Innovations in Psoriasis Management

Based on Selected Presentations from
Music City SCALE Symposium for Cosmetic Advances & Laser Education
May 9–11, 2019, Nashville, Tennessee
 by Jo Ann LeQuang
by Jo Ann LeQuang
Owner, LeQ Medical, Angleton, Texas; Director of Scientific Communications, NEMA Research, Inc., Naples, Florida; Founding Director, No Baby Blisters, Colorado Springs, Colorado.
J Clin Aesthet Dermatol 2019;12(12):S8–S19
Based on presentations by
 Adelaide A. Hebert, MD
Adelaide A. Hebert, MD
Professor, Department of Dermatology; Director, Pediatric Dermatology—University of Texas Health Science Center, Houston, Texas

Leon Kircik, MD
Clinical Associate Professor, Icahn School of Medicine, Mount Sinai, New York; Clinical Associate Professor, Indiana Medical Center, Indianapolis; Medical Director, Physicians Skin Care, PLLC and DermResearch, PLLC, Louisville, Kentucky

George Martin, MD
Dr. George Martin Dermatology Associates, Khei, Maui, Hawaii
 Alan Menter, MD
Alan Menter, MD
Dermatologist, Chief, Division of Dermatology, Baylor Scott & White; Clinical Professor of Periodontics, Baylor College of Dentistry; Clinical Professor of Dermatology, University of Texas Southwestern Medical Center, Dallas, Texas
 Jami L. Miller, MD
Jami L. Miller, MD
Assistant Professor of Medicine, Department of Medicine, Division of Dermatology—Vanderbilt University Medical Center, Nashville, Tennessee
Funding: This supplement was funded by Celgene Corporation, Summit, New Jersey.
Disclosures: Dr. Gold is the co-organizer of Music City SCALE and also has performed clinical research for Celgene.Dr. Herbert has received honoraria from Ortho Dermatologics, GSK, Mayne Pharma, and Amgen. Additionally, the University of Texas Health Science Center McGovern Medical School has received grants and funds from Amgen, Promius, Pharma, GSK, Mayne Pharma, Symbio, and Leo Pharma. Dr. Kircik has served as an investigator, consultant, and/or advisory board member for Amgen, BMS, Abbvie, Celgene, Lilly, Novartis, Mayne Pharma, Ortho Dermatologic, Sun, and Pfizer. Ms. LeQuang has no conflicts of interest relative to the content of this article. Dr. Martin has served as a speaker, consultant, and/or advisory board member for Aclaris, Aqua, Celgene, DUSA/Sun Pharma, Janssen, Pfizer, Ortho/Valeant, UCB, Aclaris, Celgene, Pfizer, and UCB. Dr. Mentor has no conflicts of interest relative to the content of this article. Dr. Miller is on the Menlos Pharmaceuticals Ad Board.
A Message from the SCALE Co-Organizers
 Michael H. Gold, MD
Michael H. Gold, MD
Program Co-Organizer, Music City SCALE meeting; Medical Director, Gold Skin Care Center and Tennessee Clinical Research Center; Assistant Clinical Professor, Department of Medicine, Division of Dermatology, Vanderbilt Univerisity School of Medicine, Nashville, Tennessee
 Brian S. Biesman, MD, FACS
Brian S. Biesman, MD, FACS
Program Co-Organizer, Music City SCALE meeting; Clinical Assistant Professor, Ophthalmology, Dermatology, and Otolaryngology-Head and Neck Surgery, Vanderbilt University Medical Center, Nashville; Clinical Associate Professor, University of Tennessee Health Sciences Center, Memphis, Tennessee; Brian S. Biesman, MD, FACS private practice, Nashville, Tennessee
Dear Colleagues:
Mark your calendars! Next summer, July 22–25, 2020, we will present the 15th Anniversary Edition of the Music City SCALE (Society for Cosmetic Advances and Laser Education) event, one of the leading medical seminars in the United States. SCALE embraces everything that is innovative and exciting in clinical, aesthetic, and cosmetic dermatology and offers something unique to everyone. Perhaps that is why attendance and industry support has continued to grow each year—the SCALE 2019 meeting hosted more than 1,000 participants and over 135 vendors, and we expect even more attendees and exhibitors at the 2020 meeting.
We have brainstormed together with Ms. Karen Dennis and her Meeting Designs team to develop an innovative, engaging agenda for SCALE 2020, featuring top-notch faculty who are leaders in their fields. Our medical dermatology sessions will highlight the latest treatment options in acne and rosacea, atopic dermatitis, psoriasis, hidradenitis, and many other challenging dermatological conditions, as well as explore cutting edge therapeutic concepts in dermatology that are changing lives, including the latest treatments in skin cancer. On the aesthetic side, we are proud to offer presentations and demonstrations by the experts in laser and energy-based devices (EBD) and injectables, from whom participants will learn cutting edge techniques and technologies in the cosmetic and aesthetic realm of dermatology. And for the best tips on business efficiency—something that is important to all of us—attendees won’t want to miss our excellent practice management sessions.
For this special supplement to the Journal of Clinical and Aesthetic Dermatology, we hand-picked pertinent SCALE 2019 presentations by experts in the field of psoriasis and summarized them just for you. Here, you’ll read about the latest in psoriasis research and clinical practice, including new and emerging drugs and topical therapies, treatment techniques, disease management in the pediatric population, cardiovascular concerns, biologics in pregnancy and lactation, and more. We hope you enjoy this special supplement on psoriasis, which is just a sample of the many exciting learning opportunities attendees of the SCALE meeting experience each year.
The Music City SCALE faculty is the best of the best. After participating in these lectures and demonstrations, you will return home with new, innovative, and refreshing ideas to improve your practices and optimize your patient outcomes. So join us next summer for 15th Anniversary Edition of the Music City SCALE meeting in Nashville—America’s “IT” city. You will love our meeting…and the country music’s not so bad either!
With regards,
Michael H. Gold, MD and Brian S. Biesman, MD, FACS
Introduction
This year’s Symposium for Cosmetic Advances and Laser Education (SCALE) was held May 9 to 11, 2019, in Nashville, Tennessee. SCALE is a multidisciplinary meeting for aesthetic and medical dermatology healthcare clinicians and researchers. Psoriasis was one of many dermatological conditions covered during the 2019 symposium. This article summarizes selected presentations by experts in the field, highlighting the latest research, therapeutic updates, and new paradigms for patient care in psoriasis diagnosis and treatment.
New Drugs for Psoriatic Arthritis
Based on a presentation by Alan Menter, MD—Dermatologist, Chief, Division of Dermatology, Baylor Scott & White; Clinical Professor of Periodontics, Baylor College of Dentistry; Clinical Professor of Dermatology, University of Texas Southwestern Medical Center, Dallas
The American College of Rheumatology (ACR) and the National Psoriasis Foundation (NPF) have updated their 2018 guidelines for the treatment of PsA. The goal of this guidance is to better equip healthcare providers and their patients with tools to make individualized treatment decisions over the trajectory of PsA.28 Dermatologists, perhaps more than other specialists, should take the lead in the early diagnosis and treatment of PsA, since dermatologists frequently see patients at known risk for PsA. The joint damage characteristics of PsA might take a decade or more to develop, but are irreversible. Meanwhile, such patients might seek treatment for cutaneous psoriasis and present dactylitis, enthesitis, or other visible signs suggestive of PsA. Early signs of PsA might be visible, particularly in the Achilles tendon and the hands.
While the prevalence of PsA is about 0.3% to 1.0% of the general population, PsA occurs in about 30 percent of patients with cutaneous psoriasis.29 Conversely, the prevalence of psoriasis among patients with other forms of arthritis is about 2 to 3 percent.30 While there are many clinical presentations, skin psoriasis generally precedes PsA by 5 or 10 years in about 80 percent of patients, but about 11 to 15 percent of patients develop both skin psoriasis and PsA concurrently. In a small number of patients, PsA can precede cutaneous psoriasis by a decade or more.30 Clinical patterns have been observed to be distal predominant, oligoarticular asymmetrical, polyarticular (similar to rheumatoid arthritis [RA]), spondylitis, and arthritis multilans.30 In some cases, it can be difficult to differentiate RA from PsA (Table 1). When differentiating PsA from osteoarthritis (OA), a very common form of rheumatic disease, it is important to evaluate the distal interphalangeal joints, which are typically involved in OA but not PsA. OA often involves the carpometacarpal thumb joint. In OA, joints tend to swell and be hard and bony, rather than inflamed and tender as seen in PsA. Over time, OA leads to cartilage loss, which narrows joint spacing.
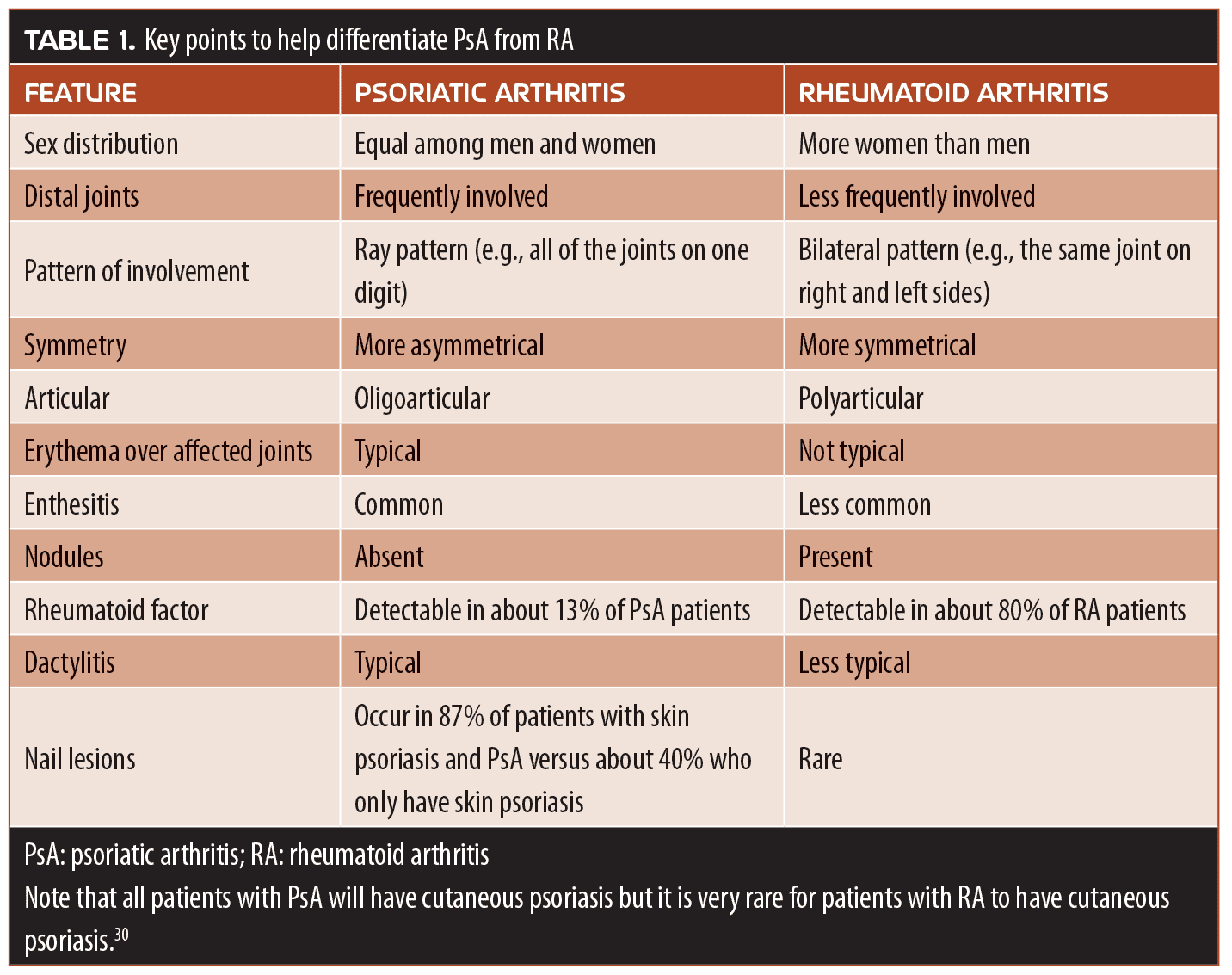
The differential diagnosis of PsA involves ruling out gout, which occurs when urate or calcium pyrophosphate crystals build up in the synovial fluids. Tophi or crystalline uric acid on the joint surfaces (or on the skin) is characteristic of gout, as are gouty erosions on the skin. Ankylosing spondylitis (AS) is part of the psoriasis disease family and is more prevalent in men than women. It typically involves the axial skeleton (sacroiliitis). The HLA-B27 blood test might be helpful in diagnosis. “Bamboo spine,” the radiological image of vertebral body fusion, is a hallmark of AS. Flexibility testing, such as Schober’s test of lower-back flexion, might also be helpful in diagnosing AS. Reactive arthritis can also develop, such as lupus, which follows 1 to 4 weeks after certain infections (e.g., salmonella, shigella, chlamydia). Keratoderma blennorhagicum is another form of reactive arthritis. Classic arthritic symptoms, urethritis, and conjunctivitis are the triad of symptoms associated with reactive arthritis. Finally, it is important when diagnosing PsA to rule out the possibility of a form of arthritis that is actually related to inflammatory bowel syndrome, Crohn’s disease, or ulcerative colitis. Patients with such forms of arthritis might find their joint disease exacerbated if treated with IL-17 inhibitors. Fibromyalgia is a painful condition characterized by diffuse, widespread pain and tenderness. The differential diagnosis of fibromyalgia can be challenging.
Since many patients with undiagnosed PsA already consult dermatologists regularly about their cutaneous symptoms, dermatologists have an excellent opportunity for early diagnosis and intervention of PsA. Since PsA is a destructive and progressive disorder, this early intervention can play a crucial role in improving outcomes. As it advances, PsA can adversely affect a patient’s health-related quality of life and contribute to painful symptoms, functional deficits, and disability. PsA can cause obvious signs in a patient, which can lead to embarrassment and concern; it is rarely discussed, but it has been argued that PsA can distort a patient’s healthy body image and create an emotional burden.31
Once PsA is diagnosed, patients can be treated with pharmacological and nonpharmacological therapies. The most recommended nonpharmacological treatments for PsA include joint protection, exercise, weight loss, and stress reduction. In some cases, patients might benefit from physical or occupational therapy. Obese and morbidly obese patients might be recommended for bariatric surgery. Orthotics can be helpful to reduce back pain. While physical therapy and exercise are often recommended for PsA patients, there are no large randomized clinical trials that offer evidence for the benefits of physical exercise in managing PsA.
Pharmacological treatments include nonsteroidal anti-inflammatory drugs (NSAIDs) for mild inflammation and the mild-to-moderate pain associated with mild joint involvement. Leflunomide is an antimetabolite that can be effective against joint disease and might even provide modest benefits for cutaneous psoriasis. While corticosteroids (i.e., intramuscular, intravenous, oral) are not recommended for the treatment of cutaneous psoriasis, they might be helpful to treat PsA. A dose of up to 7.5mg of prednisone can relieve joint pain without affecting the skin disease; prednisone doses should be kept low. While methotrexate and cyclosporine are sometimes prescribed for the treatment of PsA, there is no evidence that they slow the radiologic progression of PsA. The small molecule apremilast has demonstrated moderate effectiveness against PsA. The role of tofacitinib, a Janus kinase (JAK) inhibitor, is being evaluated.32 The pegylated TNF-alpha inhibitor CZP has been available for several years for treating PsA, and its indications have recently been expanded to cutaneous psoriasis. CZP demonstrated durable effectiveness in PsA. As a class, TNF-alpha inhibitors are approximately equally effective in treating PsA but have different degrees of effectiveness in skin psoriasis because the pathogenesis of skin psoriasis differs from that of PsA.
To date, no IL-23 inhibitor, such as tildrakizumab or guselkumab, has been approved for the treatment of PsA, but studies are ongoing. IL-17 inhibitors have been shown to be effective in the treatment of PsA. For example, brodalumab was significantly more effective than placebo in improving the symptoms of PsA in 168 patients with serious adverse events occurring in three percent and two percent of brodalumab and placebo patients, respectively, at 12 weeks.33 The FUTURE-2 study was a placebo-controlled Phase III study of secukinumab for patients with PsA. Patients were randomized to receive secukinumab 300mg, 150mg, or 75mg a day or placebo with the endpoint of an ACR20 score at 24 weeks. ACR20 was achieved by 54 percent of patients in the 300mg group, 51 percent in the 150mg group, and 29 percent in the 75mg group at 24 weeks, compared to 15 percent of patients taking the placebo.34 Ixekizumab was evaluated for PsA treatment in the SPIRIT-P1 placebo-controlled trial, which reported significantly more patients treated with ixekizumab achieved ACR20 response at 24 weeks than placebo (62.1% for ixekizumab 80mg every two weeks and 57.9% for ixekizumab 40mg every two weeks) versus 30.2 percent with placebo. All ixekizumab patients began with a loading dose of 160mg at baseline.35 This study used adalimumab as an active comparator, which showed good results against PsA compared to placebo. These results were for biologic-naïve patients, but PsA patients who previously failed TNF inhibitors showed a good response to ixekizumab as well.36
Key points. The intersection between dermatology and rheumatology has emerged as an important point in patient care. Patients might have better outcomes and higher quality of life if dermatologists diligently evaluate them for PsA and refer them faster. Any patient with cutaneous psoriasis can be considered to be at an elevated risk for PsA. Patients with cutaneous psoriasis should be examined and interviewed about possible PsA symptoms. Patient education about PsA can be helpful. For example, a patient can exhibit no obvious clinical signs of PsA but report that they experience morning stiffness upon arising for more than 30 minutes. A few minutes of stiff joints upon arising is not unusual in older patients, but benign symptoms resolve quickly, usually in five minutes or so. X-rays can be a helpful diagnostic tool if PsA is suspected. When PsA is suspected, the dermatologist might wish to consult with a rheumatologist or to refer the patient to a rheumatologist. When a PsA patient gets early, aggressive, evidence-based treatment, their PsA symptoms can resolve more rapidly and more completely than symptoms of cutaneous psoriasis.
An interesting question for us and future generations of dermatologists will be where PsA fits in when it comes to various medical specialties. Psoriasis has multiple comorbidities—should PsA count as one of them? Does it “belong” to the specialty of dermatology or should PsA routinely be a matter for rheumatologists? Do dermatologists in general feel equipped to manage PsA? Regardless of how this question is resolved in coming years, dermatologists should ask patients with cutaneous psoriasis about possible PsA symptoms and examine them for signs of the disease, knowing that about one third of patients with psoriasis will develop PsA. Early diagnosis and early intervention can help prevent, or at least delay, irreversible joint damage.
Pediatric Psoriasis
Based on a presentation by Adelaide A. Hebert, MD— Professor, Department Of Dermatology;
Director, Pediatric Dermatology—University of Texas Health Science Center, Houston
About one third of adults with psoriasis experienced the onset of their disease in childhood.37 While no pediatric guidelines were available at the time Dr. Hebert’s lecture was given, the American Academy of Dermatology–National Psoriasis Foundation (AAD–NPF) guidelines on pediatric psoriasis were made available on the AAD website (https://www.aad.org/member/clinical-quality/guidelines/psoriasis) in November of 2019 and will be published in the JAAD. Pediatric psoriasis is a prevalent condition that presents diagnostic and treatment challenges. There are no specific guidelines for the treatment of pediatric psoriasis38 and limited pharmacological options are available for this population.39 In the US, there can also be reimbursement impediments for the treatment of pediatric psoriasis.
An epidemiological study of pediatric psoriasis using a database of 1.3 million nonselected individuals reported a psoriasis prevalence of 0.71 percent in people younger than 18 years of age. However, patients with psoriasis under the age of 20 years had double the rate of psoriasis-associated comorbidities compared to people over the age of 20 years.40 In some cases, psoriasis might be evident at birth, although it is rarely diagnosed in neonates. Despite the fact that pediatric psoriasis is prevalent, it is often misdiagnosed as eczema. The clinical presentation of pediatric psoriasis can differ from that of adults, such as in terms of pruritus and in the distribution and morphology of lesions.38 Pediatric psoriasis tends to appear as plaque psoriasis. PsA can also occur in children. The signs and symptoms of pediatric PsA tend to appear between the ages of two and three years and then again between 10 and 12 years of age. It might be misdiagnosed as juvenile idiopathic arthritis. Dactylitis is a common finding for pediatric PsA. As opposed to adults who develop cutaneous psoriasis before PsA, 80 percent of pediatric PsA patients develop PsA two or three years before they have symptoms of cutaneous psoriasis.41 Overall, the prognosis for pediatric PsA patients is good with early diagnosis and treatment.
Genetic risk factors and potential enivronmental triggers for psoriasis are starting to be elucidated. The HLA-Cw6 gene, in particular, has been associated with early-onset psoriasis.42 The human leukocyte antigen Cw0602 is a gene linked to guttate psoriasis and PsA.43 Thus, family history might be important in the diagnosis of pediatric psoriasis.
Most pediatric psoriasis presents as a mild form of the disease that can be managed with topical products. In the US, pharmacological therapies approved for the treatment of pediatric psoriasis include etanercept (four years and older), ustekinumab (12 years and older), and calcipotriene/betamethasone (for scalp psoriasis, 12 years and older). A prospective study of 72 pediatric patients with psoriasis found an ointment of calcipotriol 50ug and betamethasone dipropionate 0.5mg decreased PASI scores by 15.4 percent at 12 weeks and 17.3 percent at 24 weeks, with no serious TEAEs.44 Of course, biologics have not been exhaustively studied in pediatric patients. A randomized double-blind trial of 211 pediatric patients (ages 4 to 17 years) with moderate-to-severe plaque psoriasis found 57 percent achieved PASI75 at 12 weeks versus 11 percent of placebo patients (p<0.001).45
Pediatric psoriasis is associated with comorbid conditions, which have been presented in a screening guideline.41 These comorbidities include obesity, type 2 diabetes mellitus, hyperlipidemia, hypertension, nonalcoholic fatty liver disease, polycystic ovary syndrome, gastrointestinal disorders, PsA, uveitis, mood disorders, and substance abuse. Even for children, it can be helpful to recommend lifestyle changes to reduce these comorbid conditions.
Obesity may be the most frequently observed comorbidity associated with pediatric psoriasis.46 The prevalence of childhood obesity is on the rise in many parts of the world but it is not clear as to whether childhood obesity predisposes a child to psoriasis or merely affects the severity of an appearance of the disease that would have occurred anyway.47 A pilot study of 37 pediatric patients with psoriasis with obesity reported that obesity preceded the onset of psoriasis symptoms in 93 percent of patients.47 Adiposity is associated with the expression of proinflammatory cytokines, so it sets up conditions conducive to psoriatic disease.
Therapeutic options for pediatric psoriasis include the education of parents and children, healthy lifestyle modifications (especially with respect to diet and exercise), and the use of selected products—namely, moisturizers, coal tar, and topical steroids. Phototherapy might be helpful for some children. Phototherapy induces a shift from Th-1 to Th-2 cells which, in turn, induces lymphocyte apoptosis and decreases IL-10 secretion and suppresses the IL-17 and IL-23 signaling pathways. Narrowband ultraviolet B phototherapy has resulted in durable improvements in PASI scores of up to 52 percent, with no serious adverse events.48 In older children, laser therapy, methotrexate, cyclosporine, topical or oral retinoids, and biologics might be considered as well. Vitamin D analogs can be used concomitantly with topical steroids, but there are no formal guidelines for this treatment currently. Localized irritation can occur. Systemic treatments might be appropriate in certain cases of pediatric psoriasis and, to date, the evidence for their application in pediatric patients shows promise in that results so far suggest these systemic treatments to be effective, tolerable, and safe in children.49 A retrospective review of pediatric psoriasis treatments found that medication-related adverse events occurred less frequently with TNF inhibitors than with methotrexate.50
Key points. Despite the formidable armamentarium of psoriasis treatments and growing knowledge of the disease, managing pediatric psoriasis remains a major challenge in dermatology. Pediatric therapeutic options typically lag behind treatments for adult patients. Diagnosis can be difficult, approved pharmacological options are limited, and reimbursement can be difficult. Pediatric psoriasis is not uncommon, although it is often misdiagnosed; the high rate of psoriasis-related comorbidities is an important consideration when treating this population.
My Approach to Psoriasis
Based on a presentation by Leon Kircik, MD— Clinical Associate Professor, Icahn School of Medicine, Mount Sinai, New York; Clinical Associate Professor, Indiana Medical Center, Indianapolis; Medical Director, Physicians Skin Care, PLLC and DermResearch, PLLC, Louisville, Kentucky
Optimal psoriasis care means addressing each patient individually and holistically. Despite these modern times, there remains a social and psychological stigma to this and other cutaneous diseases. Psoriasis is not infectious. Meeting with patients, the clinician should make efforts to reassure the patient with touch and see past the skin disorder to the person seeking treatment.
The collection of psoriasis available treatments is vast and varied, but every agent has potential risks that accompany their benefits. Topical treatments are often ineffective except for in mild cases of psoriasis, so most dermatologists will start a patient with a systemic drug. Consult the package insert for the product to learn about side effects, since these agents are associated with many and potentially serious adverse events, including malignancy, infection, and major adverse cardiovascular events. The package inserts for products are getting shorter—for instance, the adalimumab package insert is 101 pages long, while that for ixekizumab is 25 pages and that for secukinumab is down to 19 pages. This suggests that the side effects are being reduced with the newer agents. New drugs are coming to market all the time, making it challenging to keep up with the latest evidence for products that might benefit our patients. However, many of these new drugs are safer, better-tolerated, and increasingly effective.
The downside of so many new products is that we lack long-term efficacy data for many seemingly promising agents. This is improving with time. There are long-term safety data for secukinumab and ixekizumab. Ustekinumab is a great agent, but the newer agent brodalumab is even better for achieving PASI100. The proverbial elephant in the room with brodalumab is the risk of suicidality. Brodlumab has not been withdrawn from the market, but carries a black-box warning that gives many prescribers great pause. However, the issue of brodalumab-related suicidality remains to be elucidated further. Suicide is associated with depression, as is psoriasis. Many patients with psoriasis suffer comorbid depression regardless of which treatment they take for their psoriasis or if they receive no treatment at all. Three patients with psoriasis who took brodalumab died by suicide, but it remains unclear whether brodalumab exacerbated their suicidal ideation or not.6 In addition to adverse effects, prescribers should consider durable results when making prescribing choices. With newer drugs, these long-term results are not always available, but there are some data available for certain drugs, such as tildrakizumab (64 weeks).16
Biologic therapy should be ushering in a new era for patients with psoriasis, but many patients still must see multiple healthcare professionals and undergo several courses of ineffective treatment before being prescribed an effective new biologic. Further, some patients with psoriasis waste time with old drugs, such as methotrexate and cyclosporin. Even etanercept, which is a biologic, must be considered an old drug. Dermatologists should learn about and not be fearful of the newer biologics, as many of these drugs offer life-changing results.
When prescribing a biologic or systemic agent for a psoriasis patient, it is helpful to prescribe a topical as well. Even with phototherapy, topical treatment should be offered to patients. There are several reasons for this; combination therapy is effective, topicals offer symptomatic relief, and patients are reassured when they leave the clinic with a product they can apply right away to start their therapeutic regimen. Topical therapy is and will likely remain a mainstay in psoriasis care. If a patient has very little BSA involved (2%–5%), topical therapy might be all that they require. In moderate-to-severe cases, topical therapy should be part of a combination therapy approach (topical plus systemic or topical plus biologic). While patients initially like applying topical products to get started, topicals have a low adherence over time,82 patients sometimes complain they are messy and inconvenient, and topical therapy is not highly effective. In addition, there can be safety issues with the use of topical steroids. When selecting a topical product, it is important to recognize that vehicle characteristics can play an important role in how well the patient likes the product (and adheres to therapy) and therapeutic outcomes.83,84 The novel combination of tazarotene and halobetasol in a topical product has recently been approved. Tazarotene is an older, well-established drug used to treat other conditions, such as acne. The addition of the synthetic corticosteroid halobetasol makes the tazarotene better tolerated, less irritating, and possibly more effective.85,86 When using combination topical therapy, it is preferred to rely on so-called “fixed-dose” products that are already combined rather than “loose dose” products that the patient combines.
When treating patients with only 1 to 3 percent of the BSA affected, it is easy to forget the fact that these patients, like other patients with psoriasis, are still at risk for PsA. In other words, the risk for PsA is not associated with the severity of the extent of cutaneous psoriasis. Some patients with minimal cutaneous symptoms accompanied with the onset of PsA might resist aggressive treatment because their symptoms are minimal. A patient with 1-percent BSA of cutaneous psoriasis (about the size of the palm of the hand) and the start of PsA can benefit from biologics to stop PsA disease progression and joint damage. It is a common misperception that psoriasis treatment is intended only to address the visible skin symptoms, when in fact treatment can be crucial to address PsA progression. Patient education in this area can be helpful.
Dermatologists can play a leading role in the diagnosis and treatment of PsA because patients with PsA are often under the care of a dermatologist before the symptoms of PsA manifest. While any patient with cutaneous psoriasis (even mild cases) must be considered at risk for PsA, the risk increases 3.9-fold if the patient has scalp psoriasis, 2.9-fold in conjunction with nail dystrophy, and 2.4-fold if they have intergluteal and/or perianal lesions.87 Patients with psoriasis should be routinely evaluated for PsA. This includes a short interview with the patient about joint pain and stiffness and a visual examination of any tender or swollen joints, asymmetric inflammatory signs, dactylitis, and enthesitis. The Classification Criteria for PsA (CASPAR) has set up a scoring system with two points for current psoriasis and one point each for a history of psoriasis (if not current), family history of psoriasis, dactylitis, juxta-articular new bone formation, rheumatoid factor negativity, and nail dystrophy.88 PsA can be effectively treated with TNF inhibition or with the use of IL-17 blockers (e.g., secukinumab, brodalumab, ixekizumab, ustekinumab) as well as apremilast and tofacitinib, a JAK inhibitor.32 The older drug methotrexate does not address the axial symptoms or enthesitis or dactylitis.
Key points. Patients with psoriasis face a disease that can adversely affect their self image, confidence, and quality of life. Dermatologists must approach them with professional compassion and understanding and reassure them about therapeutic options available. Psoriasis care should be evidence-based, individualized to meet the patient’s needs, and holistic. It is true that the clinical management of psoriasis and related conditions, such as PsA, can be complex. The major impediments to psoriasis therapy tend to involve the patient; they include difficulties of living with any chronic disease, therapeutic adherence, the occurrence of flares, the management of comorbidities, and psychosocial issues. Health care providers must help their patients to navigate a wealth of therapeutic choices, many of which offer unprecedented effectiveness but carry notable side effects. Clinicians must see past the symptoms to offer help and guidance to the person seeking treatment. All patients with psoriasis, even those with mild and limited disease, must be considered to be at risk for PsA and should be treated aggressively to prevent progressive and irreversible joint damage.89
Cardiovascular Concerns in Psoriasis and Psoriatic arthritis
Based on a presentation by Alan Menter, MD—Dermatologist, Chief, Division of Dermatology, Baylor Scott & White; Clinical Professor of Periodontics, Baylor College of Dentistry; Clinical Professor of Dermatology, University of Texas Southwestern Medical Center in Dallas,, Texas
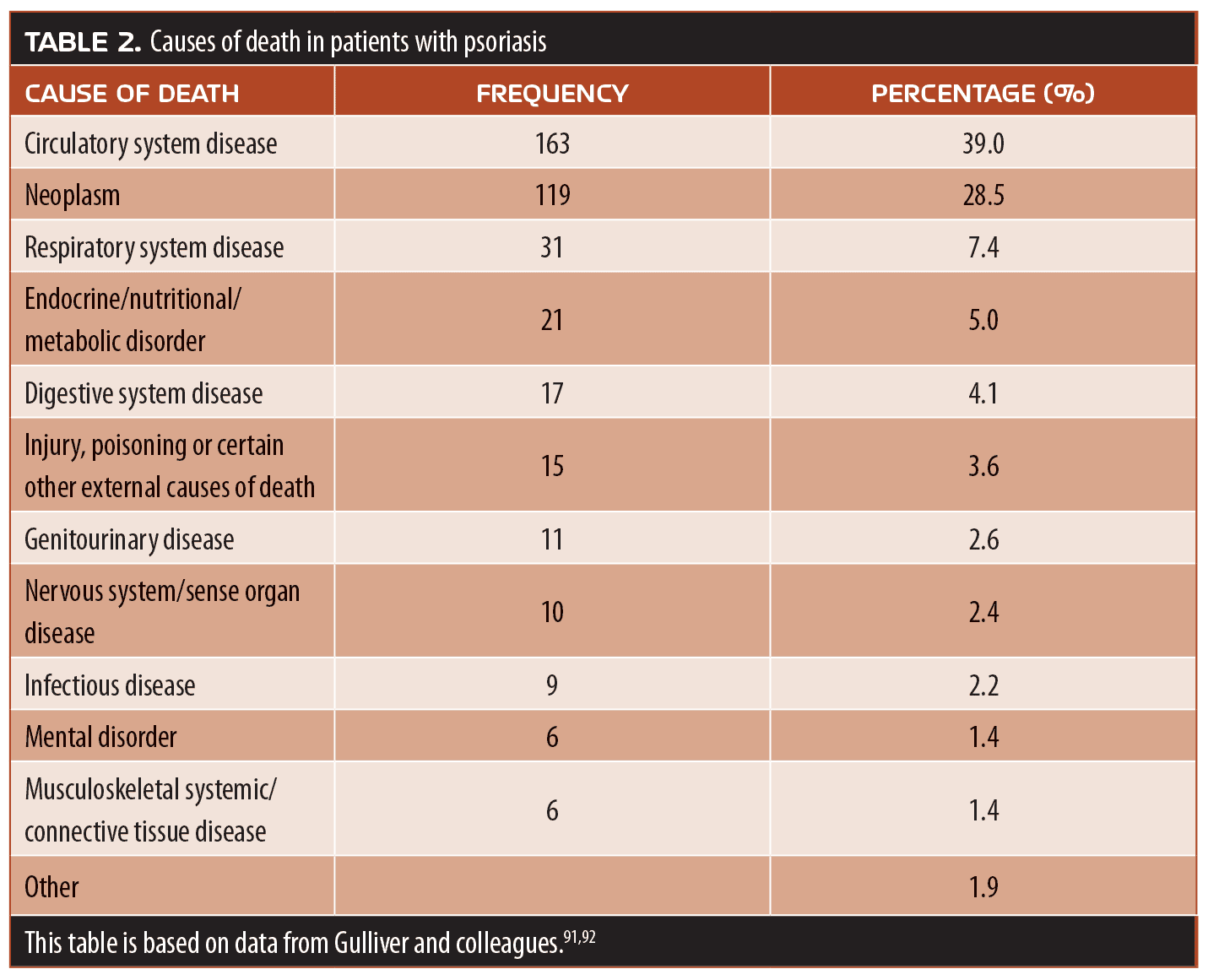
Psoriasis is not just a skin disease, but an internal disease that manifests as symptoms that appear on the skin. The American Association of Dermatology and the NPF have issued guidelines for the care of patients with psoriasis, with a special emphasis on the many and varied comorbidities associated with the condition.90 Obesity and metabolic syndrome are frequently comorbid with psoriasis. PsA is a less prevalent comorbidity, but patients with cutaneous psoriasis should be regularly screened for PsA. Immune-mediated diseases, cardiovascular conditions, and psychiatric diseases are also psoriasis comorbidities. People with psoriasis might also have comorbid sleep apnea, renal dysfunction, cancer, lymphoma, nonalcoholic steatohepatitis, and chronic obstructive pulmonary disease. Smoking and excessive alcohol consumption are personal behaviors that have been associated with psoriasis and could even be considered “comorbid” conditions.91 Finally, psoriasis is also associated with a shortened lifespan. These risks vary in prevalence and severity. For instance, many patients with psoriasis have obesity, but the cancer risk in patients with psoriasis is only slightly elevated. While the exact constellation and occurrence of comorbidities in patients with psoriasis is individual, many patients with psoriasis have more than one comorbidity, which can complicate ongoing care (Table 2).
Besides comorbid conditions, psoriasis can erode a patient’s self confidence and give him or her a distorted self image. Psoriasis and PsA can diminish the quality of life. The negative psychosocial effects of psoriasis can cause patients to feel helpless, embarrassed, and even lead to depression.31 Patients with psoriasis often require treatment to manage their comorbidities. Bariatric surgery, but not gastric banding, can improve the prognosis of psoriasis or PsA in patients with morbid obesity and psoriasis. Why weight loss can improve psoriasis (and why bariatric surgery works better than banding) has not yet been thoroughly elucidated. Patients with morbid obesity and psoriasis might be appropriate candidates to refer for bariatric procedures.93
Since many patients with psoriasis also have comorbid conditions, they often rely on polypharmacy. Care should be taken to prevent adverse drug-drug reactions and to avoid taking drugs that might exacerbate comorbid conditions. For example, methotrexate can be associated with liver problems.
By far, the comorbidities of most significant concern in the psoriatic population are cardiovascular conditions, because heart disease has high rates of morbidity and mortality. The underlying pathology of cardiovascular disease is atherosclerosis, a chronic inflammatory condition that progressively causes an accumulation of arterial plaque made of serum lipids. Psoriasis is a disease of systemic inflammation, and systemic inflammation is, in itself, a risk factor for cardiovascular disease. Atherosclerosis has been established as a precursor to coronary artery disease.94 Early cardiovascular lesions are mainly streaks of subendothelial deposits of lipids along with macrophage foam cells (cholesterol) and T-cells. Lesions build up over time, collecting cell debris and adding necrotic cells and cholesterol crystals that fibrose.95 Over time, a fibrotic cap forms and becomes inundated with activated T-cells, macrophages, and mast cells which, in turn, produce proinflammatory mediators and enzymes.96 The thickening plaque inside the vessel narrows its lumen, causing stenosis, which has the potential to lead to ischemia in the surrounding tissue. If the plaque ruptures, thrombosis can occur. The thrombus might occlude the vessel or it may detach to become an embolus, blocking blood flow at some distal point. The outcomes of such situations are potentially catastrophic, such as myocardial infarction, ischemic stroke, or another form of ischemic injury.95 Psoriasis has been described as an independent risk factor for myocardial infarction.97 A retrospective cohort study found that methotrexate therapy in 7,615 psoriasis outpatients reduced their incidence of vascular disease; it is theorized here that the anti-inflammatory effects of methotrexate reduced the rate of vascular disease.98
Cardiovascular disease is driven by powerful immunological factors and not just hyperlipidemia. In fact, many of the cytokines involved in atherosclerosis (and, by extension, cardiovascular disease) are the same ones familiar to dermatologists from psoriasis—that is, Th-1 and Th-17 cells, and regulatory T-cells (Tregs). Extravasation, the step-by-step process by which white blood cells in the bloodstream travel through the vascular endothelium into tissue, is characteristic of both heart disease and psoriasis. In a nested case-control study that evaluated six patients with psoriasis (>10% BSA) plus four matched control patients, participants underwent 18F-fluorodeoxyglucose positron-emission tomography-computed tomography (FDG-PET/CT) to examine FDG uptake in the liver, musculoskeletal system, and aorta. The study identified several foci of inflammation in the patients with psoriasis. Aortic inflammation was significantly higher in patients with psoriasis compared to controls (p<0.001).99 Separately, a prospective cohort study (n=220) reported that reductions in the severity of cutaneous psoriasis was associated with an improvement in aortic vascular inflammation as seen on FDG-PET/CT.100
In three single-center, cross-sectional studies, patients with moderate-to-severe psoriasis, but without type 2 diabetes mellitus, were compared to two other groups: patients with type 2 diabetes, but without psoriasis or other inflammatory diseases, and age-matched, sex-matched, healthy controls without psoriasis, diabetes, or other inflammatory diseases (n=387). The patients with psoriasis had cardiovascular and cardiometabolic risk factors similar to those of people with diabetes, and psoriasis was independently associated with the presence of coronary artery calcium (odds ratio: 2.35, 95% confidence interval: 1.12–4.94) in adjusted models. Thus, patients with psoriasis had a risk of coronary artery calcium similar to that of patients with type 2 diabetes.101
High levels of triglycerides, rather than just global hyperlipidemia, have been associated with coronary heart disease102 and have been demonstrated in the psoriasis population. Reducing the levels of triglycerides and low-density lipoprotein cholesterol (LDL) is associated with a reduced risk for coronary heart disease.103 Since patients with psoriasis are at particular risk for cardiovascular disease, it is important to test for hyperlipidemia and manage the condition through addressing LDL and triglyceride levels.
The risks to patients with psoriasis from comorbid conditions are not trivial. A cause-specific mortality study in patients with psoriasis or PsA from the Danish National Patient Registry reported that patients with severe psoriasis have elevated mortality compared to matched controls.104 A population-based study that included the entire population of Denmark (N=5,458,627) found the overall death rate per 1,000 patient-years was 13.8, but 17.0 and 25.4 for people with mild and severe psoriasis, respectively. Also, the age at the time of death was 76.5±14.0 years for the total population and 74.4±12.8 years and 72.0±13.4 years for those with mild and severe psoriasis, respectively. In other words, severe psoriasis shortened the lifespan by more than four years.105
Key points. Psoriasis is a systemic disease with obvious cutaneous symptoms that is often comorbid with a range of conditions, including PsA and potentially life-threatening cardiovascular conditions. Obesity is a frequently reported comorbidity in the psoriasis population and can sometimes be appropriately managed with bariatric procedures. Cardiovascular comorbidities are prevalent and of particular concern because of their potential impact on morbidity and mortality. The systemic inflammation and proinflammatory environment of psoriasis can exacerbate cardiovascular conditions. Patients with psoriasis show higher degrees of aortic inflammation compared to people without psoriasis. These risks must be recognized and managed.
Biologics in Pregnancy and Lactation
Based on a presentation by George Martin, MD—Dr. George Martin Dermatology Associates, Khei, Maui, Hawaii
Psoriasis affects about seven million Americans, with 150,000 to 260,000 new cases added every year. Of this population, about 1.5 million have moderate (3–10% BSA) to severe (>10% BSA) psoriasis.106 Psoriasis affects men and women equally and about 1.5 million psoriasis patients are likely candidates for systemic therapies. This means that many women in their childbearing years are in a position to consider systemic treatment for psoriasis.
In 2006, 49 percent of pregnancies in America were unplanned. The rate of unintended pregnancies is highest in young (18–24 years), unmarried women with a low socioeconomic status.107 In 2011, the rate of unplanned pregnancy had declined to about 45 percent, but the risk factors remained the same.108 Most women do not learn that they are pregnant until 4 to 7 weeks after conception. Many women might drink alcohol or take medications before they know they are pregnant. Even after they know they are pregnant, they might continue taking medications. In fact, the use of medicine in the first trimester of pregnancy has increased to more than 60 percent in the past 30 years and the number of pregnant women taking four or more medications has tripled.109 The first weeks of pregnancy are a critical time in fetal development. About half of all pregnant women take at least one medication during pregnancy, sometimes under medical supervision.109 Unfortunately, there are inadequate data regarding the effects of most of the medications taken during pregnancy.110
Pregnant women comprise an important but understudied patient population. There are about four million live births in America every year and about 60 million women of childbearing potential. However, the US FDA and most drug developers routinely exclude pregnant women from clinical studies.111 Women enrolled in a clinical study who become pregnant during the course of the trial are likely to be excluded. In the event that human pregnancy data are obtained for a given drug, it is typically studied after the drug is approved for the market. Data on the use of specific agents in pregnant women often come from spontaneous reports or observational studies rather than rigorously controlled clinical trials.111 Thus, there are pregnancy data for only a limited number of drugs and those data might be scant and of poor quality. There are even fewer data on breastfeeding women and infants.112 Ethical constraints limit our ability to conduct rigorous prospective studies on pregnant and nursing women, forcing clinicians to rely on limited information from inadvertent and uncontrolled exposures to certain drugs. The available data are incomplete, uncontrolled, not always accessible to clinicians, and sometimes contradictory and difficult to interpret.
In the US, between 65,000 and 107,000 women with psoriasis give birth every year and about 9,000 to 15,000 of them have moderate-to-severe psoriasis.113 The average age at which a woman is diagnosed with psoriasis is 28 years, which falls well within the childbearing years.113 Care must be taken with the use of biologics during pregnancy to treat any form of inflammatory disease, including but not limited to psoriasis. In a normal pregnancy, during the first trimester, there is a proinflammatory environment in the woman’s body caused by a proliferation of Th-1 cells associated with embryonic implantation and placental development. In the second trimester, an anti-inflammatory environment is created by Th-2 cells, which promotes rapid fetal development in the womb. The third trimester then reverts this to a proinflammatory environment with an abundance of Th-1 cytokines, TNF-alpha, and interferon-gamma. Thus, the woman’s body changes naturally from proinflammatory to anti-inflammatory and back again over the course of a normal pregnancy.
In a study comparing 47 pregnant patients with psoriasis with 27 nonpregnant, menstruating patients with psoriasis, most of the pregnant patients thought their condition improved over the course of their pregnancy (55%), while some thought it worsened (23%) and 21 percent reported no change.114 Postpartum, 65 percent of patients with psoriasis said their psoriasis got worse, nine percent reported improvements, and 26 percent stated their psoriasis was unchanged. Compared to control patients, the psoriatic BSA of pregnant patients decreased at 10 to 20 weeks of pregnancy (p<0.001) but increased six weeks after birth (p=0.001). About 40 percent of pregnant patients had a BSA of more than 10 percent and reported their psoriatic BSA decreased 83.8 percent during pregnancy. High serum levels of estrogen were associated with psoriasis improvement.114 A systematic review that included nine studies and evaluated an association between adverse pregnancy outcomes and psoriasis was equivocal.115
Immunoglobulin G (IgG) is the main source of fetal immunity. Like other antibodies, it crosses the placenta primarily during the third trimester.116 Maternal IgG increases in the fetal blood concentration starting in the second trimester and continues to increase throughout the third trimester. The mother must produce an adequate amount of IgG for an effective transfer of IgG to the fetus. IgG transfer, mediated by the Fc receptor (FcRn), crosses the placenta via the syncytiotrophoblast that covers the chorionic villi and the endothelium of fetal capillaries within the villi.117 IgG1 is the most actively transported and encompasses infliximab, adalimumab, golimumab, and ustekinumab. In a study of 80 pregnant women with inflammatory bowel disease receiving the TNF-alpha inhibitors adalimumab (n=36) or infliximab (n=44), drugs could be detected in infants up to 12 months after birth with an inverse correlation between the last exposure during pregnancy and drug concentration in the umbilical cord.118 The concentration of adalimumab or infliximab in cord blood can exceed maternal levels as much as fourfold.119 Organogenesis occurs prior to the transplacental transfer of TNF-alpha blockers, which might explain why birth defects are not frequent.
Most pregnant patients with psoriasis are very concerned about the potential for birth defects. A study from the US Centers for Disease Control and Prevention reported that about 1 in 6 children in the US is born with some form of developmental disability, including autism, attention-deficit hyperactivity disorder, and others.120 About 10 percent of birth defects can be traced back to a specific agent, biologic, or a nutritional or environmental factor. About 20 percent of birth defects, such as Down syndrome, are associated with chromosomal changes. The remainder of birth defects, about 70 percent, are of an unknown etiology.121 The US FDA pregnancy category system has been criticized, but is often the best available tool to help stratify the risk of various drugs in the pregnant population. Available guidance suggests that, among systemic agents for psoriasis, methotrexate is contraindicated in pregnancy, while biologics, such as adalimumab, alefacept, etanercept, and infliximab are associated with an “undetermined” risk.122 Topical therapies, including low-to-moderate potency steroids, are first-line treatments for pregnant and lactating women, with certain types of phototherapy considered as second-line therapy. The role of TNF-alpha inhibitors remains unclear but should be considered with caution.123
The European League Against Rheumatism (EULAR) previously issued a document based on expert consensus and a systematic review of the literature regarding points to consider when prescribing antirheumatic agents to women who are pregnant, might become pregnant, or are nursing.124 EULAR advocated for the discontinuation of methotrexate, mycophenolate, mofetil, and cyclophosphamide prior to conception, as these are known teratogenic agents. Leflunomide, tofacitinib, abatacept, rituximab, belimumab, tocilizumab, ustekinumab, and anakinra should be discontinued prior to a planned pregnancy, although the evidence is less clear about their teratogenic effects. TNF-alpha inhibitors appear to be reasonably safe for use in the first and second trimesters of pregnancy.124 It has been noted that specialists might prefer different medications; for example, primary care physicians might be more likely to discontinue a TNF-alpha blocker than gastroenterologists or dermatologists. Of course, there is a paucity of on-point literature to guide prescribing choices. A systematic review reported mixed data on TNF-alpha blockers and no data on IL-17 or IL-12/23 inhibitors. Clinicians must advise their patients and used a shared decision making paradigm to try to find a balance between the risk of treatment weighed against the risk of untreated psoriasis.125
Some data might be available in the form of registries. In 1990, the Organization of Teratology Information Services created a registry of evidence-based information of drug exposures during pregnancy, beginning initially with asthma medications.126 A prospective registry of 1,000 pregnancy outcomes in women with inflammatory bowel disease exposed to biologics or immunomodulating therapy was compiled as the PIANO registry. Women in the PIANO registry did not have significantly increased rates of underlying disease or most adverse events compared to controls, but women on TNF-alpha inhibitors had a higher incidence of cesarean sections and more of their babies stayed in the neonatal intensive care unit.127 However, infant growth rate, immune development up to one year, number of infections, and achievements of development milestones were not affected. It is not clear whether these results from inflammatory bowel disease patients are generalizable to psoriasis patients. In general, patients with inflammatory bowel disease do not discontinue their drug therapy during pregnancy.
These registries have highlighted certain important unmet needs in this field. Teratogenic effects are not well-studied and many of their mechanisms are not elucidated. Advances in reproductive technology have increased birth defects; it is not known if specific medications might have an amplified teratogenic effect in this population. Likewise, the role of genetics in terms of risk stratification for teratogenic drugs has not been well studied. It is not known if any drugs (and which ones) can have different risks and benefits depending on the trimester of the pregnancy during which they are taken. Most of the limited information available is based on oral or injected medications; far less is understood about transdermal or airborne exposures to teratogenic substances. Finally, there is very little clinical training done in understanding and identifying teratogenic substances.128 In the event that a woman with psoriasis is to be continued on biologic therapy during pregnancy, it is reasonable to consider whether one biologic might be safer than others. Biologics are transported over the placenta by the Fc receptor, but CZP does not cross the placenta.129 Babies born to mothers taking CZP have undetectable serum levels of the drug at four and eight weeks after birth.129 When treating patients with psoriasis who might be trying to get pregnant, a systematic approach to psoriasis therapy might be helpful (Table 3).
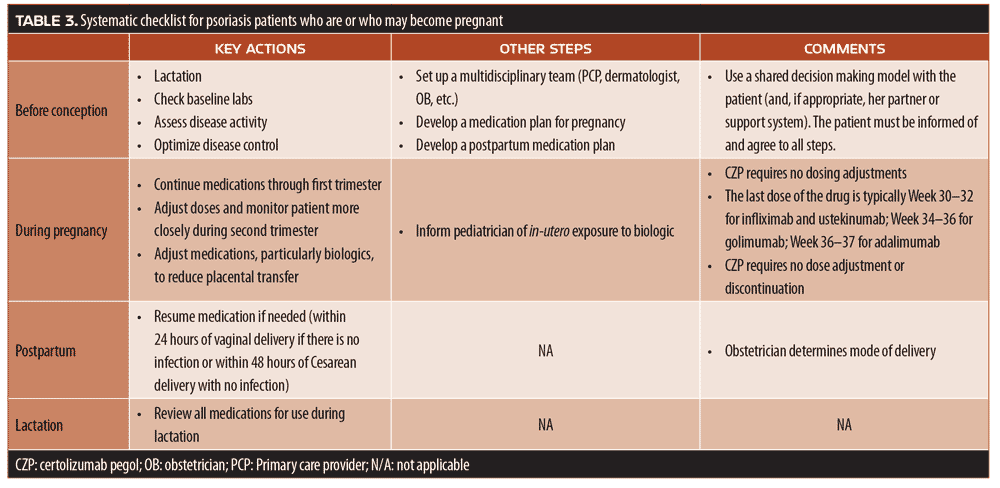
Because of potential immune suppression, babies born to a mother taking biologics should not receive any live vaccines for the first nine months of life or until drug levels are no longer detectable.130 In the US, this would only apply to the rotavirus vaccination; all other childhood vaccines could be administered on schedule. Note that a child who does not get the rotavirus vaccination before six months is not eligible for a catch-up vaccination due to the increased risk of intussusception after six months.
Key points. Unfortunately, there is a lack of robust data and controlled clinical evidence on the use of many drugs during pregnancy and lactation. Many women with moderate-to-severe psoriasis taking biologics might become pregnant and need guidance about the continuation of therapy. Their main concern is birth defects and about 70 percent of birth defects are of an unknown etiology. Psoriasis might improve during the course of pregnancy and many women report it worsens after delivery. Since biologics are transported over the placenta by the Fc receptor, CZP might be an optimal prescribing choice because it does not cross the placenta; babies born to mothers taking CZP have undetectable blood levels of CZP at four and eight weeks after delivery.
Updates in Topical Treatment
Based on a presentation by Jami L. Miller, MD— Assistant Professor of Medicine, Department of Medicine, Division of Dermatology, Vanderbilt University Medical Center, Nashville, Tennessee
Topical treatments have always been a mainstay in psoriasis treatment and, for patients with low BSA involvement and easy-to-treat areas, topical therapy might be the best option. When psoriasis is very limited and easy to cover with topicals, monotherapy with a topical might be adequate treatment. In other cases, topicals can be an important part of combination therapy. Topical cannabinoid preparations are a new development for topical products but their role in psoriasis (and other therapies) remains to be elucidated. Cannabinoid products seem to be very popular, with over 100 different brands available already.131 However, the older topical products remain important in psoriasis care.
Topical steroids first came to market in 1952 at a time when they were called “compound F,” and psoriasis was treated in a step-by-step sequence, with topicals being the first step.132 Recently, three new topical products for psoriasis came to the market: halobetasol propionate lotion 0.01% in November 2018, halobetasol propionate foam 0.05% in May 2018, and halobetasol propionate with tazarotene 0.045% in April 2019. These new topicals came to market in part to address the issue of patient adherence, which can be markedly improved with better, more elegant vehicles. Both lotions are more effective than the foam and it must be noted that the foam vehicle uses alcohol as its propellant, making it flammable. The foam dries quickly and is recommended for a course of two weeks (total dose: 50g/week). The lotions can be used for eight weeks. The lotions dry quickly and have a vehicle with micron-sized emulsion droplets designed to improve penetration. Note that the lotions are off-label for pediatric use.
Topical steroids are effective but have been associated with adverse events, including skin infections, cataracts, glaucoma, localized adverse reactions, such as atrophy or dermatitis, and hypothalamic–pituitary–adrenal (HPA) axis suppression. The incidence of skin infections ranges from 16 to 43 percent and includes any of several bacterial and fungal infections.133 Furthermore, topical steroids can promote acne. Steroids have also been associated with a type of open-angle glaucoma and patients with closed-angle glaucoma might be at a heightened risk for this condition with steroid use. Care should be exercised with the use of any topical steroid (anywhere on the body) in patients with glaucoma and such products should not be used on or around or on the eyelids for any patient.133 Atrophy and other localized reactions can occur with the use of a topical steroid, but this might be mitigated by adding an irritant (such as a retinoic acid) to the topical.134 Laboratory evidence suggests HPA axis suppression can occur in 15 to 20 percent of patients with any topical product (particularly in younger and older patients using strong drugs) and can be asymptomatic. HPA axis suppression resolves spontaneously when the product is discontinued.135
Halobetasol and tazarotene are now available as a fixed-dose combination product that might be considered as a moisturizing lotion for psoriasis.85,136 It can be used in intertriginous areas but not mucous membranes. Tazarotene should not be used in women who are pregnant, so a negative pregnancy test is appropriate before initiating therapy.
Cannabinoids are of increasing interest in many fields of medicine and have been shown to have anti-inflammatory, antipruritic, and antiproliferative properties. Cannabinoids appear to have an inhibitory effect on neutrophils and cytokines.137 The skin contains cannabinoid receptors, making a topical cannabinoid product for the treatment of psoriasis appear to be of special interest.138 It should be noted that these are new products with very limited clinical data, many are not legal in all parts of the country, and their use in psoriasis care is still being explored. More news on cannabinoid topicals for psoriasis is expected in the future.
Key points. Topicals have been and likely will remain one of the pillars of psoriasis care. While they are rarely effective as monotherapy, except in patients with a limited affected BSA, they can be important adjuncts. Newer, fast-drying products can improve patient adherence and offer better cutaneous penetration. Although topical therapy for psoriasis might be considered an older established treatment modality, three new products came to market in the past year or so. Elegant new vehicles have been an important improvement in topical products for patients with psoriasis. The advent of cannabinoids in medicine can add to the armamentarium of topical products, particularly due to the possible anti-inflammatory effect of cannabinoids.
Conclusion
The ongoing renaissance in dermatology offers clinicians an unprecedented opportunity to offer patients more effective, longer-lasting, and better-tolerated treatments for psoriasis. The growing elucidation of the underlying pathophysiology of psoriasis is being confronted by a rapid evolution of highly effective new biologic agents. Dermatologists have access to a large and expanding armamentarium of new and old agents, but must still balance risks against benefits when making prescribing choices for individual patients. Topicals remain a pillar of psoriasis care and new products are entering that space as well. Dermatologists must equip themselves to help manage psoriasis in patients who are pregnant and nursing, although there are limited data on this subject. Dermatologists are at the forefront of care for patients at an elevated risk for PsA and many other comorbidities as it is increasingly realized that psoriasis is not just a cutaneous condition, but rather is a systemic disease with skin symptoms.
References
- Haider AS, Cohen J, Fei J, et al. Insights into gene modulation by therapeutic TNF and IFNgamma antibodies: TNF regulates IFNgamma production by T cells and TNF-regulated genes linked to psoriasis transcriptome. J Invest Dermatol. 2008;128(3):
655–666. - Hawkes JE, Chan TC, Krueger JG. Psoriasis pathogenesis and the development of novel targeted immune therapies. J Allergy Clin Immunol. 2017;140(3):645–653.
- Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med. 2014;371(4):326–338.
- Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375(4):345–356.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373(14):1318–1328.
- Valeant. Brodalumab injection briefing document. Available at: https://www.fda.gov/media/99043/download. Accessed June 14, 2019.
- Beck KM, Koo J. Brodalumab for the treatment of plaque psoriasis: up-to-date. Expert Opin Biol Ther. 2019;19(4):287–292.
- Kreymborg K, Bohlmann U, Becher B. IL-23: changing the verdict on IL-12 function in inflammation and autoimmunity. Expert Opin Ther Targets. 2005;9(6):1123–1136.
- Oppmann B, Lesley R, Blom B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13(5):715–725.
- Papp KA, Blauvelt A, Kimball AB, et al. Patient-reported symptoms and signs of moderate-to-severe psoriasis treated with guselkumab or adalimumab: results from the randomized VOYAGE 1 trial. J Eur Acad Dermatol Venereol. 2018;32(9):1515–1522.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate-to-severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76(3):405–417.
- Gordon KB, Armstrong AW, Han C, et al. Anxiety and depression in patients with moderate-to-severe psoriasis and comparison of change from baseline after treatment with guselkumab vs. adalimumab: results from the phase 3 VOYAGE 2 study. J Eur Acad Dermatol Venereol. 2018;32(11):1940–1949.
- Foley P, Gordon K, Griffiths CEM, et al. Efficacy of guselkumab compared with adalimumab and placebo for psoriasis in specific body regions: a secondary analysis of 2 randomized clinical trials. JAMA Dermatol. 2018;154(6):676–683.
- Langley R, Blauvelt A, Armstrong A, et al. Guselkumab demonstrates superior long-term responses to secukinumab at Week 48 in the treatment of moderate to severe psoriasis: results from the ECLIPSE trial. Available at: https://www.events.mondial.at/ei/2018/Downloads/Late_Breaking_Abstracts_1-8.pdf. Accessed March 15, 2019.
- Susman E. Tremfya tops Cosentyx in Head-to-Head Psoriasis Trial. Available at: https://www.medpagetoday.com/meetingcoverage/isds/76905. Accessed June 15, 2019.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390(10091):276–288.
- Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392(10148):650–661.
- Dermatology World. IL-23, 33 inhibitors show promise in phase 3. Available at: https://aadmeetingnews.org/2018-annual-meeting-dailies/il-23-33-inhibitors-show-promise-in-phase-3/. Accessed June 15, 2019.
- Clowse ME, Forger F, Hwang C, et al. Minimal to no transfer of certolizumab pegol into breast milk: results from CRADLE, a prospective, postmarketing, multicentre, pharmacokinetic study. Ann Rheum Dis. 2017;76(11):1890–1896.
- Pasut G. Pegylation of biological molecules and potential benefits: pharmacological properties of certolizumab pegol. BioDrugs. 2014;28 Suppl 1:S15–S23.
- Shim H. One target, different effects: a comparison of distinct therapeutic antibodies against the same targets. Exp Mol Med. 2011;43(10):539–549.
- Nesbitt A, Fossati G, Bergin M, et al. Mechanism of action of certolizumab pegol (CDP870): in vitro comparison with other anti-tumor necrosis factor alpha agents. Inflamm Bowel Dis. 2007;13(11):1323–1332.
- Gottlieb AB, Blauvelt A, Thaci D, et al. Certolizumab pegol for the treatment of chronic plaque psoriasis: results through 48 weeks from 2 phase 3, multicenter, randomized, double-blinded, placebo-controlled studies (CIMPASI-1 and CIMPASI-2). J Am Acad Dermatol. 2018;79(2):302–314.e306.
- Keating GM. Apremilast: a review in psoriasis and psoriatic arthritis. Drugs. 2017;77(4):459–472.
- Kavanaugh A, Gladman DD, Edwards CJ, et al. Long-term experience with apremilast in patients with psoriatic arthritis: 5-year results from a PALACE 1-3 pooled analysis. Arthritis Res Ther. 2019;21(1):118.
- Smith RL. Pediatric psoriasis treated with apremilast. JAAD Case Rep. 2016;2(1):89–91.
- Vitale A, Rigante D, Lopalco G, et al. New therapeutic solutions for Behcet’s syndrome. Expert Opin Investig Drugs. 2016;25(7): 827–840.
- Singh JA, Guyatt G, Ogdie A, et al. Special Article: 2018 American College of Rheumatology/National Psoriasis Foundation Guideline for the Treatment of Psoriatic Arthritis. Arthritis Care Res (Hoboken). 2019;71(1):2–29.
- Zacharie H. Prevalence of joint disease in patients with psoraisis: implications for therapy. Am J Clin Dermtol. 2003;4(7): 441–447.
- Gladman DD, Antoni C, Mease P, et al. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 2005;64 Suppl 2:ii14–ii17.
- Mease PJ, Menter MA. Quality-of-life issues in psoriasis and psoriatic arthritis: outcome measures and therapies from a dermatological perspective. J Am Acad Dermatol. 2006;54(4):685–704.
- Raychaudhuri SP, Wilken R, Sukhov AC, et al. Management of psoriatic arthritis: early diagnosis, monitoring of disease severity and cutting edge therapies. J Autoimmun. 2017;76:21–37.
- Mease PJ, Genovese MC, Greenwald MW, et al. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med. 2014;370(24):2295–2306.
- McInnes IB, Mease PJ, Kirkham B, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386(9999):1137–1146.
- Mease PJ, van der Heijde D, Ritchlin CT, et al. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann Rheum Dis. 2017;76(1):79–87.
- Nash P, Kirkham B, Okada M, et al. Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: results from the 24-week randomised, double-blind, placebo-controlled period of the SPIRIT-P2 phase 3 trial. Lancet. 2017;389(10086): 2317–2327.
- Relvas M, Torres T. Pediatric psoriasis. Am J Clin Dermatol. 2017;18(6):797–811.
- Bronckers IM, Paller AS, van Geel MJ, et al. Psoriasis in children and adolescents: diagnosis, management and comorbidities. Paediatr Drugs. 2015;17(5):
373–384. - Dogra S, Mahajan R. Biologics in pediatric psoriasis—efficacy and safety. Expert Opin Drug Saf. 2018;17(1):9–16.
- Augustin M, Glaeske G, Radtke MA, et al. Epidemiology and comorbidity of psoriasis in children. Br J Dermatol. 2010;162(3): 633–636.
- Osier E, Wang AS, Tollefson MM, et al. Pediatric psoriasis comorbidity screening guidelines. JAMA Dermatol. 2017;153(7): 698–704.
- Harden JL, Krueger JG, Bowcock AM. The immunogenetics of psoriasis: a comprehensive review. J Autoimmun. 2015;64:66–73.
- McGonagle D, Ash Z, Dickie L, et al. The early phase of psoriatic arthritis. Ann Rheum Dis. 2011;70 Suppl 1:i71–i76.
- van Geel MJ, Mul K, Oostveen AM, et al. Calcipotriol/betamethasone dipropionate ointment in mild-to-moderate paediatric psoriasis: long-term daily clinical practice data in a prospective cohort. Br J Dermatol. 2014;171(2):363–369.
- Paller AS, Siegfried EC, Langley RG, et al. Etanercept treatment for children and adolescents with plaque psoriasis. N Engl J Med. 2008;358(3):241–251.
- Paller AS, Schenfeld J, Accortt NA, Kricorian G. A retrospective cohort study to evaluate the development of comorbidities, including psychiatric comorbidities, among a pediatric psoriasis population. Pediatr Dermatol. 2019;36(3):290–297.
- Becker L, Tom WL, Eshagh K, et al. Excess adiposity preceding pediatric psoriasis. JAMA Dermatol. 2014;150(5):573–574.
- Zamberk P, Velazquez D, Campos M, et al. Paediatric psoriasis—narrowband UVB treatment. J Eur Acad Dermatol Venereol. 2010;24(4):415–419.
- Marqueling AL, Cordoro KM. Systemic treatments for severe pediatric psoriasis: a practical approach. Dermatol Clin. 2013;31(2):267–288.
- Bronckers I, Seyger MMB, West DP, et al. Safety of systemic agents for the treatment of pediatric psoriasis. JAMA Dermatol. 2017;153(11):1147–1157.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361(5):496–509.
- Bachelez H. Pustular psoriasis and related pustular skin diseases. Br J Dermatol. 2018;178(3):614–618.
- Naldi L, Gambini D. The clinical spectrum of psoriasis. Clin Dermatol. 2007;25(6):510–518.
- Merola JF, Li T, Li WQ, et al. Prevalence of psoriasis phenotypes among men and women in the USA. Clin Exp Dermatol. 2016;41(5):486–489.
- Raposo I, Torres T. Palmoplantar psoriasis and palmoplantar pustulosis: current treatment and future prospects. Am J Clin Dermatol. 2016;17(4):349–358.
- Farley E, Masrour S, McKey J, Menter A. Palmoplantar psoriasis: a phenotypical and clinical review with introduction of a new quality-of-life assessment tool. J Am Acad Dermatol. 2009;60(6):1024–1031.
- Rivard J, Janiga J, Lim HW. Tacrolimus ointment 0.1% alone and in combination with medium-dose UVA1 in the treatment of palmar or plantar psoriasis. J Drugs Dermatol. 2006;5(6):505–510.
- Marsland AM, Chalmers RJ, Hollis S, et al. Interventions for chronic palmoplantar pustulosis. Cochrane Database Syst Rev. 2006(1):Cd001433.
- Brunasso AM, Puntoni M, Aberer W, et al. Clinical and epidemiological comparison of patients affected by palmoplantar plaque psoriasis and palmoplantar pustulosis: a case series study. Br J Dermatol. 2013;168(6):1243–1251.
- Kromer C, Wilsmann-Theis D, Gerdes S, et al. Drug survival and reasons for drug discontinuation in palmoplantar pustulosis: a retrospective multicenter study. J Dtsch Dermatol Ges. 2019;17(5):503–516.
- Capella G, Fracchiolla C, Frigerio E, Altomare G. A controlled study of comparative efficacy of oral retinoids and topical betamehasone/salicylic acid for chronic hyperkertotic palmoplantar dermatitis. J Dermatol Treatment. 2004;15(2):88–93.
- Bissonnette R, Haydey R, Rosoph LA, et al. Apremilast for the treatment of moderate-to-severe palmoplantar psoriasis: results from a double-blind, placebo-controlled, randomized study. J Eur Acad Dermatol Venereol. 2018;32(3):403–410.
- Menter A, Warren RB, Langley RG, et al. Efficacy of ixekizumab as compared with etanercept and placebo in patients with moderate-to-severe plaque psoriasis and non-pustular palmoplantar involvement: results from three phase 3 trials (UNCOVER-1, UNCOVER-2 and UNCOVER-3). J Eur Acad Dermatol Venereol. 2017;31(10):1686–1692.
- Haugh I, Preston A, Kivelevitch D, Menter A. Risankizumab: an anti-IL-23 antibody for the treatment of psoriasis. Drug Des Devel Ther. 2018;12:3879–3883.
- Gottlieb A, Sullivan J, van Doorn M, et al. Secukinumab shows significant efficacy in palmoplantar psoriasis: results from GESTURE, a randomized controlled trial. J Am Acad Dermatol. 2017;76(1):70–80.
- Mrowietz U, Bachelez H, Burden AD, et al. Secukinumab for moderate-to-severe palmoplantar pustular psoriasis: results of the 2PRECISE study. J Am Acad Dermatol. 2019;80(5):1344–1352.
- Saunier J, Debarbieux S, Jullien D, et al. Acrodermatitis continua of Hallopeau treated successfully with ustekinumab and acitretin after failure of tumour necrosis factor blockade and anakinra. Dermatology. 2015;230(2):97–100.
- Cymerman RM, Cohen DE. Treatment of acrodermatitis continua of Hallopeau with ustekinumab as monotherapy. JAMA Dermatol. 2016;152(3):346–348.
- Papp K, Berth-Jones J, Kragballe K, et al. Scalp psoriasis: a review of current topical options. J Eur Acad Dermatol Venereol. 2007;21: 1151–1160.
- Chan S, Van Voorhees AS, Lebwohl M, et al. Treatment of severe scalp psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2009;60(6):962–971.
- Bagel J, Lynde C, Tyring S, et al. Moderate to severe plaque psoriasis with scalp involvement: a randomized, double-blind, placebo-controlled study of etanercept. J Am Acad Dermatol. 2012;67(1):
86–92. - Fotiadou C, Lazaridou E, Sotiriou E, et al. Scalp psoriasis and biologic agents: a retrospective, comparative study from a tertiary psoriasis referral centre. J Eur Acad Dermatol Venereol. 2016;30(12):2091–2096.
- Bagel J, Duffin KC, Moore A, et al. The effect of secukinumab on moderate-to-severe scalp psoriasis: Results of a 24-week, randomized, double-blind, placebo-controlled phase 3b study. J Am Acad Dermatol. 2017;77(4): 667–674.
- Czuczwar P, Stepniak A, Goren A, et al. Genital psoriasis: a hidden multidisciplinary problem—a review of literature. Ginekol Pol. 2016;87(10):
717–721. - Ryan C, Sadlier M, De Vol E, et al. Genital psoriasis is associated with significant impairment in quality of life and sexual functioning. J Am Acad Dermatol. 2015;72(6):978–983.
- Ryan C. Genital psoriasis: the failure of dermatologists to identify genital involvement. Br J Dermatol. 2019;180(3): 647–656.
- Ryan C, Menter A, Guenther L, et al. Efficacy and safety of ixekizumab in a randomized, double-blinded, placebo-controlled phase IIIb study of patients with moderate-to-severe genital psoriasis. Br J Dermatol. 2018;179(4):844–852.
- Langenbruch A, Radtke MA, Krensel M, et al. Nail involvement as a predictor of concomitant psoriatic arthritis in patients with psoriasis. Br J Dermatol. 2014;171(5):1123–1128.
- Piraccini BM, Starace M. Optimal management of nail disease in patients with psoriasis. Psoriasis (Auckl). 2015;5:25–33.
- Rich P, Gooderham M, Bachelez H, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with difficult-to-treat nail and scalp psoriasis: results of 2 phase III randomized, controlled trials (ESTEEM 1 and ESTEEM 2). J Am Acad Dermatol. 2016;74(1):134–142.
- Reich K, Sullivan J, Arenberger P, et al. Effect of secukinumab on the clinical activity and disease burden of nail psoriasis: 32-week results from the randomized placebo-controlled TRANSFIGURE trial. Br J Dermatol. 2018 Oct 26. [Epub ahead of print].
- Kircik LH, Stein Gold LF, Pariser DM. Improving adherence to topical therapies through improved clinician–patient communication and shared decision making. Cutis. 2019;103(4S):S13–S15.
- Stein Gold LF, Kircik LH, Pariser DM. Understanding topical therapies for psoriasis. Cutis. 2019;103(4S):S8–S12.
- Del Rosso JQ, Kircik LH, Zeichner J, Stein Gold L. The clinical relevance and therapeutic benefit of established active ingredients incorporated into advanced foam vehicles: vehicle characteristics can influence and improve patient outcomes. J Drugs Dermatol. 2019;18(2S):s100–S107.
- Del Rosso JQ, Kircik L, Lin T, Pillai R. Halobetasol 0.01%/tazarotene 0.045% fixed-combination lotion in the treatment of plaque psoriasis: sensitization and irritation potential. J Clin Aesthet Dermatol. 2019;12(1):11–15.
- Bhatia ND, Pariser DM, Kircik L, et al. Safety and efficacy of a halobetasol 0.01%/tazarotene 0.045% fixed combination lotion in the treatment of moderate-to-severe plaque psoriasis: a comparison with halobetasol propionate 0.05% Cream. J Clin Aesthet Dermatol. 2018;11(11):15–19.
- Wilson FC, Icen M, Crowson CS, et al. Incidence and clinical predictors of psoriatic arthritis in patients with psoriasis: a population-based study. Arthritis Rheum. 2009;61(2):233–239.
- Taylor W, Gladman D, Helliwell P, et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006;54(8):2665–2673.
- Lebwohl M. Innovations in the treatment of psoriasis. J Am Acad Dermatol. 2004;51(1 Suppl):S40–S41.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80(4):1073–1113.
- Gulliver W. Long-term prognosis in patients with psoriasis. Br J Dermatol. 2008;159 Suppl 2:2–9.
- Gulliver WP, Macdonald D, Gladney N, et al. Long-term prognosis and comorbidities associated with psoriasis in the Newfoundland and Labrador founder population. J Cutan Med Surg. 2011;15(1): 37–47.
- Egeberg A, Sorensen JA, Gislason GH, et al. Incidence and prognosis of psoriasis and psoriatic arthritis in patients undergoing bariatric surgery. JAMA Surg. 2017;152(4):344–349.
- Kivelevitch D, Schussler JM, Menter A. Coronary plaque characterization in psoriasis. Circulation. 2017;136(3):277–280.
- Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12(3):204–212.
- Hansson GK, Robertson AK, Soderberg-Naucler C. Inflammation and atherosclerosis. Annu Rev Pathol. 2006;1:297–329.
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296(14):1735–1741.
- Prodanovich S, Ma F, Taylor JR, et al. Methotrexate reduces incidence of vascular diseases in veterans with psoriasis or rheumatoid arthritis. J Am Acad Dermatol. 2005;52(2):262–267.
- Mehta NN, Yu Y, Saboury B, et al. Systemic and vascular inflammation in patients with moderate to severe psoriasis as measured by [18F]-fluorodeoxyglucose positron emission tomography-computed tomography (FDG-PET/CT): a pilot study. Arch Dermatol. 2011;147(9):1031–1039.
- Dey AK, Joshi AA, Chaturvedi A, et al. Association between skin and aortic vascular inflammation in patients with psoriasis: a case-cohort study using positron emission tomography/computed tomography. JAMA Cardiol. 2017;2(9):1013–1018.
- Mansouri B, Kivelevitch D, Natarajan B, et al. Comparison of coronary artery calcium scores between patients with psoriasis and Type 2 diabetes. JAMA Dermatol. 2016;152(11):1244–1253.
- Navar AM. The evolving story of triglycerides and coronary heart disease risk. JAMA. 2019;321(4):347–349.
- Ference BA, Kastelein JJP, Ray KK, et al. Association of triglyceride-lowering LPL variants and LDL-C-lowering LDLR variants with risk of coronary heart disease. JAMA. 2019;321(4):364–373.
- Skov L, Thomsen SF, Kristensen LE, et al. Cause-specific mortality in patients with psoriasis and psoriatic arthritis. Br J Dermatol. 2019;180(1):
100–107. - Salahadeen E, Torp-Pedersen C, Gislason G, et al. Nationwide population-based study of cause-specific death rates in patients with psoriasis. J Eur Acad Dermatol Venereol. 2015;29(5):1002–1005.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70(3):512–516.
- Finer LB, Zolna MR. Unintended pregnancy in the United States: incidence and disparities, 2006. Contraception. 2011;84(5):478–485.
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med. 2016;374(9):843–852.
- Mitchell AA, Gilboa SM, Werler MM, et al. Medication use during pregnancy, with particular focus on prescription drugs: 1976–2008. Am J Obstet Gynecol. 2011;205(1):51.e51–e58.
- Thorpe PG, Gilboa SM, Hernandez-Diaz S, et al. Medications in the first trimester of pregnancy: most common exposures and critical gaps in understanding fetal risk. Pharmacoepidemiol Drug Saf. 2013;22(9):1013–1018.
- United States Food and Drug Administration. Communicating information about risks in pregnncy in product labeling for patients and providers to make informed decisions about the use of drugs in pregnancy. Risk Communication Advisor Committee Meeting, March 5–6, 2018. Available at: https://www.fda.gov/media/110905/download. Accessed June 21, 2019.
- Dinatale M. Two years in: lessons learned with the pregnancy and lactation labeling rule-aproaches to human data. Available at: https://www.fda.gov/media/111774/download. Accessed June 21, 2019.
- Nicholson A. Psoriasis and pregnancy. Available at: https://plaquepsoriasis.com/psoriasis-affect-health/pregnancy-risk-complications/. Accessed June 22, 2019.
- Murase JE, Chan KK, Garite TJ, et al. Hormonal effect on psoriasis in pregnancy and post partum. Arch Dermatol. 2005;141(5): 601–606.
- Bobotsis R, Gulliver WP, Monaghan K, et al. Psoriasis and adverse pregnancy outcomes: a systematic review of observational studies. Br J Dermatol. 2016;175(3):464–472.
- Kane SV, Acquah LA. Placental transport of immunoglobulins: a clinical review for gastroenterologists who prescribe therapeutic monoclonal antibodies to women during conception and pregnancy. Am J Gastroenterol. 2009;104(1):228–233.
- Simister NE. Placental transport of immunoglobulin G. Vaccine. 2003;21(24):3365–3369.
- Julsgaard M, Christensen LA, Gibson PR, et al. Concentrations of adalimumab and infliximab in mothers and newborns, and effects on infection. Gastroenterology. 2016;151(1):110–119.
- Mahadevan U, Wolf DC, Dubinsky M, et al. Placental transfer of anti-tumor necrosis factor agents in pregnant patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013;11(3):286–292; quiz e224.
- Boyle CA, Boulet S, Schieve LA, et al. Trends in the prevalence of developmental disabilities in US children, 1997–2008. Pediatrics. 2011;127(6):
1034–1042. - Cleveland Clinic. Birth defects. Available at: https://my.clevelandclinic.org/health/diseases/12230-birth-defects. Accessed June 22, 2019.
- Lam J, Polifka JE, Dohil MA. Safety of dermatologic drugs used in pregnant patients with psoriasis and other inflammatory skin diseases. J Am Acad Dermatol. 2008;59(2):295–315.
- Bae YS, Van Voorhees AS, Hsu S, et al. Review of treatment options for psoriasis in pregnant or lactating women: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67(3):459–477.
- Gotestam Skorpen C, Hoeltzenbein M, Tincani A, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis. 2016;75(5):795–810.
- Pottinger E, Woolf RT, Exton LS, et al. Exposure to biological therapies during conception and pregnancy: a systematic review. Br J Dermatol. 2018;178(1):95–102.
- Scialli AR. The Organization of Teratology Information Services (OTIS) registry study. J Allergy Clin Immunol. 1999;103(2 Pt 2):
S373–S376. - A SPECIAL MEETING REVIEW EDITION: Highlights in Crohn’s Disease and Ulcerative Colitis Digestive Disease Week 2012 May 19–22, 2012 • San Diego, California. Gastroenterol Hepatol (N Y). 2012;8(8 Suppl 5):1–24.
- Holmes LB. Human teratogens: update 2010. Birth Defects Res A Clin Mol Teratol. 2011;91(1):1–7.
- Mariette X, Forger F, Abraham B, et al. Lack of placental transfer of certolizumab pegol during pregnancy: results from CRIB, a prospective, postmarketing, pharmacokinetic study. Ann Rheum Dis. 2018;77(2):228–233.
- Matro R, Martin CF, Wolf D, Shah SA, Mahadevan U. Exposure concentrations of infants breastfed by women receiving biologic therapies for inflammatory bowel diseases and effects of breastfeeding on infections and development. Gastroenterology. 2018;155(3):696–704.
- Adler BL, DeLeo VA. Allergenic ingredients in commercial topical cannabinoid preparations. J Am Acad Dermatol. 2019;81(3):847–848.
- Sulzberger MB, Witten VH. The effect of topically applied compound F in selected dermatoses. J Invest Dermatol. 1952;19(2):101–102.
- Coondoo A, Phiske M, Verma S, Lahiri K. Side-effects of topical steroids: a long overdue revisit. Indian Dermatol Online J. 2014;5(4):416–425.
- Lesnik RH, Mezick JA, Capetola R, Kligman LH. Topical all-trans-retinoic acid prevents corticosteroid-induced skin atrophy without abrogating the anti-inflammatory effect. J Am Acad Dermatol. 1989;21(2 Pt 1):186–190.
- Levin E, Gupta R, Butler D, et al. Topical steroid risk analysis: differentiating between physiologic and pathologic adrenal suppression. J Dermatolog Treat. 2014;25(6):501–506.
- Sugarman JL, Gold LS, Lebwohl MG, et al. A phase 2, multicenter, double-blind, randomized, vehicle controlled clinical study to assess the safety and efficacy of a halobetasol/tazarotene fixed combination in the treatment of plaque psoriasis. J Drugs Dermatol. 2017;16(3):197–204.
- Mabou Tagne A, Marino F, Legnaro M, et al. A novel standardized Cannabis sativa L. extract and its constituent cannabidiol inhibit human polymorphonuclear leukocyte functions. Int J Mol Sci. 2019;20(8). pii: E1833.
- Del Giorno R, Skaper S, Paladini A, et al. Palmitoylethanolamide in fibromyalgia: results from prospective and retrospective observational studies. Pain Ther. 2015;4(2):169-178.

