by Jacob Beer, MD; Sarah Hermak, MS; and Omar Noor, MD, FAAD
by Jacob Beer, MD; Sarah Hermak, MS; and Omar Noor, MD, FAAD
Dr. Beer and Ms. Hermak are with the Dr. Phillip Frost Department of Dermatology and Cutaneous Surgery at the University of Miami in Miami, Florida. Dr. Noor is a board-certified dermatologist practicing in New York and New Jersey and is the co-owner of Rao Dermatology.
Introduction
Psoriasis is a chronic, immune-mediated, systemic inflammatory skin disease associated with several comorbidities, including cardiovascular disease, inflammatory bowel disease, and psychiatric disorders with significant disease burden.1
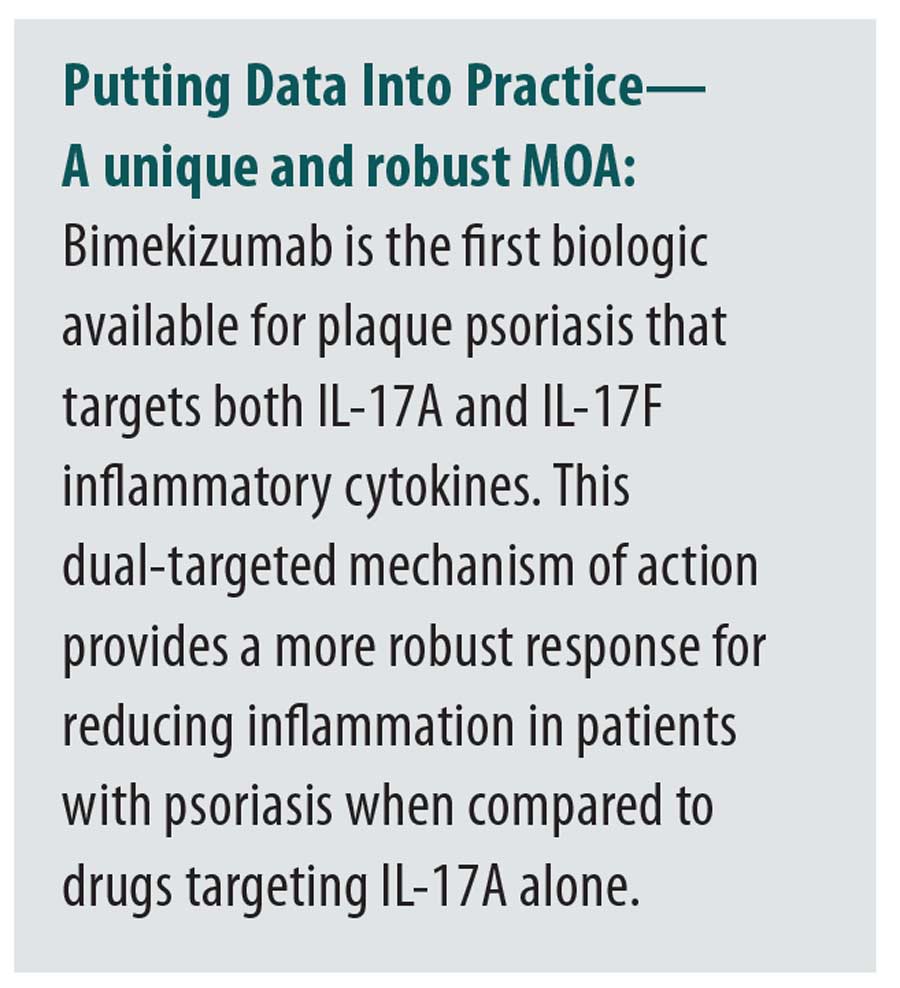
Psoriasis is driven by the interleukin (IL)-23/IL-17 immunologic pathway, which stimulate feed-forwarding circuits of T cell-mediated inflammation.2 The IL-17 family is constituted by isoforms from A to F. The pro-inflammatory cytokines IL-17A, IL-17C, and IL-17F are all increased in psoriatic lesions. IL-17A and IL-17F are the most structurally similar and are expressed as both homodimers and heterodimers (IL-17A/IL-17F).3 IL-17A is the most biologically active and potent in the pathophysiology of psoriasis, while IL-17F is the more abundantly expressed of the two in psoriasis.2 Although IL-17A and IL-17F act individually as pro-inflammatory mediators in psoriasis, they also work together to promote increased inflammation.4 This suggests that inhibition of both IL-17A and IL-17F may bemore effective than inhibition of IL-17A alone in the treatment of psoriasis.3,4
Biologic therapies inhibit specific proinflammatory cytokines involved in the pathophysiology of psoriasis. Bimekizumab is a monoclonal IgG1 antibody that selectively binds and neutralizes both IL-17A and IL-17F.3 Bimekizumab demonstrated superior efficacy and good safety profile in Phase III clinical trials and has been approved by the United States Food and Drug Administration for the treatment of moderate to severe plaque psoriasis.5–10
Therapeutic Efficacy of Bimekizumab
Bimekizumab has demonstrated high levels of efficacy and a favorable safety profile in the treatment of moderate to severe plaque psoriasis.5–10 Below is a review of clinical results from all bimekizumab Phase III studies conducted.
BE READY (NCT03410992) was a Phase III, 56-week multicenter, randomized, double-blinded, placebo-controlled clinical trial.5 Patients with moderate to severe psoriasis were randomly assigned (4:1) at Week 0 to receive bimekizumab 320mg every four weeks or placebo every four weeks. Patients treated with bimekizumab who achieved 90 percent or greater on the Psoriasis Area and Severity Index (PASI90) at Week 16 were reallocated (1:1:1) to receive bimekizumab 320mg every four weeks, every eight weeks, or placebo for Weeks 16 to 56 (i.e., randomized withdrawal period). Patients who did not achieve PASI90 at Week 16 received bimekizumab 320mg every four weeks. Patients receiving placebo who achieved PASI90 at Week 16 continued to receive placebo every four weeks.5 Co-primary endpoints of the BE READY study were the proportion of patients achieving PASI90 and a clear or almost clear (score 0 or 1) response on the Investigator’s Global Assessment (IGA) at Week 16. A greater proportion of the patients treated with bimekizumab achieved PASI90 (91%) and an IGA score of 0 or 1 (93%) compared to those receiving placebo (1%; 1%; p<0.0001) at Week 16. Compared to only 16 percent of patients receiving placebo, PASI90 was maintained at Week 52 by 87 percent of patients treated with bimekizumab 320mg every four weeks and 91 percent of patients treated with bimekizumab 320mg every eight weeks (all bimekizumab vs. placebo: p<0.0001). As early as Week 4, significantly more patients achieved PASI75 after only one dose of bimekizumab compared to placebo (75%; 1%; p<0.0001). Among the treatment withdrawal group, 40 percent maintained PASI90 at Week 40 and 16 percent at Week 52. In patients with IGA score of 0 or 1 response within the PASI90 responder group, 87 percent treated with bimekizumab 320mg every four weeks and 90 percent treated with bimekizumab 320mg every eight weeks maintained an IGA score of 0 or 1 response at Week 52 compared to placebo (24%). At Week 56, response rates were maintained with both bimekizumab-treated groups, but reduced to 11 percent in the placebo group. The median time to relapse (PASI<75) was 28 weeks (32 weeks from the last dose of bimekizumab; p<0.0001).5
BE VIVID (NCT03370133) was a 52-week, multicenter, randomized, double-blind, active comparator and placebo-controlled Phase III trial.6 Patients were randomized (4:2:1) to receive bimekizumab, ustekinumab, or placebo, respectively. At Week 16, patients receiving placebo were switched to bimekizumab. Co-primary endpoints were the proportion of patients achieving PASI90 and the proportion of patients with an IGA response of 0 or 1 at Week 16. At Week 16, significantly more patients (85%) in the bimekizumab group achieved PASI90 compared to ustekinumab (50%; 53%; p<0.0001) and placebo (4.5%; 4.5%; p<0.0001). Additionally, a significantly greater proportion of patients receiving bimekizumab (59%) had complete skin clearance (PASI100) at Week 16 than those receiving ustekinumab or placebo (21%; 0%; p<0.0001). A greater proportion of patients treated with bimekizumab achieved an IGA score of 0 or 1 at Week 16 compared to ustekinumab and placebo (4%, 53%, and 5% respectively; p<0.0001). Of note, bimekizumab showed a faster and greater response at Week 4 than ustekinumab or placebo. After only a single dose of bimekizumab, significantly more patients (77%) had PASI75 compared to ustekinumab or placebo (15%; 2%; p<0.000). At Week 52, a greater proportion of patients receiving bimekizumab achieved PASI90 and IGA response than patients who received ustekinumab (82% vs. 56%; 78% vs. 61%, respectively; p<0.0001). At Week 52, a higher number of patients (65%) in the bimekizumab group had PASI100 than ustekinumab group (65% vs. 38% respectively; p<0.000).6
BE RADIANT (NCT03536884) was a 48-week, randomized, multicenter, double- blinded, Phase IIIb active comparator-controlled trial.7 Patients were randomized (1:1) to receive bimekizumab 320mg every four weeks or secukinumab 300mg weekly until Week 4 then every four weeks until Week 48. At Week 16, patients treated with bimekizumab were randomized (2:1) to continue dosing at four weeks or every eight weeks to Week 48. The primary endpoint was a PASI100 score at Week 16. A greater proportion of patients treated with bimekizumab had PASI100 at Week 16 compared to the secukinumab group (61.7% vs. 48.9%, respectively; p<0.001). At Week 48, a higher proportion of patients treated with bimekizumab had a PASI100 response compared to secukinumab (67.0% vs. 46.2%; p<0.001). At Week 4, after only one dose of bimekizumab, 71 percent of patients achieved PASI75 compared to 47.3 percent in the secukinumab group, which required four doses (p<0.001).7
BE SURE (NCT03412747) was a 56-week, double-blinded, multicenter, Phase III trial comparing bimekizumab to adalimumab.8 Patients were randomized (1:1:1) to receive bimekizumab 320mg every four weeks for 56 weeks; bimekizumab 320mg every four weeks for 16 weeks, then every eight weeks to Week 56; or adalimumab 40mg every two weeks for 24 weeks, followed by bimekizumab 320mg every four weeks to Week 56. The primary endpoints were a PASI90 response and IGA score of 0 or 1 at Week 16. At Week 4, after one dose of treatment, 76.5 percent of bimekizumab-treated patients had a PASI75 response compared to 31.4 percent of patients treated with adalimumab (p<0.001).8 At Week 16, a significantly higher proportion of patients reached PASI90 in combined bimekizumab groups (86.2%) than with adalimumab (47.2%, p<0.001). Additionally, more patients treated with bimekizumab (85.3%) had an IGA score of 0 or 1 than with adalimumab (57.2%, p<0.001). At Week 56, complete clearance (PASI100) was achieved in 72.2 percent of patients receiving bimekizumab every four weeks, 70.2 percent receiving bimekizumab every four then every eight weeks, and 66.7 percent in patients treated with adalimumab followed by bimekizumab at Week 24.8
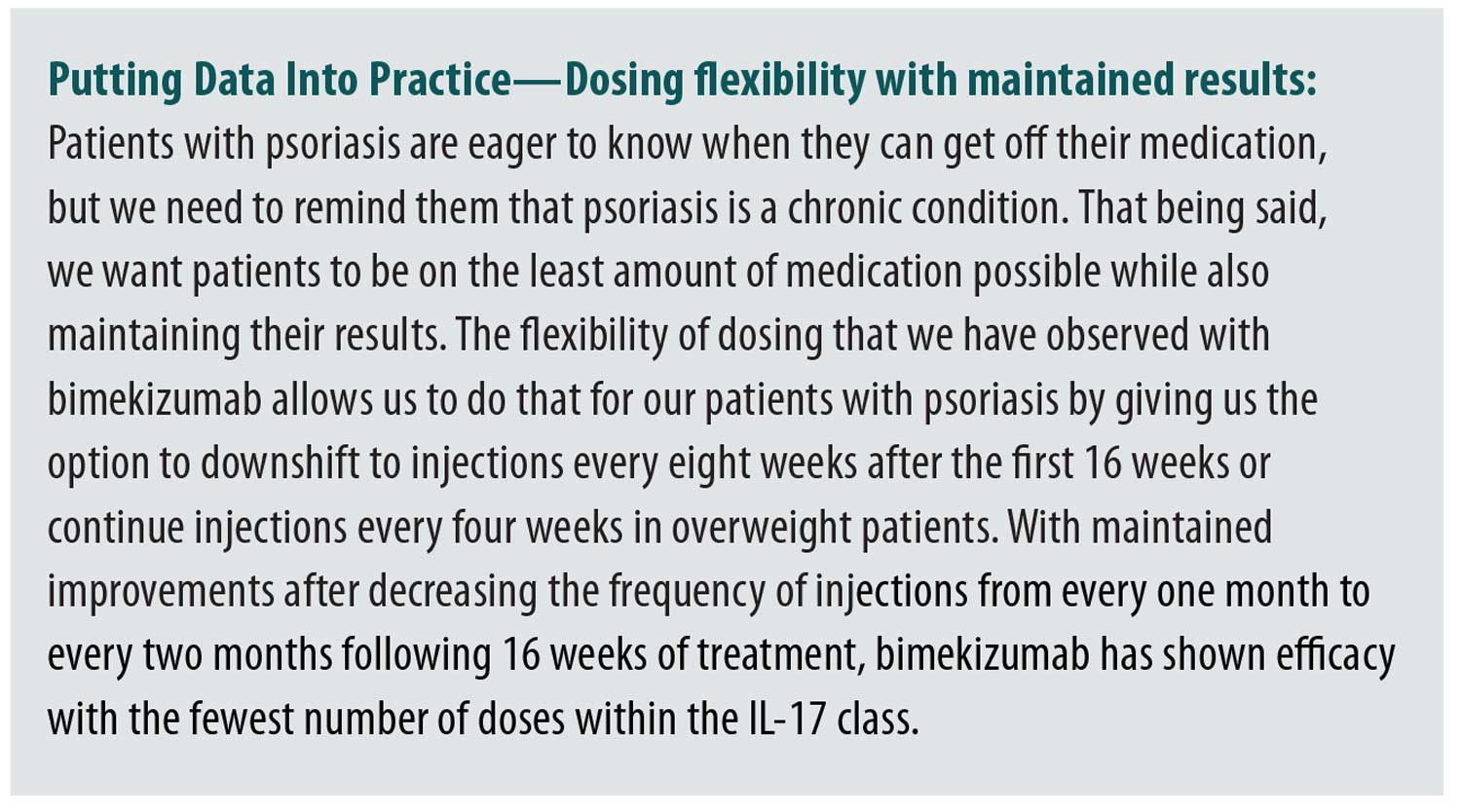
BE BRIGHT (NCT03598790) is an ongoing, multicenter, open-label extension (OLE) study assessing long-term safety, efficacy, and tolerability of bimekizumab in patients who completed BE READY, BE VIVID, or BE SURE studies.5,6,8,10 The analysis included patients randomized to bimekizumab 320mg every four weeks in the aforementioned Phase III trials, achieved a response at Week 16, continued bimekizumab 320mg every four or eight weeks as a maintenance dose and enrolled in the BE BRIGHT trial. Patients who received bimekizumab 320mg every four weeks and achieved PASI90 were randomized (4:1) at the end of the feeder study to receive bimekizumab 320mg every four or every eight weeks. Patients who received bimekizumab 320mg every eight weeks and achieved PASI90 continued with the same regimen in OLE. Regardless of maintenance dose at the end of the feeder study, patients who had not yet reached PASI90 received bimekizumab 320mg every four weeks. At Week 24 of the OLE, patients who had achieved PASI90 on bimekizumab 320mg every four weeks could be switched to receiving treatment every eight weeks. At Week 48 of the OLE, all patients who had remained on bimekizumab 320mg every four weeks were switched to every eight weeks. The objective of the study was to evaluate maintenance of Week 16 responses through three years in patients treated with bimekizumab.10 High levels of responses observed at Week 16 were maintained in most patients though three years of bimekizumab treatment, with 93.0 percent maintaining PASI90, 80.8 percent maintaining PASI100, and 94.0 percent maintaining PASI≤2 and 90.3 percent maintained BSA≤1% responses. It is worth highlighting that patients maintained good outcomes after decreasing the frequency of injections from every four weeks to every eight weeks. In the eight-week maintenance dosing schedule of bimekizumab, a high proportion of patients, 93.6 percent and 82.0 percent, maintained PASI100 after 1 year and 3 years, respectively.10
Bimekizumab efficacy in biologic-experienced participants. In head-to-head trials, bimekizumab fared better than adalimumab, ustekinumab, and secukinumab.11 Among biologic-experienced patients who had not achieved PASI90, bimekizumab demonstrated high rates of PASI90 and PASI100 achievement after a single dose (67% and 33% in adalimumab to bimekizumab, 79% and 42% in ustekinumab to bimekizumab and 53% and 21% in secukinumab to bimekizumab, respectively).11
Bimekizumab compared to other IL-17 inhibitors. A network meta-analysis showed that bimekizumab 320mg was superior in achieving PASI90 and PASI100 compared with other biologics, including other IL-17 inhibitors, ixekizumab, brodalumab, and secukinumab.12
Safety
In Phase III clinical trials and their OLE, bimekizumab has demonstrated a favorable safety profile. The most common adverse events reported across all Phase III trials were oral candidiasis, nasopharyngitis, and upper respiratory tract infection, none of which were serious and few led to discontinuations of bimekizumab.5–9
IL-17 inhibitors are known to increase risk of oral candidiasis.13 Oral candidiasis was frequently reported in bimekizumab-treated patients; however, most cases were mild or moderate and were easily treated.5–9 While rates of oral candidiasis with bimekizumab were greater than those with of IL-17 inhibitors, incidence of oral candidiasis was lower in patients treated with bimekizumab every eight weeks compared to four weeks.9 Additionally, rates of oral candidiasis decreased with longer duration of bimekizumab treatment. Serious infection rates were low across all Phase III trials and were comparable to other IL-17 inhibitors.9
New diagnoses of inflammatory bowel disease have been reported with interleukin-17A inhibitors.14,15 Similarly, new or exacerbated cases of inflammatory bowel disease were reported with bimekizumab.5–9 However, incidence of new-onset IBD was low in Phase III clinical trials with only five cases assessed as treatment-related leading to bimekizumab discontinuation.9
Five major adverse cardiovascular events (MACE) were reported in the bimekizumab-treated BE VIVID study patients, including a fatal cardiac arrest.6 A non-fatal myocardial infarction was reported in one patient in the BE READY study.5 However, all patients had pre-existing cardiovascular risk factors and none of these events were assessed to be bimekizumab related.5,6 Over three years, the incidence rate of MACE was low and remained low with longer bimekizumab exposure.9
Occurrence of malignancies were low, with predominately reports of nonmelanoma skin cancer, only two malignancies (one breast and one prostate) were assessed as bimekizumab-related by investigators.5–9 Phase III clinical trials in bimekizumab had low rates of serious hypersensitivity reactions and injection-site reactions. No anaphylactic reactions associated with bimekizumab were reported.5–9
Hepatic events during bimekizumab treatment were mainly reported as an increase in liver enzymes, however, these elevations were predominately transient, and returned to baseline with continued treatment or shortly after discontinuation.9,16
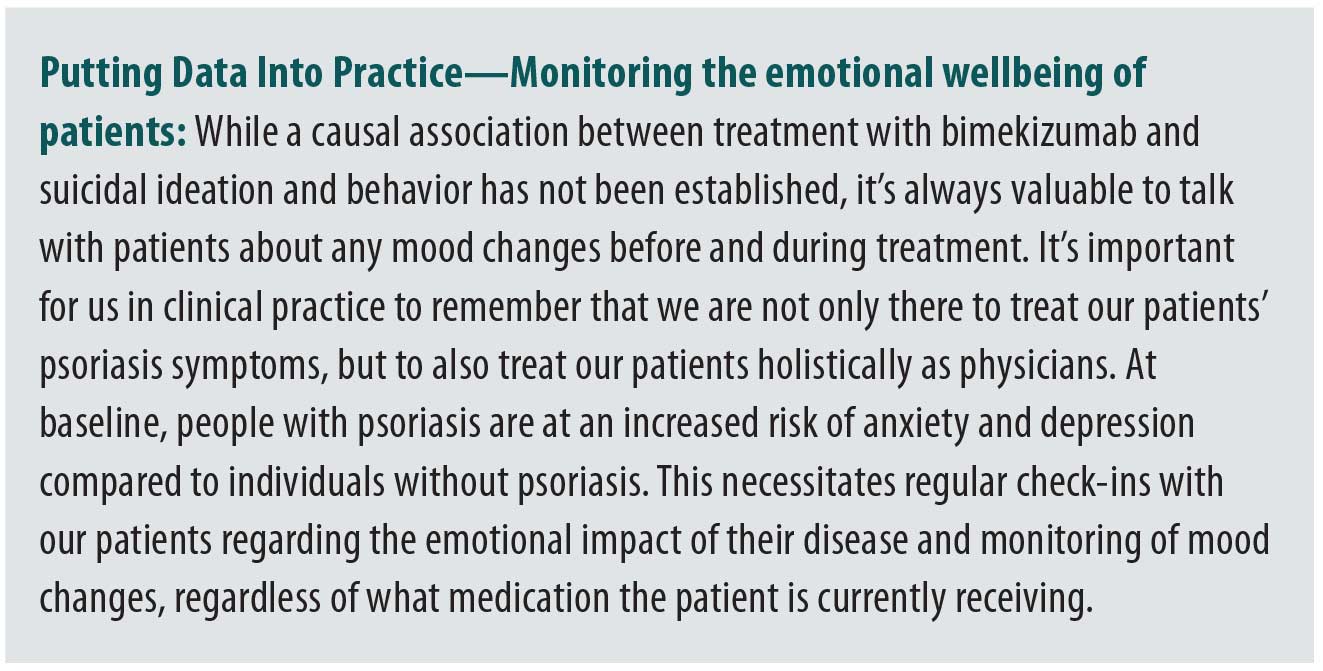
Throughout bimekizumab clinical trials for plaque psoriasis, there have been a few cases of reported suicidal ideation episodes and one completed suicide. The case of completed suicide occurred in the OLE trial after 718 days of treatment; the patient had no known psychiatric medical history, however, financial issues were reported as risk factors. None of these events were assessed to be related to treatment with bimekizumab.9,16
Funding
Funding for the preparation of this article was provided by UCB Pharma.
Disclosures
Dr. Noor has served as a speaker for UCB Pharma. Dr. Beer and Ms. Hermak have no relevant disclosures.
References
- Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: Epidemiology. J Am Acad Dermatol. Mar 2017;76(3):377-390. doi:10.1016/j.jaad.2016.07.064
- Blauvelt A, Chiricozzi A. The Immunologic Role of IL-17 in Psoriasis and Psoriatic Arthritis Pathogenesis. Clin Rev Allergy Immunol. Dec 2018;55(3):379-390. doi:10.1007/s12016-018-8702-3
- Adams R, Maroof A, Baker T, et al. Bimekizumab, a Novel Humanized IgG1 Antibody That Neutralizes Both IL-17A and IL-17F. Front Immunol. 2020;11:1894. doi:10.3389/fimmu.2020.01894
- Brembilla NC, Senra L, Boehncke WH. The IL-17 Family of Cytokines in Psoriasis: IL-17A and Beyond. Front Immunol. 2018;9:1682. doi:10.3389/fimmu. 2018.01682
- Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. Feb 6 2021;397(10273):475-486. doi:10.1016/s0140-6736(21)00126-4
- Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet. Feb 6 2021;397(10273):487-498. doi:10.1016/s0140-6736(21)00125-2
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus Secukinumab in Plaque Psoriasis. N Engl J Med. Jul 8 2021;385(2):142-152. doi:10.1056/NEJMoa2102383
- Warren RB, Blauvelt A, Bagel J, et al. Bimekizumab versus Adalimumab in Plaque Psoriasis. N Engl J Med. Jul 8 2021;385(2):130-141. doi:10.1056/NEJMoa2102388
- Gordon KB, Langley RG, Warren RB, et al. Bimekizumab safety in patients with moderate-to-severe plaque psoriasis: pooled data from up to 3 years of treatment in randomized phase III trials. Br J Dermatol. Mar 15 2024;190(4):477-485. doi:10.1093/bjd/ljad429
- Strober B, Tada Y, Mrowietz U, et al. Bimekizumab maintenance of response through 3 years in patients with moderate-to-severe plaque psoriasis: results from the BE BRIGHT open-label extension trial. Br J Dermatol. May 24 2023;188(6):749-759. doi:10.1093/bjd/ljad035
- Kokolakis G, Warren RB, Strober B, et al. Bimekizumab efficacy and safety in patients with moderate-to-severe plaque psoriasis who switched from adalimumab, ustekinumab or secukinumab: results from phase III/IIIb trials. Br J Dermatol. Feb 22 2023;188(3):330-340. doi:10.1093/bjd/ljac089
- Armstrong A, Fahrbach K, Leonardi C, et al. Efficacy of Bimekizumab and Other Biologics in Moderate to Severe Plaque Psoriasis: A Systematic Literature Review and a Network Meta-Analysis. Dermatol Ther (Heidelb). Aug 2022;12(8):1777-1792. doi:10.1007/s13555-022-00760-8
- Davidson L, van den Reek J, Bruno M, et al. Risk of candidiasis associated with interleukin-17 inhibitors: A real-world observational study of multiple independent sources. Lancet Reg Health Eur. Feb 2022;13:100266. doi:10.1016/j.lanepe.2021.100266
- Fieldhouse KA, Ukaibe S, Crowley EL, Khanna R, O’Toole A, Gooderham MJ. Inflammatory bowel disease in patients with psoriasis treated with interleukin-17 inhibitors. Drugs Context. 2020;9doi:10.7573/dic.2020-2-1
- Hohenberger M, Cardwell LA, Oussedik E, Feldman SR. Interleukin-17 inhibition: role in psoriasis and inflammatory bowel disease. J Dermatolog Treat. Feb 2018;29(1):13-18. doi:10.1080/09546634.2017.1329511
- Gordon KB, Langley RG, Warren RB, et al. Bimekizumab Safety in Patients With Moderate to Severe Plaque Psoriasis: Pooled Results From Phase 2 and Phase 3 Randomized Clinical Trials. JAMA Dermatology. 2022;158(7):735-744. doi:10.1001/jamadermatol.2022.1185