Based on a presentation by Zoe Draelos, MD
Based on a presentation by Zoe Draelos, MD
Watch the video of this Skincare Academy presentation featuring Dr. Draelos at https://jcad.tv/sca-zoe-draelos-novel-sunscreen-insights/
Dr. Draelos is with Dermatology Consulting Services, PLLC and is Consulting Professor, Department of Dermatology, at Duke University School of Medicine in Durham, North Carolina.
There are a lot of commonly asked sunscreen questions that we get as dermatologists. Why do I still burn when I wear sunscreen? What’s the best order for sunscreen application? Why are sunscreens so sticky and greasy? Why do sunscreens make me so hot? What is the added value of a tinted sunscreen? Let’s delve into the science behind these seemingly simplistic but very important questions.
Why do I still burn when I wear sunscreen?
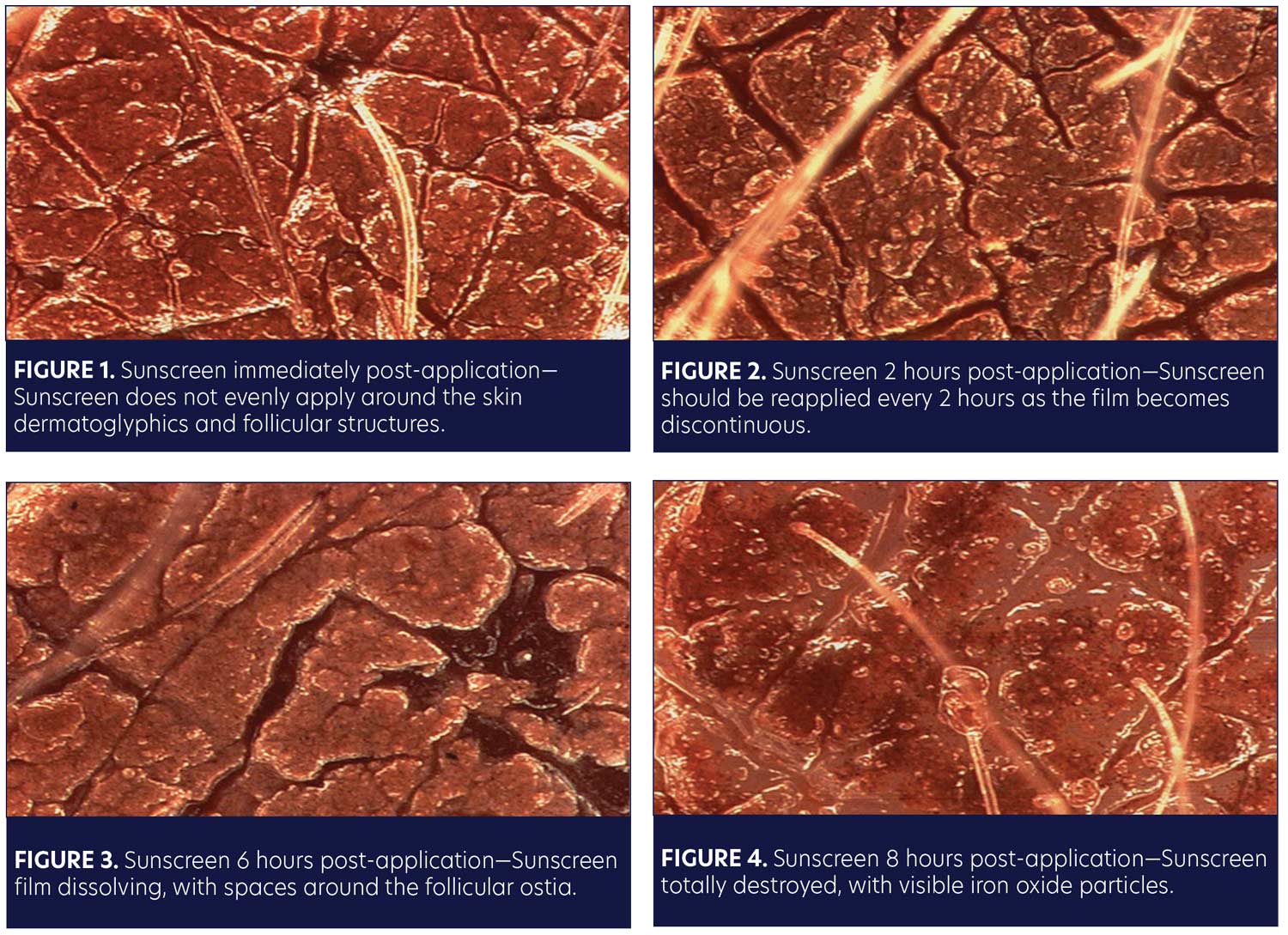
This is a common complaint of patients who come into my office. We all know that sunscreen must be applied as a thin, continuous, even film over the skin’s surface. Any discontinuity in that film will leave the skin unprotected. Over time, sunscreens tend to migrate as they mix with perspiration and sebum. This is why sunscreens need to be reapplied every two hours. Iron oxides can be combined with sunscreens to demonstrate this migration and exactly what happens to sunscreens as they’re applied to the skin and change over time.
Figure 1 shows skin immediately following application of a sunscreen with iron oxide particles. Notice that right from the start, the sunscreen does not evenly coat the skin; there is an area devoid of sunscreen around the follicular orifices. Additionally, the sunscreen does not sit in the dermatoglyphics of the skin. Thus, even though you have an even application, you do not have an even application when you look under a microscope.
Two hours following application (Figure 2), the dermatoglyphics have become even less protected. We can observe widening spaces where the skin markings are present. We can see individual iron oxide particulates and widening spaces, especially where the hair follicle exits the skin at the follicular ostia, as well as at the dermatoglyphics. Additionally, the sunscreen starts to migrate up the hair shaft itself. The film has become discontinuous. This is why sunscreen needs to be reapplied every two hours.
What about six hours following application (Figure 3)? The sunscreen film is completely dissolved. The sunscreen has been completely broken down by sebum and perspiration. Again, we can observe widening spaces around the follicular ostia. We also see a sebaceous gland dumping into the skin at the far right of Figure 3. We can observe the continued widening and loss of complete sun protection over the dermatoglyphics.
Finally, at eight hours, you can see the sebum has completely dissolved the film (Figure 4). We observe individual iron oxide particles, the sunscreen, sebum, and perspiration migrate up the hair shaft; at this stage, the film has been entirely broken down and the skin is completely unprotected.
What is the best order for sunscreen application?
How should we advise our patients when it comes to layering their products with their sunscreen to achieve optimal protection? Keep in mind that sunscreens do not need to touch the skin to work. As a matter of fact, they work best as a coating over everything else that has been applied to the face. Therefore, sunscreens work much better when they’re on top of the skin. With regard to application order, treatment serums, such as antioxidants or vitamin C serums, should be applied first after cleansing. A moisturizer should be applied next, then a sunscreen. Often, many facial foundations include sun protection. However, if the selected facial foundation does not contain sun protection then a separate facial foundation would be applied following sunscreen.
Applying the products in this order is important because it allows the sunscreen to function properly, while providing the best functional appearance benefits.
Why are sunscreens sticky and greasy?
Sunscreens can contain both organic and inorganic filters. Organic filters undergo degradation when they absorb a photon of ultraviolet (UV) radiation. Organic filters work by absorbing a photon of UV radiation leading to a chemical reaction known as resonance delocalization. This process breaks down the organic filter. This breakdown is another reason why sunscreens need to be applied every two hours. Octyl methoxycinnamate, also known as octinoxate, is a widely used organic filter. Eighty-six percent of the sunscreens in the current marketplace use octyl methoxycinnamate. It has optimal absorption, somewhere around 305 nanometers, and it has excellent stability. Only 4.5 percent degrades after UV exposure. Octyl methoxycinnamate is a clear, thick, greasy oil. Many organic filters are greasy oils, making for a greasy sunscreen formulation. Sunscreens with higher SPF contain higher concentrations of organic filters, making them even greasier. Sunscreen formulation is an art; the formulator must combine filters and vehicle ingredients in such a way that the sunscreen performs and protects excellently and stays in place on the skin, with minimal greasiness.
Why do sunscreens make me feel hot?
Many patients will come in and tell you that they feel fine until they put sunscreen on, and then they overheat. In fact, sunscreens do produce heat. Inorganic filters, like zinc oxide and titanium dioxide, mainly reflect light energy from the skin, but they also absorb photons of UV energy that are then dispersed into heat, making the skin warm. Organic filters take photons of light energy and transform it into heat through a chemical double bond breaking reaction. Therefore, organic filters actually produce heat as a byproduct. That heat is present on the skin’s surface because that’s where the sunscreen is placed and that’s where the skin gets warm. Heat production can be minimized by avoiding sunscreens with organic filters and using products with zinc oxide or titanium dioxide, which do not undergo much of a chemical reaction and produce less heat than organic filters.
What is the added value of a tinted sunscreen?
Zinc oxide, one of the inorganic sunscreen filters, is a white powder. When a photon of UV radiation strikes zinc oxide, it generates secondary oxygen radicals or reactive oxygen species. To quell these reactive oxygen species, most of the zinc oxide used in sunscreens today is coated with silicone. The silicone-coated zinc oxide neutralizes those radicals and allows less secondary oxygen radical production. This can also be achieved by adding antioxidants to sunscreen formulations. Titanium dioxide, another inorganic filter, is also a white powder. These ingredients can leave a white film on the skin’s surface. Tinting titanium dioxide and zinc oxide can make them more cosmetically elegant. As a bonus, iron oxides, which are used to tint sunscreens, also provide photoprotection. Visible light has been implicated in the production of melasma and dyspigmentation, especially in Fitzpatrick Skin Types 4, 5, and 6.
In summary
In summary, sunscreens must be reapplied every two hours. It should be applied after you moisturize the face, but before colored cosmetics. Unfortunately, sunscreens are indeed sticky due to the presence of organic filters, and they do create heat as light energy is transformed into heat energy. Finally, sunscreens can be tinted with iron oxide, not only to provide enhanced protection from visible light, but also to create a better cosmetic appearance.