been published. They provide detailed recommendations for improvements of a range of skin quality issues, including laxity, superficial wrinkles, roughness, and acne scars.10,14 The following protocol was established in accordance with these recommendations.10,14 The panel recommended administering diluted or hyperdiluted CaHA to the immediate subdermal plane for treating skin laxity, acne scars, superficial wrinkles and pore size, and increasing skin firmness and thickness (Table 3). As in published guidelines,10,14 the preferred dilution for effective biostimulation is 1:1; hyperdilution up to ratios of 1:3 may be used in areas of thin skin. The panel members recommended using the retrograde linear fanning technique, gently scraping the underside of the dermis with a 22–25G cannula for more precise delivery of a thin, uniform coating of diluted product to the treatment area. Similar to published guidelines,10, 14 the panel recommended assessing response within 3 to 4 months after treatment and reinject as necessary. One to three sessions are usually required at treatment initiation, followed by maintenance treatment every 12 to 18 months.
Treatment protocol for using HA fillers. HA fillers have been shown to be safe and effective for improving skin hydration, elasticity, tone and glow, as well as decreasing surface roughness, sebum production, and enlarged pore size.11,17,18,32,49,50 Upon superficial injection of HA filler with low viscoelasticity, a condensed network with the ECM is established to increase hydration and stimulate fibroblast activation. This in turn triggers production of collagen, elastin and hyaluronic acid, and promote the release of stromal-derived factor 1 (which plays a role in inhibiting cutaneous pigmentation), resulting in increased skin hydration and elasticity, decreased surface roughness, and improved skin tone.51,52 In addition, HA fillers play an important role inhibiting sebum production via CD44/RhoA signaling.17
Numerous formulations of HA fillers have been developed over the years, which are manufactured with different cross-linking technologies resulting in HA fillers with specific characteristics and rheological properties.53–55 CPM-HA fillers are monophasic polydensified non-particulate gels manufactured using a patented dynamic two-stage cross-linking technology, which results in gels with variable densities of crosslinked HA.54 This allows for homogeneous tissue integration, resulting in favorable aesthetic outcomes.11,18,56-58 CPM-HA fillers are available in different HA concentrations and cross-linking ratios, with different viscoelasticity and projection capacity for use in a wide range of aesthetic applications.18,55,59 Products with low viscoelasticity are suited for superficial injections for skin hydration and rejuvenation, whereas those with moderate or high viscoelasticity and good projection capacity are useful for soft-tissue augmentation (Supplementary Table 1).18,55,59 By combining CPM-HA fillers with different rheological properties in different tissue planes, a more harmonized and effective treatment outcome could be achieved with minimum quantity of fillers.59
The following protocol was established by the panel. To increase skin hydration and elasticity, improve skin tone and glow, as well as decrease surface roughness, sebum production and pore size, the panel recommended using the micropuncture technique11 to deliver CPM-HA filler with low viscoelasticity to the dermis to immediate subdermal plane (Table 3). The product can be administered in aliquots of 0.01–0.02 mL per puncture, spaced 1 to 2 cm apart using a 4-mm 31–33G needle. A total of 1 mL of the product can be injected per cheek. Two to three sessions spaced one month apart are usually required at treatment initiation. The panel recommended assessing patient’s response within 1 to 3 months after the last injection, followed by retreatment every 6 to 9 months thereafter. To address volume loss and structural deficits in deeper tissue planes, which can in turn improve skin appearance, the panel recommended injecting CPM-HA filler with moderate or high viscoelasticity into the supraperiosteal and/or subcutaneous (superficial and deep fat compartments) plane in accordance with published consensus and published literature on safe and effective augmentation with CPM-HA fillers (Table 3).59–61 The choice of filler and the dose are dependent on the skin thickness, the area being treated, and the severity of volume loss and structural deficits. Depending on the target treatment area and treatment goals, the product can be administered slowly using the bolus or vertical depot technique with a 27–30G needle and/or the retrograde linear fanning technique with a 25G or larger cannula. The panel recommended assessing patient’s response within 2 to 4 weeks of initial treatment and touch up if required. Repeat treatment is usually required every 6 to 18 months, depending on the type of filler injected.
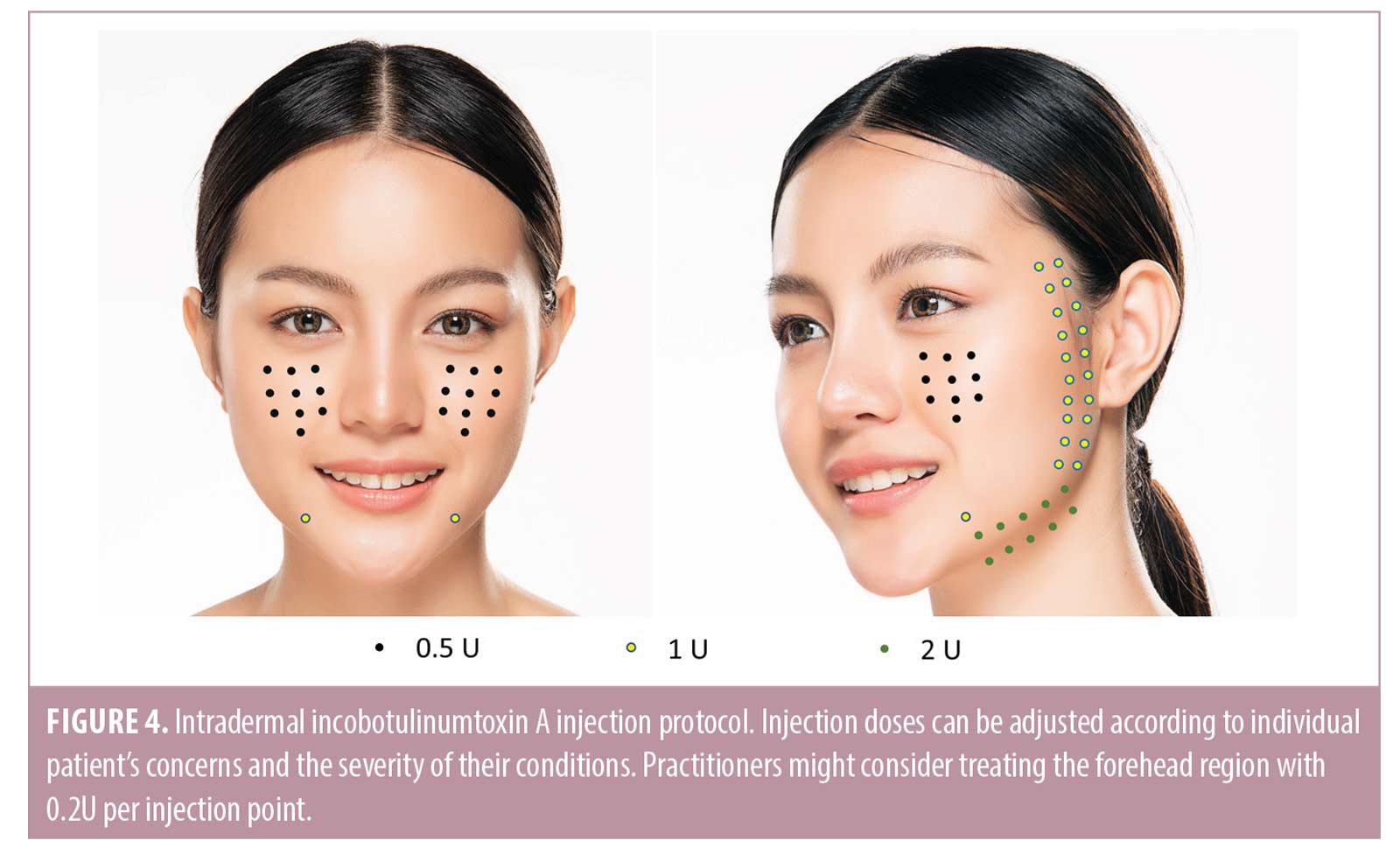
Treatment protocol using BoNT.Encouraging results have been reported with intradermal BoNT for treating a variety of skin quality issues, including excess sebum production, enlarged pores, erythema, and facial skin laxity.12,62-66 These positive effects could possibly be mediated via acetylcholine inhibition, leading to the paralysis of arrector pili muscles and depressor muscles, and reduction in exocrine gland activity (details in Supplementary Table 1).64,67-73 Additional mechanisms of action proposed include the inhibition of other neuropeptides, such as vasoactive intestinal peptide, and stimulation of fibroblast contraction.74,75 However, intradermal injections are regarded as more immunogenic than intramuscular injections because the dermis contains large numbers of dendritic cells, which play a pivotal role in inducing immune responses through antigen capture and presentation to T-lymphocytes.76 This physiological concept is applied in immunology, where intradermal vaccination has been used for certain populations that do not respond well to intramuscular vaccination e.g. hepatitis B vaccine in hemodialysis patients.77 As such, the panel agreed that it is advisable to choose a BoNT formulation containing only the active 150 kDa neurotoxin molecule to reduce the risk of immunogenicity.12 INCO is a highly purified and precisely manufactured BoNT-A formulation, which contains only the active neurotoxin, free of unnecessary bacterial peptides and impurities.78,79 Given its low immunogenic potential79,80 and positive clinical experience in improving sebum control and facial skin laxity, and reducing pore size and erythema,12,62,74 INCO can be considered a rational choice for intradermal injections to address these skin quality issues. The panel recommended reconstituting 100-U of INCO with 5mL of saline and administering the product using the microdroplet injection technique12 with a 31–34G needle into the dermis (Table 3). The suggested injection protocol was described in a recent publication12 and is provided in Figure 4. Practitioners may consider including injections of 0.2-U per point in the forehead region, as well as intramuscular injections into the glabella, mentalis, and masseter to complement the full face intradermal injection protocol. As the exact doses vary according to individual patient’s concerns and the severity of their conditions, practitioners should tailor treatment to meet the needs of their individual patients. The panel recommended assessing response within 2 to 4 weeks after treatment, followed by retreatment every 3 to 4 months thereafter.
Conclusion
To our knowledge, this is the first report describing the current skin quality and clinical practice trends in the Asia Pacific region and the first practical guide for skin quality improvement. Improving facial skin quality can have a positive impact on overall attractiveness, which can favorably affect one’s self-confidence, wellbeing, and overall health. The survey findings showed that uneven skin tone, skin surface unevenness, skin laxity, as well as sebaceous gland hyperactivity and enlarged pores were the most common skin quality issues encountered by patients in the Asia Pacific region. Practitioners recognized four concepts—skin tone evenness, skin surface evenness, skin firmness, and skin glow—as key attributes of good skin quality and attractive skin. The same four skin quality attributes were identified in a recent global consensus on skin quality and were described as EPCs. Although clinical evidence supports the use of MFU-V, biostimulators, superficial HA injections, intradermal BoNT as part of treatment plans to enhance facial skin quality, the survey revealed low adoption of MFU-V and biostimulators for managing certain skin quality issues in Asia Pacific, suggesting low awareness of these modalities, or lack of knowledge and expertise on how to use or combine these modalities among practitioners in the region. These findings provide valuable insights on the current skin quality trends and gaps in clinical practice, which can aid in improving existing practices for skin quality improvement.
The overall perception of skin quality is influenced by multiple attributes or parameters, which are interrelated and can affect each other. Morphological and structural deficits in deeper tissue planes can also affect the appearance of the skin surface. Therefore, the panel recommended a multimodal approach targeting different interrelated issues across the tissue planes involved (as opposed to only treating one issue and only the skin surface) for achieving balanced results. The practical guide relied on available evidence and the panel’s collective clinical experience with various treatment modalities to provide specific guidance on the appropriate combination and sequence of treatment. Treatment protocols were created to assist practitioners in tailoring their treatment plans to the needs of their patients. The guide focuses on an “inside-out” approach, treating first the deeper underlying tissues prior to the superficial layers, to achieve harmonious and balanced outcomes. Future studies are needed to generate clinical evidence to support recommended treatment protocols for skin quality improvement, specifically in Asian patients.
Acknowledgments
Medical writing and editorial support were provided by Tech Observer Asia Pacific Pte Ltd and was funded by Merz Aesthetics.
References
- Samson N, Fink B, Matts P. Interaction of skin color distribution and skin surface topography cues in the perception of female facial age and health. J Cosmet Dermatol. 2011;10(1):78-84.
- Fink B, Neave N. The biology of facial beauty. Int J Cosmet Sci. 2005;27(6):317–325.
- Samson N, Fink B, Matts PJ. Visible skin condition and perception of human facial appearance. Int J Cosmet Sci. 2010;32(3): 167–184.
- Thomas DR. Psychosocial effects of acne. J Cutan Med Surg. 2004;8 Suppl 4:3-5.
- Balkrishnan R, McMichael AJ, Hu JY, et al. Correlates of health-related quality of life in women with severe facial blemishes. Int J Dermatol. 2006;45(2):111–115.
- Goldie K, Kerscher M, Fabi SG, et al. Skin Quality – A Holistic 360° View: Consensus Results. Clin Cosmet Investig Dermatol. 2021;14:643–654.
- Krueger N, Luebberding S, Oltmer M, et al. Age-related changes in skin mechanical properties: a quantitative evaluation of 120 female subjects. Skin Res Technol. 2011;17(2): 141–148.
- Gierloff M, Stöhring C, Buder T, et al. Aging changes of the midfacial fat compartments: a computed tomographic study. Plast Reconstr Surg. 2012;129(1):263–273.
- Shaw RB, Jr., Katzel EB, Koltz PF, et al. Aging of the facial skeleton: aesthetic implications and rejuvenation strategies. Plast Reconstr Surg. 2011;127(1):374–383.
- Goldie K, Peeters W, Alghoul M, et al. Global Consensus Guidelines for the Injection of Diluted and Hyperdiluted Calcium Hydroxylapatite for Skin Tightening. Dermatol Surg. 2018;44 Suppl 1:S32–S41.
- Hertz-Kleptow D, Hanschmann A, Hofmann M, et al. Facial skin revitalization with CPM((R))-HA20G: an effective and safe early intervention treatment. Clin Cosmet Investig Dermatol. 2019;12:563–572.
- Park JY, Cho SI, Hur K, et al. Intradermal Microdroplet Injection of Diluted Incobotulinumtoxin-A for Sebum Control, Face Lifting, and Pore Size Improvement. J Drugs Dermatol. 2021;20(1):49–54.
- Casabona G. Combined use of microfocused ultrasound and a calcium hydroxylapatite dermal filler for treating atrophic acne scars: A pilot study. J Cosmet Laser Ther. 2018;20(5):301–306.
- de Almeida AT, Figueredo V, da Cunha ALG, et al. Consensus Recommendations for the Use of Hyperdiluted Calcium Hydroxyapatite (Radiesse) as a Face and Body Biostimulatory Agent. Plast Reconstr Surg Glob Open. 2019;7(3):e2160.
- Fabi SG, Few JW, Jr, Moinuddin S. Practical Guidance for Optimizing Patient Comfort During Microfocused Ultrasound with Visualization and Improving Patient Satisfaction. Aesthetic Surgery Journal. 2019;40(2):208–216.
- Fabi SG, Joseph J, Sevi J,et al. Optimizing Patient Outcomes by Customizing Treatment With Microfocused Ultrasound With Visualization: Gold Standard Consensus Guidelines from an Expert Panel. J Drugs Dermatol. 2019;18(5):426–432.
- Jung YR, Hwang C, Ha JM, et al. Hyaluronic Acid Decreases Lipid Synthesis in Sebaceous Glands. J Invest Dermatol. 2017;137(6):1215–1222.
- Prasetyo AD, Prager W, Rubin MG, et al. Hyaluronic acid fillers with cohesive polydensified matrix for soft-tissue augmentation and rejuvenation: a literature review. Clin Cosmet Investig Dermatol. 2016;9:257–280.
- Vachiramon V, Kositkuljorn C, Leerunyakul K, et al. A Study of Botulinum Toxin A for Ultraviolet-Induced Hyperpigmentation: A Randomized Controlled Trial. Dermatol Surg. 2021;47(5):e17–e178.
- Carruthers J, Burgess C, Day D, et al. Consensus Recommendations for Combined Aesthetic Interventions in the Face Using Botulinum Toxin, Fillers, and Energy-Based Devices. Dermatol Surg. 2016;42(5):586–597.
- Cuerda-Galindo E, Palomar-Gallego MA, Linares-Garcíavaldecasas R. Are combined same-day treatments the future for photorejuvenation? Review of the literature on combined treatments with lasers, intense pulsed light, radiofrequency, botulinum toxin, and fillers for rejuvenation. J Cosmet Laser Ther. 2015;17(1):49–54.
- Park JY, Lin F, Suwanchinda A, et al. Customized Treatment Using Microfocused Ultrasound with Visualization for Optimized Patient Outcomes: A Review of Skin-tightening Energy Technologies and a Pan-Asian Adaptation of the Expert Panel’s Gold Standard Consensus. J Clin Aesthet Dermatol. 2021;14(5):E70–e79.
- Kerscher M, Nurrisyanti AT, Eiben-Nielson C, et al. J. Skin physiology and safety of microfocused ultrasound with visualization for improving skin laxity. Clin Cosmet Investig Dermatol. 2019;12:71–79.
- Alhaddad M, Wu DC, Bolton J, et al. A Randomized, Split-Face, Evaluator-Blind Clinical Trial Comparing Monopolar Radiofrequency Versus Microfocused Ultrasound With Visualization for Lifting and Tightening of the Face and Upper Neck. Dermatol Surg. 2019;45(1):131–139.
- Alam M, White LE, Martin N, et al. Ultrasound tightening of facial and neck skin: a rater-blinded prospective cohort study. J Am Acad Dermatol. 2010;62(2):262–269.
- Lu PH, Yang CH, Chang YC. Quantitative Analysis of Face and Neck Skin Tightening by Microfocused Ultrasound With Visualization in Asians. Dermatol Surg. 2017;43(11): 1332–1338.
- Lee HS, Jang WS, Cha YJ, et al. Multiple pass ultrasound tightening of skin laxity of the lower face and neck. Dermatol Surg. 2012;38(1):20–27.
- Werschler WP, Werschler PS. Long-term Efficacy of Micro-focused Ultrasound with Visualization for Lifting and Tightening Lax Facial and Neck Skin Using a Customized Vectoring Treatment Method. J Clin Aesthet Dermatol. 2016;9(2): 27–33.
- Dayan SH, Fabi SG, Goldman MP, et al. Prospective, Multi-Center, Pivotal Trial Evaluating the Safety and Effectiveness of Micro-Focused Ultrasound with Visualization (MFU-V) for Improvement in Lines and Wrinkles of the Décolletage. Plastic and Reconstructive Surgery. 2014;134(4S-1):123–124.
- Lee HJ, Lee KR, Park JY, et al. The efficacy and safety of intense focused ultrasound in the treatment of enlarged facial pores in Asian skin. J Dermatolog Treat. 2015;26(1):73–77.
- Lowe S. Single Treatment, Single Depth Superficial Microfocused Ultrasound with Visualization for Rhytid Improvement. Plast Reconstr Surg Glob Open. 2021;9(7):e3662–e3662.
- Vachiramon V, Namasondhi A, Anuntrangsee T, et al. A study of combined microfocused ultrasound and hyaluronic acid dermal filler in the treatment of enlarged facial pores in Asians. J Cosmet Dermatol. 2021;n/a(n/a).
- Laubach HJ, Makin IR, Barthe PG, et al. Intense focused ultrasound: evaluation of a new treatment modality for precise microcoagulation within the skin. Dermatol Surg. 2008;34(5):727–734.
- White W, Makin I, Slayton M, et al. Selective transcutaneous delivery of energy to porcine soft tissues using intense ultrasound (IUS). Lasers in Surgery and Medicine. 2008;40.
- Suh DH, Shin MK, Lee SJ, et al. Intense focused ultrasound tightening in Asian skin: clinical and pathologic results. Dermatol Surg. 2011;37(11):1595–1602.
- Berlin A, Cohen JL, Goldberg DJ. Calcium hydroxylapatite for facial rejuvenation. Semin Cutan Med Surg. 2006;25(3):132–137.
- Yutskovskaya YA, Kogan EA. Improved Neocollagenesis and Skin Mechanical Properties After Injection of Diluted Calcium Hydroxylapatite in the Neck and Décolletage: A Pilot Study. J Drugs Dermatol. 2017;16(1):68–74.
- Chao YY, Kim JW, Kim J, et al. Hyperdilution of CaHA fillers for the improvement of age and hereditary volume deficits in East Asian patients. Clin Cosmet Investig Dermatol. 2018;11:357–363.
- Casabona G, Pereira G. Microfocused Ultrasound with Visualization and Calcium Hydroxylapatite for Improving Skin Laxity and Cellulite Appearance. Plast Reconstr Surg Glob Open. 2017;5(7):e1388.
- Casabona G, Marchese P. Calcium Hydroxylapatite Combined with Microneedling and Ascorbic Acid is Effective for Treating Stretch Marks. Plast Reconstr Surg Glob Open. 2017;5(9):e1474.
- Lapatina NG, Pavlenko T. Diluted Calcium Hydroxylapatite for Skin Tightening of the Upper and Abdomen. J Drugs Dermatol. 2017;16(9):900–906.
- Rovatti PP, Pellacani G, Guida S. Hyperdiluted Calcium Hydroxylapatite 1: 2 for Mid and Lower Facial Skin Rejuvenation: Efficacy and Safety. Dermatol Surg. 2020;46(12):e112–e117.
- Amselem M. Radiesse(®): a novel rejuvenation treatment for the upper arms. Clin Cosmet Investig Dermatol. 2016;9:9–14.
- Guida S, Longhitano S, Shaniko K, et al. Hyperdiluted calcium hydroxylapatite for skin laxity and cellulite of the skin above the knee: A pilot study. Dermatologic Therapy. 2020;33(6):e14076.
- Yutskovskaya Y, Kogan E, Leshunov E. A randomized, split-face, histomorphologic study comparing a volumetric calcium hydroxylapatite and a hyaluronic acid-based dermal filler. J Drugs Dermatol. 2014;13(9):1047–1052.
- Zerbinati N, Calligaro A. Calcium hydroxylapatite treatment of human skin: evidence of collagen turnover through picrosirius red staining and circularly polarized microscopy. Clin Cosmet Investig Dermatol. 2018;11:29–35.
- Courderot-Masuyer C, Robin S, Tauzin H,et al. Evaluation of lifting and antiwrinkle effects of calcium hydroxylapatite filler. In vitro quantification of contractile forces of human wrinkle and normal aged fibroblasts treated with calcium hydroxylapatite. J Cosmet Dermatol. 2016;15(3):260–268.
- González N, Goldberg DJ. Evaluating the Effects of Injected Calcium Hydroxylapatite on Changes in Human Skin Elastin and Proteoglycan Formation. Dermatol Surg. 2019;45(4):547–551.
- Qian W, Zhang YK, Hou Y, et al. Effect analysis of intradermal hyaluronic acid injection to treat enlarged facial pores. J Cosmet Dermatol. 2018;17(4):596–599.
- Lim HK, Suh DH, Lee SJ, et al. Rejuvenation effects of hyaluronic acid injection on nasojugal groove: prospective randomized split face clinical controlled study. J Cosmet Laser Ther. 2014;16(1):32–36.
- Belmontesi M, De Angelis F, Di Gregorio C, et al. Injectable Non-Animal Stabilized Hyaluronic Acid as a Skin Quality Booster: An Expert Panel Consensus. J Drugs Dermatol. 2018;17(1): 83–88.
- Yoon JE, Kim Y, Kwon S,et al. Senescent fibroblasts drive ageing pigmentation: A potential therapeutic target for senile lentigo. Theranostics. 2018;8(17):4620–4632.
- Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol. 2013;6:295–316.
- Micheels P, Sarazin D, Tran C, et al. Effect of Different Crosslinking Technologies on Hyaluronic Acid Behavior: A Visual and Microscopic Study of Seven Hyaluronic Acid Gels. JDD. 2016;15:600–606.
- Betemps JB, Marchetti F, Lim T, et al. Projection capacity assessment of hyaluronic acid fillers. Plastic and Aesthetic Research. 2018;5:19.
- Taufig AZ, Szöke A, Kühnel W. A new strategy to detect intradermal reactions after injection of resorbable dermal fillers. J Asth Chir. 2009;2.
- Choi M-S, Kwak S, Kim J, et al. Comparative Analyses of Inflammatory Response and Tissue Integration of 14 Hyaluronic Acid-Based Fillers in Mini Pigs. Clin Cosmet Investig Dermatol. 2021;14:765–778.
- Tran C, Carraux P, Micheels P, et al. In vivo bio-integration of three hyaluronic acid fillers in human skin: a histological study. Dermatology. 2014;228(1):47–54.
- Lim T, Frank K, Hadjab B. Target-Specific Sandwich Technique: Facial rejuvenation leveraging CPM technology. J Cosmet Dermatol. 2021.
- Rho NK, Chang YY, Chao YY, et al. Consensus Recommendations for Optimal Augmentation of the Asian Face with Hyaluronic Acid and Calcium Hydroxylapatite Fillers. Plast Reconstr Surg. 2015;136(5):940–956.
- van Loghem J, Sattler S, Casabona G, et al. Consensus on the Use of Hyaluronic Acid Fillers from the Cohesive Polydensified Matrix Range: Best Practice in Specific Facial Indications. Clin Cosmet Investig Dermatol. 2021;14:1175–1199.
- Luque A, Rojas AP, Ortiz-Florez A, et al. Botulinum Toxin: An Effective Treatment for Flushing and Persistent Erythema in Rosacea. J Clin Aesthet Dermatol. 2021;14(3):42–45.
- Sayed KS, Hegazy R, Gawdat HI, et al. The efficacy of intradermal injections of botulinum toxin in the management of enlarged facial pores and seborrhea: a split face-controlled study. J Dermatolog Treat. 2020:1–7.
- Shuo L, Ting Y, KeLun W, et al. Efficacy and possible mechanisms of botulinum toxin treatment of oily skin. J Cosmet Dermatol. 2019;18(2):451–457.
- Wanitphakdeedecha R, Ungaksornpairote C, Kaewkes A, et al. The comparison between intradermal injection of abobotulinumtoxinA and normal saline for face-lifting: a split-face randomized controlled trial. J Cosmet Dermatol. 2016;15(4):452–457.
- Bloom BS, Payongayong L, Mourin A, et al. Impact of intradermal abobotulinumtoxinA on facial erythema of rosacea. Dermatol Surg. 2015;41 Suppl 1:S9–16.
- Li ZJ, Park SB, Sohn KC, et al. Regulation of lipid production by acetylcholine signalling in human sebaceous glands. Journal of Dermatological Science. 2013;72(2):116–122.
- Roh M, Han M, Kim D, et al. Sebum output as a factor contributing to the size of facial pores. British Journal of Dermatology. 2006;155(5):890–894.
- Shah AR. Use of intradermal botulinum toxin to reduce sebum production and facial pore size JDD. 2008;7(9):847–850.
- Kim YS, Hong ES, Kim HS. Botulinum Toxin in the Field of Dermatology: Novel Indications. Toxins (Basel). 2017;9(12).
- Carmichael NME, Dostrovsky JO, Charlton MP. Peptide-mediated transdermal delivery of botulinum neurotoxin type A reduces neurogenic inflammation in the skin. PAIN®. 2010;149(2):316–324.
- Petchngaovilai C. Midface lifting with botulinum toxin: intradermal technique. J Cosmet Dermatol. 2009;8(4):312–316.
- Lee SK. Multiple intradermal small bolus injection of botulinum toxin: the limit and the potentiality. J Cosmet Laser Ther. 2012;14(6):304–306.
- Dayan SH, Ashourian N, Cho K. A Pilot, Double-Blind, Placebo-Controlled Study to Assess the Efficacy and Safety of IncobotulinumtoxinA Injections in the Treatment of Rosacea. J Drugs Dermatol. 2017;16(6):549–554.
- Wanitphakdeedecha R, Kaewkes A, Ungaksornpairote C, et al. The effect of botulinum toxin type A in different dilution on the contraction of fibroblast-In vitro study. J Cosmet Dermatol. 2019;18(5):1215–1223.
- Malissen B, Tamoutounour S, Henri S. The origins and functions of dendritic cells and macrophages in the skin. Nat Rev Immunol. 2014;14(6):417–428.
- Zhang L, Wang W, Wang S. Effect of vaccine administration modality on immunogenicity and efficacy. Expert Rev Vaccines. 2015;14(11):1509–1523.
- Kerscher M, Wanitphakdeedecha R, Trindade de Almeida A, et al. IncobotulinumtoxinA: A Highly Purified and Precisely Manufactured Botulinum Neurotoxin Type A. J Drugs Dermatol. 2019;18(1):52–57.
- Park J-Y, Sunga O, Wanitphakdeedecha R, et al. Neurotoxin Impurities: A Review of Threats to Efficacy. Plastic and Reconstructive Surgery – Global Open. 2020;8(1):e2627.
- Dressler D. Five-year experience with incobotulinumtoxinA (Xeomin®): the first botulinum toxin drug free of complexing proteins. Eur J Neurol. 2012;19(3):385–389.

