 J Clin Aesthet Dermatol. 2023;16(5):43-46.
J Clin Aesthet Dermatol. 2023;16(5):43-46.
by Eman M. Sanad, MD; Mays Ibrahim, MBBCh; Maha Rachwan, MD; and Ghada M. Shams, MD
Dr. Sanad and Dr. Shams are with the Department of Dermatology, Venereology and Andrology, and Faculty of Medicine at Benha University, in Banha, Egypt. Mr. Ibrahim is with the Faculty of Medicine at Benha University in Banha, Egypt. Dr. Rachwan is with the Department of Clinical and Chemical Pathology, Faculty of Medicine at Benha University in Banha, Egypt.
FUNDING: No funding was provided for this article
DISCLOSURES: The authors report no conflicts on interest relevant to the content of this article.
ABSTRACT: Objective. Pityriasis rosea (PR) is a self-limiting acute rash with unclear etiology and pathogenesis. The cytokine profile of PR is an infrequently investigated field of research. The aim of this study was to assess the level of IL-36 in sera of patients with PR and its possible interrelation with disease severity.
Methods. Forty patients with PR were included in this case-control study, and 40 comparable healthy control subjects. Severity was assessed using pityriasis rosea severity score (PRSS) and serum IL-36 was assessed using ELISA.
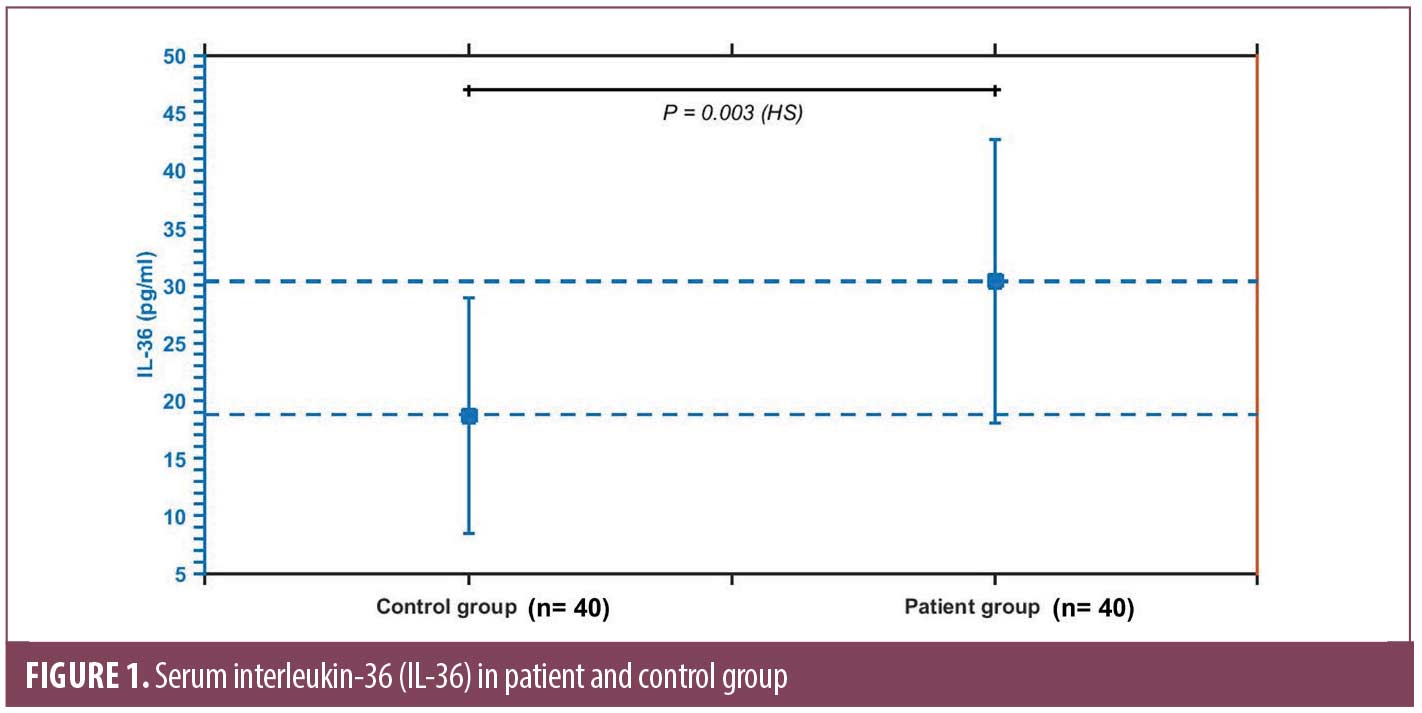
Results. Serum IL-36 was significantly higher in patients (30.36±12.35) pg/mL compared to control subjects (18.76±10.24) pg/mL (P=0.003). It correlates positively with severity as assessed by PRSS (r= 627, P= 0.003). Patients who reported a history of COVID-19 had significantly higher levels of IL-36 (32.66±11.79) pg/mL compared to those who have not (17.33±2.08) pg/mL (P= 0.000).
Conclusion. Serum IL-36 could be considered a potential biomarker for pityriasis rosea that correlates with the disease severity.
Keywords: Pityriasis rosea, Interleukin-36, COVID-19
Pityriasis rosea (PR) is a distinctive skin rash that primarily affects young adults, with an acute, usually self-limiting course, yet unclear etiopathogenesis1. Among multiple suggested etiological factors, infectious agents are accused, particularly viruses.2 The role of HHV6 was first suggested in 1997,3 but since then, no definite data has emerged. With the emergence of the SARS-CoV-2 (COVID-19) pandemic, pityriasiform eruptions have been observed and the possible viral role was once again proposed.4 Some cases were also linked to COVID-19 vaccination.5
Interleukin 36 (IL-36) is a proinflammatory cytokine that is thought to coordinate innate and adaptive immune responses. IL-36 receptor agonists stimulate the efflux of pro-inflammatory mediators such as cytokines and chemokines in keratinocytes, and phagocytic cells.6 Disturbed expression of IL-36 was suggested in inflammatory dermatoses, with psoriasis being the most abundantly studied condition.7 A crucial role of IL-36 was also reported in microbial diseases, in both in-vivo and in-vitro laboratory work various bacteria and fungi were able to induce IL-36 cytokines in lung tissue. Viral infections can also alter serum IL-36 levels.8–10
Growing evidence suggests that IL-36 may be considered a double-edged sword that defends and clear pathogens while amplifying an immune reaction that causes marked tissue damage.11 SARS-CoV-2 infection in endothelial cells is capable of triggering IL-36 efflux, resulting in leukocyte infiltration, which may be -in part- responsible for skin symptoms reported in COVID-19 patients.12 We investigated levels of IL-36 in sera of patients with PR in order to elucidate the etiological and pathological pathway of the disease and contribute more information to the possible link with COVID-19 infection, if any.
Methods
This case control study included 40 patients clinically diagnosed with typical PR with varying degrees of severity and 40 healthy age- and sex-matched participants as a control group. Subjects were selected from those attending the Outpatient Clinic of the Dermatology, Venereology and Andrology Department, Benha University Hospital within six months during the period from September 2021 to March 2022. The local ethical committee approved the scheme of this study (MS: 30-2-2020) in accordance with the Helsinki Declaration. Informed consents were obtained from participating subjects.
Participants with other dermatological conditions or psychiatric disorders were excluded from the study. Detailed clinical history was obtained from each patient, including history of PR rash, systemic illnesses, and medications. Thorough general and dermatological examination was performed. Severity of PR was evaluated using Pityriasis Rosea Severity Score (PRSS) which was developed based on the Psoriasis Area Severity Score (PASI).13
Pruritus was evaluated by each patient on the 12- Item Pruritus Severity Scale (12- PSS) developed by Reich and colleagues.14 The scale utilizes 12 questions with score ranged from 3 to 22 points. A score of 3 to 6 points denoted mild pruritus, 7 to 11 for moderate pruritus, and severe pruritus was considered at a score of 12 to 22 points.
Laboratory investigations. A 5-mL blood sample was obtained from each subject by venipuncture. A 1.5-hour solid phase IL-36 ELISA kit (Human Interleukin 36 ELISA Kit, Catalog # MBS755062) designed for the quantitative determination of human IL-36 was used according to the manufacture instructions.
Statistical analysis. Data were collected, revised, coded, and introduced into the IBM SPSS version 20 of Statistical Package for Social Science (SPSS Inc., Chicago, Illinois). The Chi-square test was utilized to compare two groups with qualitative data, and the Fisher exact test was used instead when the expected count in any cell was less than five. The Independent t-test was used to compare two independent groups with quantitative data and parametric distribution. The confidence interval was set to 95%, and the acceptable margin of error was set to 5%. As a result, the p-value was deemed significant when p≤0.05.
Results
The mean age of patient group (n=40) was 24.35±6.04 years, and of control group (n=40) was 23.35±5.42 years. Forty percent (n=16) of patients were females, while 45 percent of control subjects were females. Patient and control subjects were age- and sex- matched (P= 0.438 and 0.651 respectively). Respiratory tract infection was reported as a precipitating factor in 45 percent (n=14) of patients, wearing new clothes in 30 percent (n= 12), psychological factors in 20 percent (n=8), pregnancy in 10 percent (n= 4) and following insect bite in 5 percent (n=2) of patients. Similar attacks were reported in 60 percent (n=24) of patients, while family history was positive only in two cases (5%).
All patients (n= 40) reported sudden onset. There were 22 (55.0%) patients with regressive course, 10 (25.0%) patients with stationary course, and there were eight (20.0%) patients with progressive course.
The site of herald patches was back and thigh (20%), trunk (10%) and 5 percent for each of the following: abdomen, below knee, chest, face, knee, loin, neck, and upper limb. The mean size of patches were 3.62±0.81cm. The duration between herald patch and emergence of generalized eruption ranged from 7 to 16 days (mean 10.90 ±2.71 days). The mean PRSS was 20.98±8.08.
Pruritus severity was mild in half of the patients (n= 20), moderate in 5 percent of patients (n= 2), severe in 5 percent (n= 2), while 40 percent of patients (n=16) reported no itching. Two patients (5.0%) reported history of COVID-19 infection prior to skin rash, and two patients (5.0%) reported appearance of rash following COVID-19 vaccination.
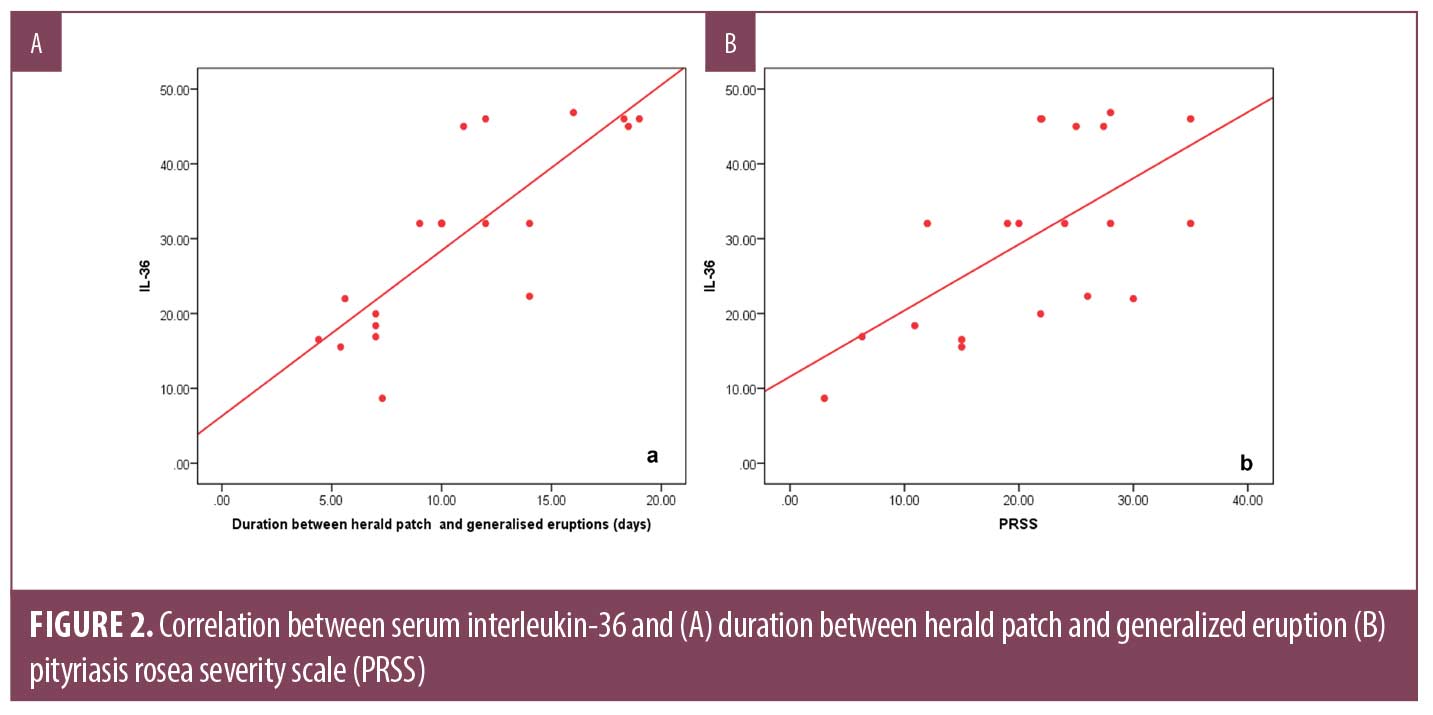
A significantly higher serum level of IL-36 was found in PR patients (30.36±12.35) pg/mL compared to control subjects (18.76±10.24) pg/mL (P=0.003) (Figure 1). Serum IL-36 correlates positively with the duration between herald patch appearance and the emergence of generalized eruption (r=0.838, P=0.000), and the PRSS (r=627, P=0.003) (Figure 2), while no significant correlation was demonstrated with age of patients (r= 0.299, P=0.201) or size of herald patch (r=0.046, P=0.857).
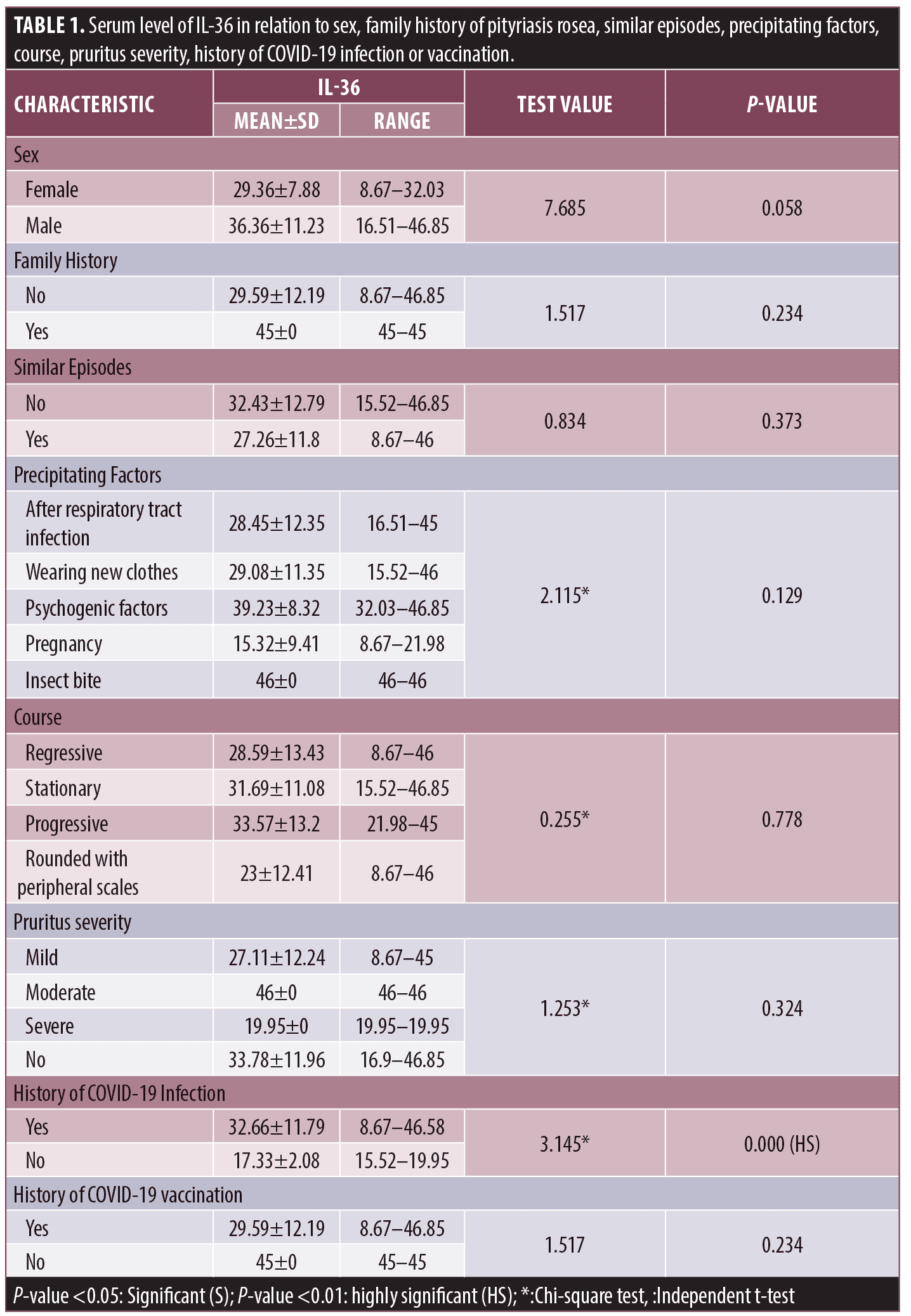
Among other clinical variables, IL-36 failed to show significant relation to sex of patients, family history of PR, similar episodes, precipitating factors, course, or pruritus severity. A significantly higher serum level was detected in PR patients with history of COVID-19 infection (32.66±11.79) pg/mL compared to those without (17.33±2.08) pg/mL (P=0.000) (Figure 3), while no significant change in IL-36 serum level in relation to COVID-19 vaccination (Table 1).
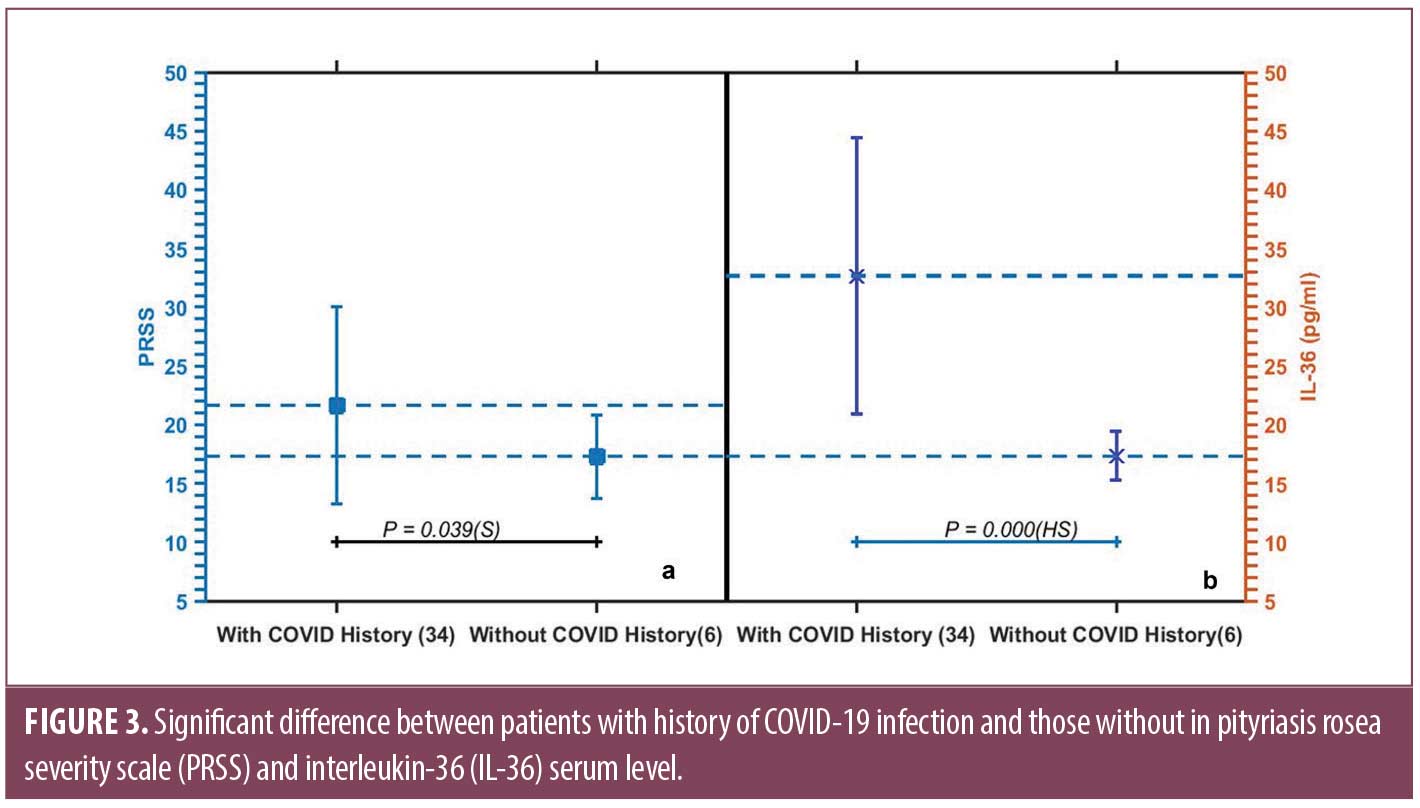
Of note, PRSS was significantly higher in patients with COVID-19 infection history (21.64±8.40) than those without (17.30±3.56) (P=0.039) (Figure 3).
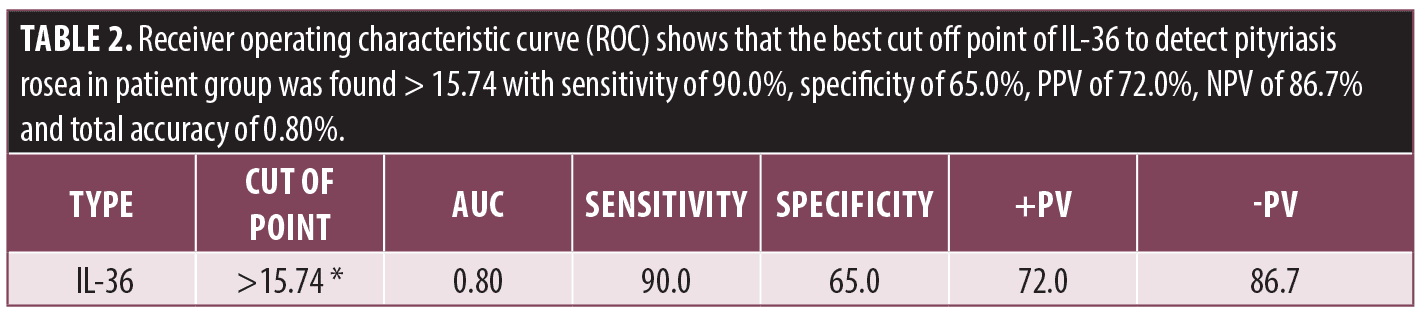
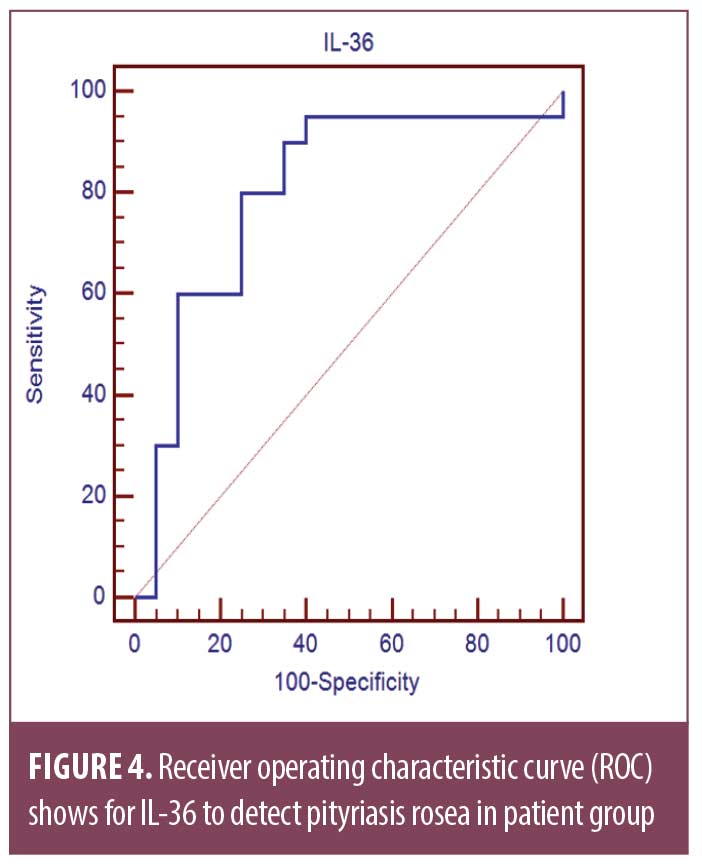
Receiver operating characteristic curve (ROC) shows the best cut off point of IL-36 to detect PR in patient group was found to be greater than 15.74 with sensitivity of 90.0 percent, specificity of 65.0 percent, PPV of 72.0 percent, NPV of 86.7 percent, and total accuracy of 0.80 percent (Table 2, Figure 4).
Discussion
Though PR is among the most common skin eruptions, the exact etiology remains undetermined with unclear cytokine profile. We investigated the serum level of IL-36 in patients with pityriasis rosea, and relation to different clinical variables including severity of disease and any accused precipitating factors.
The results of current work revealed a significantly increased serum IL-36 level in patients with PR compared to matched healthy control subjects. Moreover, the serum level correlates positively with the severity of the disease as assessed by PRSS. No correlations were found with age nor sex of the patient.
The maximum IL-36 activities are most frequently encountered at barrier sites, particularly skin, namely keratinocyte in epidermal layer, suggesting a role of IL-36 -as a pro-inflammatory cytokine- in defending the body from external attackers with a potential antimicrobial function.6 IL-36 signalling does seem to be activator of keratinocytes, antigen presenting cells (APCs), and T-cell proliferation.15 Wang and colleagues reported IL-36 regulated immune response to viral infections in mouse and human keratinocytes.16
This may explain the increased serum level in our patients than comparable controls, particularly if infectious agent is suspected as a triggering factor. We detected a significantly higher level of IL-36 in patients of PR with history of COVID-19 infection (P= 0.000) -but not with vaccination- and more severe disease as assessed by PRSS (P= 0.039) despite the history of COVID-19 infection was only reported in 5 percent of patients (n=2). SARS-CoV-2 infection in endothelial cells has been suggested to trigger IL-36 efflux, resulting in leukocyte infiltration and skin symptoms in COVID-19 patients.17 IL-36 upregulated expression of angiotensin-converting-enzyme-2 (ACE2) in keratinocytes which may be responsible for exaggerated COVID-19-associated dermatoses.12
Considering that IL-36 is a member of the IL-1 superfamily, it has been suggested as an important regulator of host defence response against micro-pathogens, not only in humans, but within different other species.18 Liu and colleagues reported increased predominant IL-36 upon exposure of skin to bacteria namely Staphylococcus aureus.19 Research also investigated the pro-inflammatory role of IL-36 in cutaneous viral infections, namely herpes simplex virus (HSV), an increased IL-36 levels was demonstrated in HSV-infected skin of mice and humans.20
Accumulating evidence suggested IL-36 as a potential biomarker for inflammatory skin conditions, namely psoriasis.21 The IL-17/IL-23 axis is thought to regulate IL-36 cytokines during the course of psoriasis.22
Apart from psoriasis, data regarding investigating IL-36 in other inflammatory skin diseases seem contradictory. Quaranta et al23 concluded increased IL-36 in psoriatic not atopic dermatitis lesions, while Suárez-Fariñas and colleagues reported increased IL-36 levels in atopic dermatitis lesions compared to non-lesional skin.24 IL-36 was also investigated in allergic contact dermatitis25 and in some blistering diseases including bullous pemphigoid and dermatitis herpetiformis.26
Scant literature is found when it comes to the role of cytokines in PR pathogenesis. To the best of our knowledge, we are the first to investigate the level of IL-36 in sera of patients with PR.
The relatively small sample size, lack of assessment of history of COVID-19 infection, and vaccination in control subjects, and lack of assessment of therapy effect on serum IL-36 levels limits the generalizability of the results of this work.
Conclusion
Serum level of IL-36 in patients with PR could be considered a potential biomarker for disease activity. Further research is needed to elucidate the link between PR and COVID-19 infection, if any.
References
- Mahajan K, Relhan V, Relhan AK, et al. Pityriasis Rosea: An Update on Etiopathogenesis and Management of Difficult Aspects. Indian J Dermatol. 2016 Jul-Aug;61(4):375–384.
- Chuh A, Chan H, Zawar V. Pityriasis rosea–evidence for and against an infectious aetiology. Epidemiol Infect. 2004 Jun;132(3):381–390.
- Drago F, Ranieri E, Malaguti F, et al. Human herpesvirus 7 in patients with pityriasis rosea. Electron microscopy investigations and polymerase chain reaction in mononuclear cells, plasma and skin. Dermatology. 1997;195(4):374–378.
- Veraldi S, Romagnuolo M, Benzecry V. Pityriasis rosea-like eruption revealing COVID-19. Australas J Dermatol. 2021 May;62(2):e333–e334.
- Khattab E, Christaki E, Pitsios C. Pityriasis Rosea Induced by COVID-19 Vaccination. Eur J Case Rep Intern Med. 2022 Feb 3;9(2):003164.
- Buhl AL, Wenzel J. Interleukin-36 in Infectious and Inflammatory Skin Diseases. Front Immunol. 2019 May 24;10:1162.
- Towne JE, Sims JE. IL-36 in psoriasis. Curr Opin Pharmacol. 2012 Aug;12(4):486–490.
- Vos JB, van Sterkenburg MA, Rabe KF, et al. Transcriptional response of bronchial epithelial cells to Pseudomonas aeruginosa: identification of early mediators of host defense. Physiol Genomics. 2005 May 11;21(3):324–336.
- Gresnigt MS, Rösler B, Jacobs CW, et al. The IL-36 receptor pathway regulates Aspergillus fumigatus-induced Th1 and Th17 responses. Eur J Immunol. 2013 Feb;43(2):416–426.
- Gong Y, Tingxi Z, Qing L, et al. Elevated production of IL-36α in chronic hepatitis B virus-infected patients correlates with viral load. Microb Pathog. 2017 Dec;113:412–415.
- Wein AN, Dunbar PR, McMaster SR, et al. IL-36γ Protects against Severe Influenza Infection by Promoting Lung Alveolar Macrophage Survival and Limiting Viral Replication. J Immunol. 2018 Jul 15;201(2):573–582.
- Wang X, Yi P, Liang Y. The Role of IL-36 in Infectious Diseases: Potential Target for COVID-19? Front Immunol. 2021 May 13;12:662266.
- Leenutaphong V, Jiamton S. UVB phototherapy for pityriasis rosea: a bilateral comparison study. J Am Acad Dermatol. 1995 Dec;33(6):996–999.
- Reich A, Bożek A, Janiszewska K, et al. 12-Item Pruritus Severity Scale: Development and Validation of New Itch Severity Questionnaire. Biomed Res Int. 2017;2017:3896423.
- Foster AM, Baliwag J, Chen CS, et al. IL-36 promotes myeloid cell infiltration, activation, and inflammatory activity in skin. J Immunol. 2014 Jun 15;192(12):6053–6061.
- Wang P, Gamero AM, Jensen LE. IL-36 promotes anti-viral immunity by boosting sensitivity to IFN-α/β in IRF1 dependent and independent manners. Nat Commun. 2019 Oct 16;10(1):4700.
- Xue X, Mi Z, Wang Z, et al. High Expression of ACE2 on Keratinocytes Reveals Skin as a Potential Target for SARS-CoV-2. J Invest Dermatol. 2021 Jan;141(1):206-209.e1.
- Jensen LE. Interleukin-36 cytokines may overcome microbial immune evasion strategies that inhibit interleukin-1 family signaling. Sci Signal. 2017 Aug 15;10(492):eaan3589.
- Liu H, Archer NK, Dillen CA, et al. Staphylococcus aureus Epicutaneous Exposure Drives Skin Inflammation via IL-36-Mediated T Cell Responses. Cell Host Microbe. 2017 Nov 8;22(5):653–666.e5.
- Milora KA, Uppalapati SR, Sanmiguel JC, et al. Interleukin-36β provides protection against HSV-1 infection, but does not modulate initiation of adaptive immune responses. Sci Rep. 2017 Jul 19;7(1):5799.
- D’Erme AM, Wilsmann-Theis D, Wagenpfeil J, Hölzel M, et al. IL-36γ (IL-1F9) is a biomarker for psoriasis skin lesions. J Invest Dermatol. 2015 Apr;135(4):1025–1032.
- Carrier Y, Ma HL, Ramon HE, et al. Inter-regulation of Th17 cytokines and the IL-36 cytokines in vitro and in vivo: implications in psoriasis pathogenesis. J Invest Dermatol. 2011 Dec;131(12):2428–2437.
- Quaranta M, Knapp B, Garzorz N, et al. Intraindividual genome expression analysis reveals a specific molecular signature of psoriasis and eczema. Sci Transl Med. 2014 Jul 9;6(244):244ra90.
- Suárez-Fariñas M, Ungar B, Correa da Rosa J, et al. RNA sequencing atopic dermatitis transcriptome profiling provides insights into novel disease mechanisms with potential therapeutic implications. J Allergy Clin Immunol. 2015 May;135(5):1218–1227.
- Mattii M, Ayala F, Balato N, et al. The balance between pro- and anti-inflammatory cytokines is crucial in human allergic contact dermatitis pathogenesis: the role of IL-1 family members. Exp Dermatol. 2013 Dec;22(12):813–819.
- Żebrowska A, Woźniacka A, Juczyńska K, et al. Correlation between IL36α and IL17 and Activity of the Disease in Selected Autoimmune Blistering Diseases. Mediators Inflamm. 2017;2017:8980534.

