 J Clin Aesthet Dermatol. 2023;16(1):14-17.
J Clin Aesthet Dermatol. 2023;16(1):14-17.
by Karen Konkel, MD; Melissa Reyes, MD, MPH, FAAD; Ida-Lina Diak, PharmD, MS;
Kelly Cao, PharmD; and Lynda McCulley, PharmD, BCPS
Drs. Konkel, Reyes, Diak, and McCulley are with the Division of Pharmacovigilance, Office of Surveillance and Epidemiology at the FDA Center for Drug Evaluation and Research in Silver Spring, Maryland. Dr. Cao was with the Office of Unapproved Drugs and Labeling Compliance, and the Office of Compliance at the FDA Center for Drug Evaluation and Research in Silver Spring, Maryland, at the time the work was conducted.
ABSTRACT: Objective. We sought to describe skin injuries associated with unapproved topical mole and skin tag removers containing concentrated salicylic acid, Sanguinaria canadensis, or other caustic agents.
Methods. We identified skin injuries associated with unapproved non-device topical mole and skin tag removers reported to the US Food and Drug Administration (FDA) through October 30, 2021 or described in Amazon consumer product reviews between 2019 and 2021.
Results. We identified 38 cases, including 30 from Amazon consumer product reviews and eight reported to the FDA. Twenty-eight were from 2021. The most common reason for use was for mole and/or skin tag removal. Listed ingredients included salicylic acid, Sanguinaria canadensis, botanicals (includes homeopathic products), and calcium oxide. Seven cases involved products without ingredients listed. Adverse events included burns, pain, and ulceration, some resulting in permanent scarring and disfigurement. There were 14 facial injuries, including four adjacent to the eye. Reported treatments included antibiotics, hospital care, wound care, and dermatology advice to have a skin graft.
Limitations. Limitations include underreporting of adverse events to the FDA, limited clinical details and potential bias in consumer reviews, and poor replicability of review searches due to the dynamic nature of the Amazon website.
Conclusion. Unapproved, non-device topical mole and skin tag removers are associated with serious skin injuries. We found Amazon consumer reviews to be a novel and useful data source for safety surveillance of these types of skin products. When dermatologists are consulted about skin injuries, exposure to these products should be considered in the differential diagnosis.
Keywords. Acrochordon, adverse drug reactions, black salve, chemical burn, diagnostic self-evaluation, drug-related side effects, mole, mole remover, mole removal, nevus, nonprescription, over the counter (OTC), salicylic acid, Sanguinaria canadensis, self-medication, skin injury, skin tag, skin tag remover, skin tag removal, topical product, unapproved
The American Academy of Dermatology (AAD) advises the public to “never try to remove a mole at home” due to risks of scarring, infection, and the possibility of malignancy in the mole of concern.1 There are no FDA-approved drugs indicated to treat moles or skin tags. The FDA has warned against using over the counter (OTC) products marketed for self-removal of moles or skin tags and has also issued warning letters to firms who sold such products.2–5 Awareness of these products will allow dermatologists to counsel patients to avoid them and seek appropriate interventions instead.
A recent publication described skin injury related to a salicylic acid (SA)-containing mole and skin tag remover product.6 Two additional published case reports described scarring following the use of an unapproved mole and skin tag remover containing cashew and fig, which may cause skin irritation, and greater celandine, a plant containing the escharotic alkaloid sanguinarine, also found in Sanguinaria canadensis.7,8 There are also case reports describing harm from S. canadensis, including cosmetic disfigurement, and death due to progression of inadequately treated malignancy.9,10
The FDA has received cases from consumers who reported purchasing mole and skin tag remover products from Amazon.com. Ingredients included concentrated SA 25%. They applied the product to lesions on the face, neck, torso, and buttocks as directed and sustained burns with resulting scars. These cases prompted our further review of this issue.
SA is a keratolytic at concentrations between 3%–6%, becoming more destructive at higher concentrations.11 The FDA allows inclusion of SA in topical OTC drug products marketed pursuant to OTC Drug Monographs at concentrations ranging from 0.5%–40%, depending on the indications, which include acne (0.5%–2%), psoriasis (1.8%–3%), and hyperkeratotic conditions like corns, calluses, and warts (5%–40%).12 The OTC Monograph for wart remover drug products requires inclusion of the following warning in the Drug Facts Label: “Do not use on moles, birthmarks, warts with hair growing from them, genital warts, or warts on the face or mucous membranes.”12
Online consumer reviews are a potential data source for pharmacovigilance. For example, there are publications that used Amazon.com online consumer reviews to identify adverse events associated with dietary supplements and to determine consumer perceptions about skin products.13,14 We found no articles utilizing Amazon.com consumer reviews to detect adverse events associated with skin products. We hypothesized that Amazon.com consumer reviews might augment adverse event reports submitted to FDA. We searched for reviews on other large online retailer websites, but none had the quantity and depth of information that was available on Amazon.com.
Methods
We searched FDA’s Adverse Event Reporting System (FAERS) and Center for Food Safety and Applied Nutrition Adverse Event Reporting System (CAERS) through October 30, 2021, for skin injuries related to products with names containing “mole” or “skin tag”. We also searched and found many products on Amazon.com with “mole remover”, “mole corrector”, or “skin tag remover” in the name. Products selected from Amazon.com were those with at least one hundred consumer reviews and that included images and/or a clear description of the skin injury. From reviews posted between 2019 and 2021, representative cases depicting skin injury were chosen for inclusion. We reviewed product information provided by sellers to identify purported ingredients and product treatment claims. We excluded cases from all sources that did not describe a skin injury or that were associated with a device (e.g., cryotherapy device).
Results
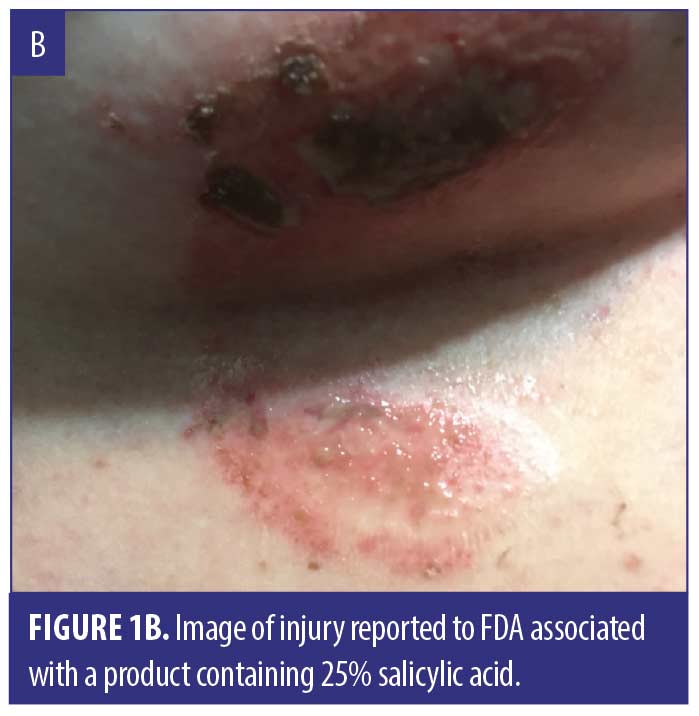
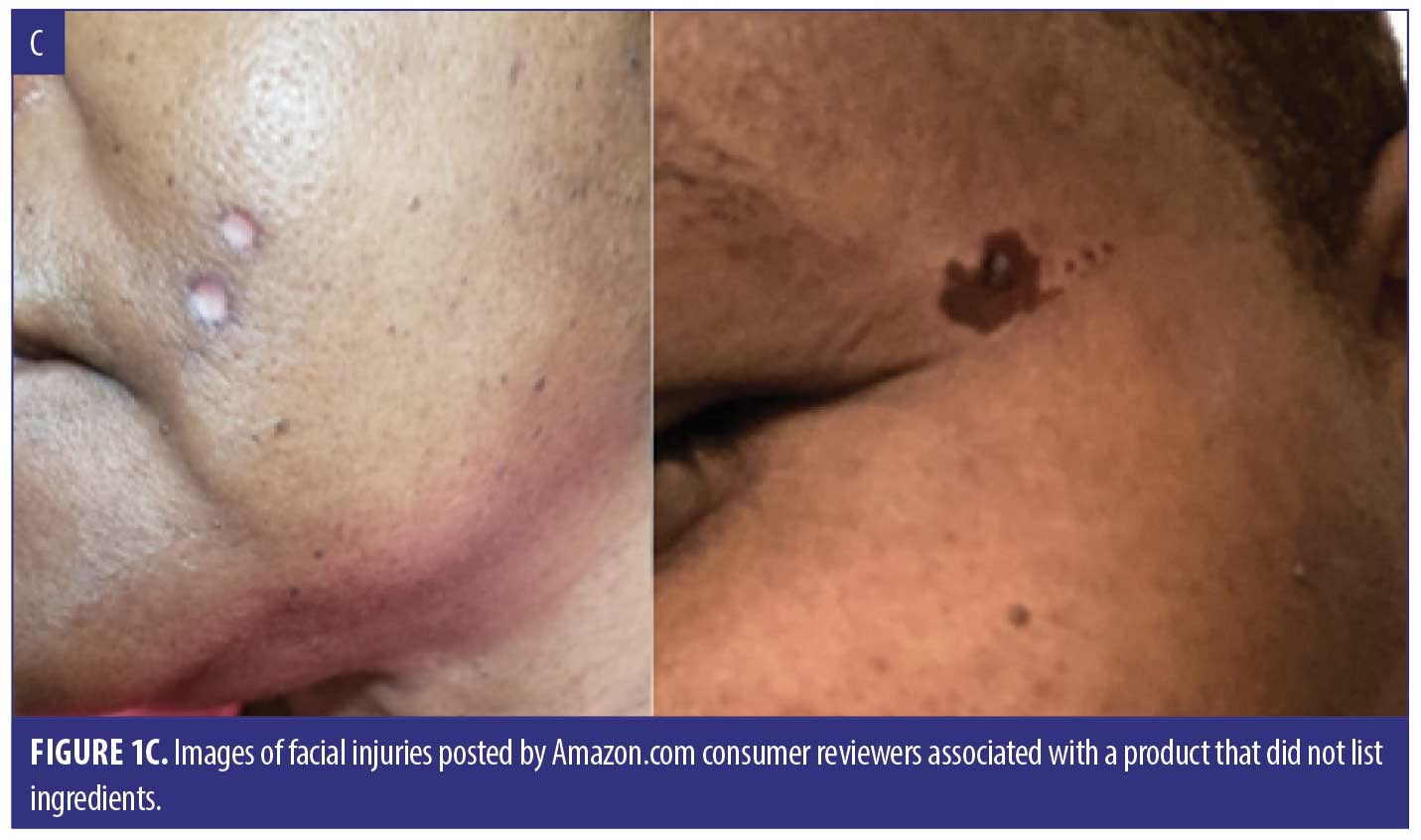
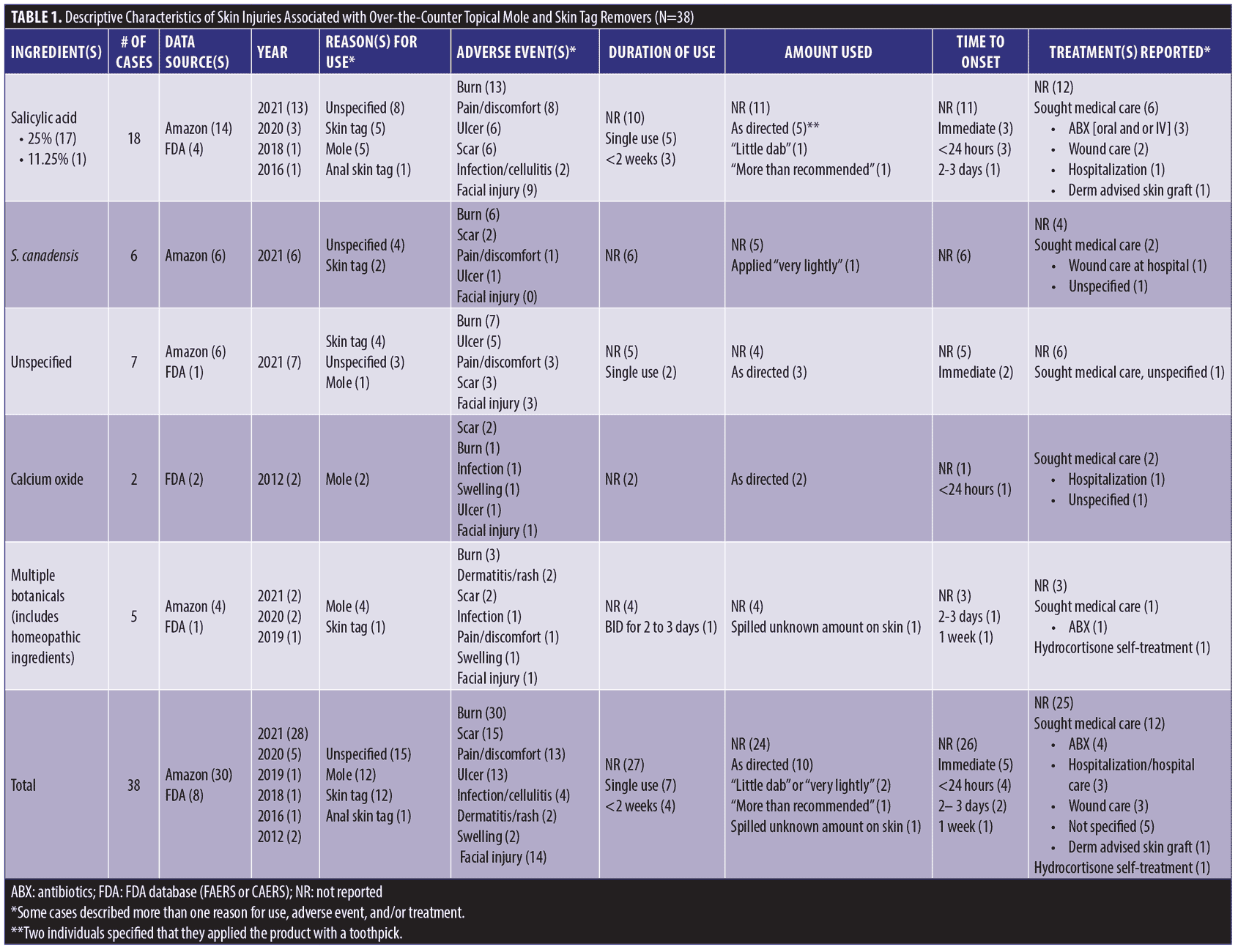
We identified 38 cases summarized in Table 1, including 30 (sex/age uncertain) from Amazon.com and 8 (4 females, 3 males, 1 not reported; median age 51 years) reported to the FDA. Listed ingredients included SA (18), S. canadensis (6), multiple botanical or “homeopathic” ingredients (5), calcium oxide (2), and unspecified ingredients (7). Reasons for use included moles (12), skin tags (12), anal skin tag (1), and unspecified lesions (15). Adverse events (not mutually exclusive) included burn (30), scar (15), pain/discomfort (13), ulcer (13), infection/cellulitis (4), dermatitis/rash (2), and swelling (2). Fourteen injuries involved the face, including four adjacent to the eye. Seven consumers developed skin injury following a single application, which in several instances was described as a small amount, including two who applied the product with a toothpick. When reported, time to symptom onset ranged from immediate (5) to within 24 hours (4), to 2 to 3 days (2) to one week (1). Twelve cases stated or implied that they sought medical care. Several required antibiotics (4), wound care (3), and/or hospital care (3). A dermatologist advised one person to have a skin graft. Figure 1 depicts representative injuries.
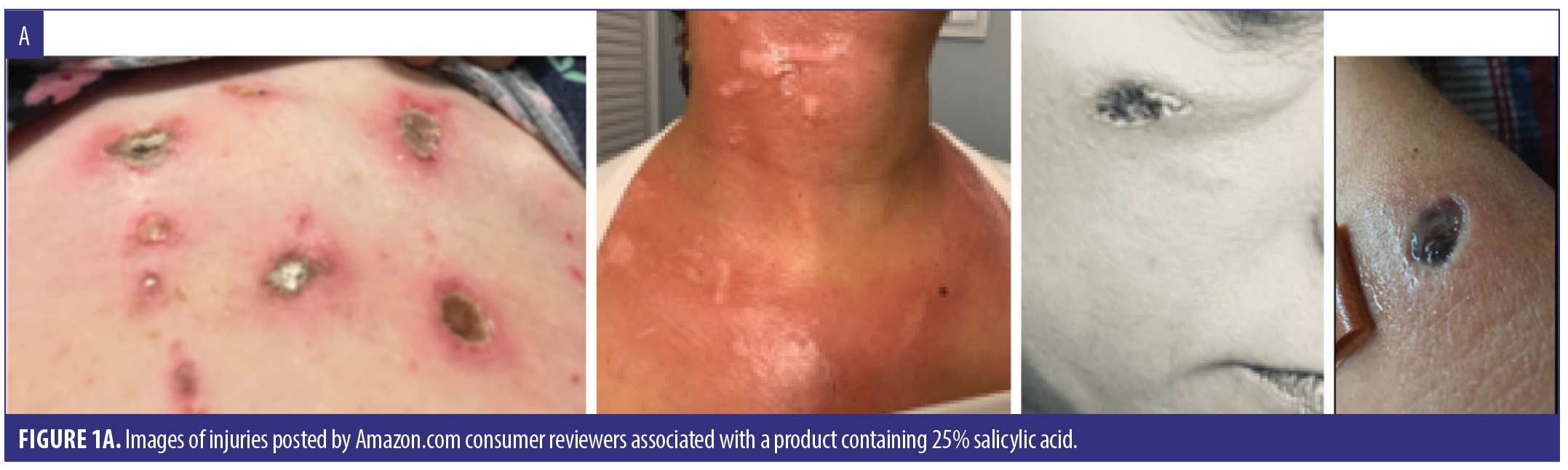
Discussion
Traditional data sources used by the FDA to identify drug safety issues include spontaneous reporting databases like FAERS and case reports in the medical literature. Amazon consumer reviews are a novel data source that allowed detection of skin injuries associated with unapproved mole and skin tag removers. Many reviews were posted in 2021, indicating recent and ongoing product use. Suspect products frequently contained concentrated SA, and reported injuries are consistent with SA’s capacity to destroy cutaneous epithelium. However, some injuries were associated with products listing multiple botanical ingredients (including homeopathics) or no ingredients at all.
We made several observations about product packages and descriptions. Similar appearing products are marketed under many names, commonly sold as a kit containing a bottle labeled as mole and skin tag remover, a second labeled as repair lotion, and directions to apply the product with included toothpick-like applicators. Accompanying images often show facial lesions before and after product use, and some explicitly state that the product is safe to use on the face. This is misleading because less prominent information on some packages states that it should not be applied to facial lesions and that it can be caustic. Product descriptions include terms such as “gentle”, “safe”, “natural”, “homeopathic”, or “plant based”; however, some ingredients are irritant botanicals (e.g., S. canadensis, greater celandine, cashew plant).7 The products may also include undeclared caustic substances.
Limitations. Our analysis has limitations. First, manufacturers of unapproved products likely fail to report adverse events to FDA despite a requirement to do so; therefore, underreporting is likely. In our case series, seven of eight FDA reports of skin injury were submitted directly by consumers, and only one was submitted by a manufacturer. Second, it is not possible to obtain additional details from Amazon.com consumer reviewers or to identify factors that might confound the adverse event. Third, consumer reviews may be biased or edited. Some explicitly state that the seller offered incentives for positive reviews or revision of negative reviews. Finally, the dynamic nature of the website limits replicability.
Unapproved mole and skin tag removers are easily accessible online, and sellers claim that they are useful for a range of skin lesions. Photographs and descriptions posted directly by consumers demonstrate serious skin injuries, some of which appeared perilously close to the eye. Although we did not capture any consumer reviews that described using a product on a potentially malignant lesion, associated tissue injury could potentially interfere with or delay a diagnosis of skin cancer.
We have found that exploring Amazon’s online consumer product reviews for adverse drug events is a useful pharmacovigilance tool that can augment and provide context when combined with traditional data sources.
Conclusion
Unapproved topical mole and skin tag removers may be caustic and cause serious skin injuries. The images identified in our series, including those from Amazon.com consumer reviews, demonstrate similar skin injuries across various products. Dermatologists may be consulted about acute ulcers or disfiguring scars, and exposure to these products should be considered in the differential diagnosis for such skin findings.
References
- American Academy of Dermatology Association. Moles: Diagnosis and Treatment. [2022; May 23, 2022]. Available from: https://www.aad.org/public/diseases/a-z/moles-treatment.
- US Food and Drug Administration. Warning Letter Oneness Labs MARCS-CMS 608918 — OCTOBER 06, 2020. [October 13, 2020; May 23, 2022]. Available from: https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/oneness-labs-608918-10062020
- US Food and Drug Administration. Warning Letter Haloderm, Inc. MARCS-CMS 608852 — OCTOBER 06, 2020. [October 13, 2020; May 23, 2022]. Available from: https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/haloderm-inc-608852-10062020
- US Food and Drug Administration. Nature Relief is Conducting a Voluntary Recall of Nature Relief Instant Wart and Mole Remover- For Immediate Release June 22,2011. [February 13, 2014; May 23, 2022]. Available from: https://wayback.archive-it.org/7993/20170406112945/https://www.fda.gov/Safety/Recalls/ArchiveRecalls/2013/ucm260817.htm
- US Food and Drug Administration. Do Not Use: Black Salve is Dangerous and Called by Many Names. [October 13, 2020; May 23, 2022]. Available from: https://www.fda.gov/consumers/consumer-updates/do-not-use-black-salve-dangerous-and-called-many-names
- Fisher MH, Hill MK, Hugh J. Necrotic Ulcerations After the Use of an Over-the-counter Mole and Skin Tag Removal Product. Cutis. 2022;109(2):E27–E28.
- Clayton R, Turner R. Cosmetic surgery: who needs surgeons when you’ve got creams? Br J Dermatol. 2007 Jun;156(6):1383–1384. eng. Epub 2007/04/27.
- Hilton S, Reinerth G, Heise H, et al. Hypopigmented scar formation after application of over-the-counter wart and mole removal cream. Wiener Klinische Wochenschrift. 2011;123(5-6):183–185. English.
- Thambi L, Konkel K, Diak IL, et al. Cosmetic disfigurement from black salve. Drugs Ther Perspect. 2020 Nov;36(11). eng. Epub 2021/01/29.
- Duncanson A, Kopstein M, Skopit S. Advanced Presentation of Homeopathically treated Giant Melanoma: A Case Report. J Clin Aesthet Dermatol. 2019;12(1):28–31.
- Micromedex® (electronic version). Greenwood Village, Colorado, USA: IBM Watson Health; June 16. Available from: https://www.micromedexsolutions.com/.
- US Food and Drug Administration. OTC Monographs at FDA. [April 8, 2022]. Available from: https://www.accessdata.fda.gov/scripts/cder/omuf/.
- Sullivan R, Sarker A, O’Connor K, et al. Finding Potentially Unsafe Nutritional Supplements From User Reviews With Topic Modeling [Article]. Pacific Symposium on Biocomputing Pacific Symposium on Biocomputing. 2016;21:528–539. English.
- Kwa M, Xu S, Agarwal A, et al. Sunscreens Consumers love-product performance, product variability, and drivers of satisfaction [Conference Abstract]. Journal of the American Academy of Dermatology. 2017;76(6):AB243. English.

