J Clin Aesthet Dermatol. 2019;12(1):11–15
 by James Q. Del Rosso, DO; Leon Kircik, MD; Tina Lin PharmD; and Radhakrishnan Pillai, PhD
by James Q. Del Rosso, DO; Leon Kircik, MD; Tina Lin PharmD; and Radhakrishnan Pillai, PhD
Dr. Del Rosso is with JDR Dermatology Research/Thomas Dermatology in Las Vegas, Nevada. Dr. Kircik is with the Indiana University School of Medicine in Indianapolis, Indiana; Physicians Skin Care, PLLC in Louisville, Kentucky; and the Icahn School of Medicine at Mount Sinai in New York, New York. Dr. Lin is with Ortho Dermatologics in Bridgewater, New Jersey. Dr. Pillai is with Dow Pharmaceutical Sciences Inc. in Petaluma, California.
Funding: Ortho Dermatologics funded the activities of Konic Ltd. (West Sussex, England, United Kingdom) pertaining to this manuscript. Konic Ltd. provided editorial services.
Disclosures: Dr. Del Rosso is a researcher, consultant, and speaker for Ortho Dermatologics. Dr. Kircik is an adviser, consultant, and investigator with Ortho Dermatologics. Drs. Lin and Pillai are employees of Bausch Health.
Abstract: Background.Psoriasis is a chronic inflammatory skin disorder often managed with topical therapy. However, the most widely used treatments, including topical corticosteroids and tazarotene, can cause limiting skin irritation, contact sensitization, and/or present long-term safety concerns. Recently, clinical data on a new fixed combination of halobetasol 0.01%/tazarotene 0.045% (HP/TAZ) lotion were published.
Objectives. We evaluated the results from two studies that assessed the potential for skin irritation (Study A) and contact sensitization (Study B) of HP/TAZ lotion.
Methods.HP/TAZ lotion and vehicle were studied in 244 healthy volunteers for the potential to induce contact skin sensitization. An additional 21-day cumulative irritation study was performed in 40 healthy volunteers to compare HP/TAZ lotion, vehicle, tazarotene 0.05% (Tazorac®; Allergan, Dublin, Ireland) cream, and positive/negative controls. Cutaneous reactions at the application sites were evaluated in both studies clinically.
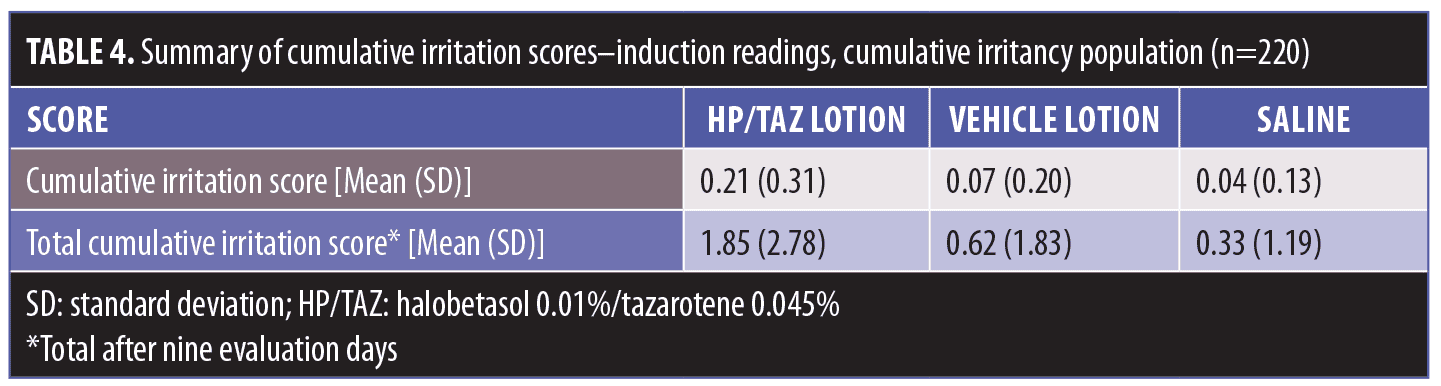
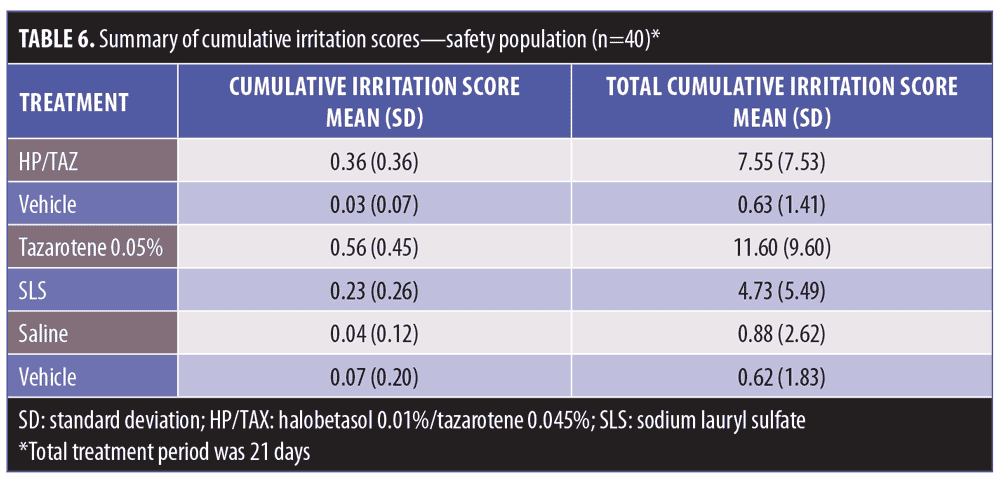
Results.There was no evidence of contact sensitization, while the HP/TAZ lotion exhibited a low level of irritation in the induction phase. Mean cumulative irritation scores were 0.21 (HP/TAZ), 0.07 (vehicle), and 0.04 (saline). In the separate 21-day irritation study, mean cumulative irritation scores were 0.36 (HP/TAZ), 0.56 (tazarotene), 0.03 (vehicle), 0.23 (sodium lauryl sulfate), and 0.04 (saline), while mean total irritation scores were 7.55 (HP/TAZ), 11.60 (tazarotene, p=0.0013 versus HP/TAZ), 0.63 (vehicle), 4.73 (sodium lauryl sulfate), and 0.88 (saline). HP/TAZ was “slightly irritating” but significantly less so than tazarotene (p=0.0009).
Conclusion.HP/TAZ lotion caused only minimal skin irritation. It was significantly less irritating than tazarotene cream.
Keywords: Psoriasis, topical, halobetasol, tazarotene, clinical trial
Psoriasis is a chronic, immune-mediated disease that varies widely in clinical expression; it affects 1 to 2 percent of the population worldwide, including predominantly adults.1 Treatment options focus on relieving signs and symptoms through a reduction of inflammation, induration, and scaling, thus leading to a decrease in the extent of the disease so that it no longer interferes with the personal, social, and occupational wellbeing of the patient. Overall, the majority of psoriasis patients can be treated successfully with topical regimens.2
Topical corticosteroids (TCS), especially higher potency TCS, are the mainstay of psoriasis treatment. However, long-term safety remains a concern when their administration is prolonged.2–4 Tachyphylaxis or tolerance can occur with lengthy use and, although uncommon, the risk of contact sensitization also exists.5 Local adverse effects of TCS use include skin atrophy, telangiectasias, persistent erythema, leukoderma, and folliculitis.6
Halobetasol propionate 0.05% ointment has been shown to be more effective than clobetasol propionate 0.05% ointment,7 betamethasone dipropionate ointment,8 and betamethasone valerate 0.1% ointment9 in plaque psoriasis. However, because of the risks associated with prolonged TCS use,10 labelling recommends daily application be limited to 14 days in the case of super-potent TCS such as halobetasol propionate, clobetasol propionate, and augmented betamethasone dipropionate.6
Tazarotene is also indicated for the topical treatment of plaque psoriasis; however, local, dose-dependent irritation can limit its use in some individuals.11–14
Several clinical studies have shown enhanced efficacy and skin tolerability with TCS therapy used in combination with topical tazarotene in the treatment of plaque psoriasis.15–17 Recently, data on a fixed-combination halobetasol propionate 0.01%/tazarotene 0.045% (HP/TAZ) lotion were published.18 In the investigation, HP/TAZ lotion was more effective than its individual active ingredients and vehicle and the reported safety data appear consistent with the known profiles of halobetasol propionate and tazarotene, respectively.
This article reviews outcomes from further research on the safety and tolerability of HP/TAZ lotion, specifically considering contact sensitization and skin irritation potential.
Methods
Study oversight. Subjects provided written, informed consent before study-related procedures were performed, and protocols and consent were approved by the Institutional Review Board (IntegReview, Austin, Texas). The studies were conducted in accordance with the principles of Good Clinical Practice (GCP) and the Declaration of Helsinki.
Study A: contact sensitization. A randomized, single-center, evaluator-blinded study in healthy adult volunteers (aged 18–70 years) was conducted to examine the potential of HP/TAZ lotion, vehicle, and negative control (0.9% saline; Medline Industries, Inc., Northfield, Illinois) to induce contact sensitization following repeated skin application. The study included a number of discreet phases, as follows: induction (3 weeks), rest period (2 weeks), challenge (1 week), a second rest period (1 week), and rechallenge (if deemed necessary by the study investigator) (Figure 1).
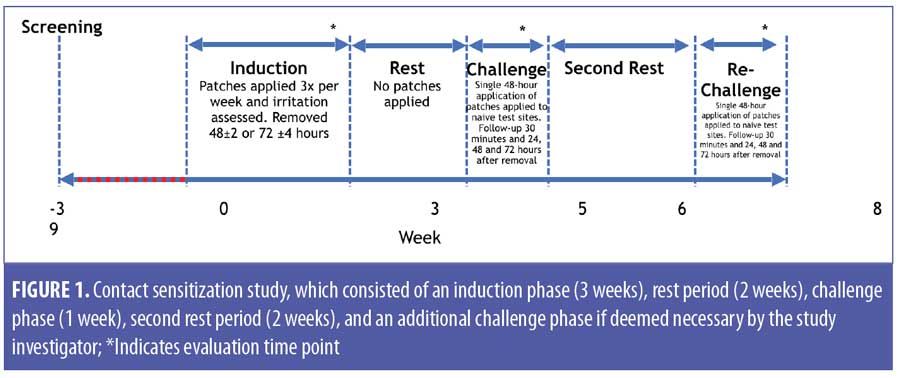
Induction phase. Test and control formulations were applied to adjacent sites on the infrascapular area of the back. Baseline evaluations were performed immediately prior to application of the patches to ensure that no skin conditions, markings, or coloration of the skin could interfere with interpretation of the study results.
Nine repetitive applications were evaluated under semiocclusive patch conditions on the same site over a period of three weeks. Patches were applied on Mondays, Wednesdays, and Fridays and removed after 48 hours; patches applied on Fridays were worn for 72 hours (±4hrs) until the following Monday.
Rest period. Subjects did not receive any application of the study drug or test articles for approximately two weeks (10–14 days).
Challenge phase. This was composed of one 48-hour semiocclusive patch application to a naïve site on the opposite side of the back, with evaluation at 30 minutes and at approximately 24 hours, 48 hours, and 72 hours following patch removal. Only subjects who underwent nine applications of the study material and had no fewer than eight subsequent readings during the induction phase and one application followed by subsequent readings during the challenge phase were considered “completed cases” and were used to assess sensitization.
Rechallenge. Patches were to be applied at least two weeks after the challenge phase, in a similar manner. Subjects who exhibited skin reactivity suggestive of induced contact sensitization (i.e., definite erythema with papules and/or edema) were rechallenged for 48 hours, with assessments occurring at 30 minutes and at approximately 24 hours, 48 hours, and 72 hours after removal.
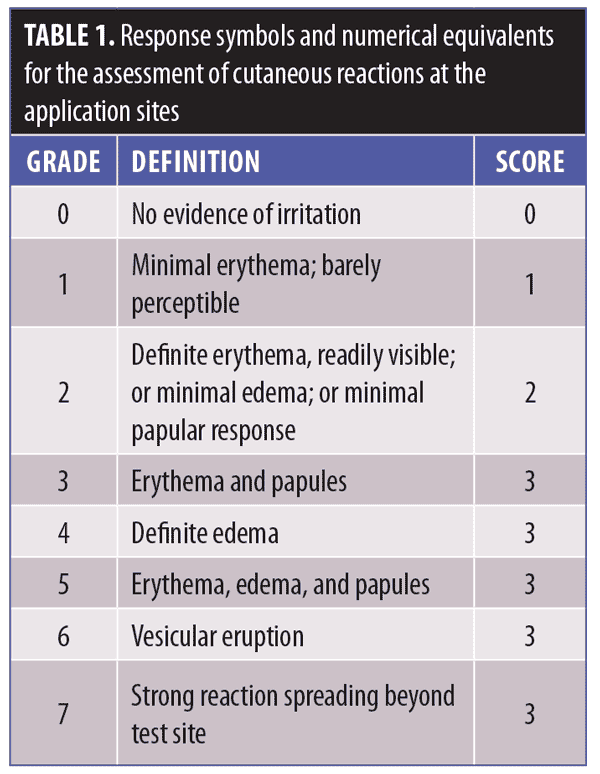
Evaluation. Cutaneous reactions at the application sites were assessed clinically using a scale rating the degree of erythema, edema, and other signs of cutaneous irritation (Table 1). The primary measure of contact sensitization induction was determined through assessments of the application sites during the challenge and (if applicable) rechallenge phases. At the end of the challenge period, the investigators assessed the occurrence of any induced contact allergic reactions. Other local skin reactions and adverse events (AEs) were reported individually.
Repeated insult patch test (RIPT) evaluation is a predictive patch study that can detect weak sensitizers that require multiple applications to induce a cell-mediated (Type IV) immune response sufficient to cause an allergic reaction. Irritant reactions can also be detected via this method, although it is not the primary purpose of the procedure. Nevertheless, a total cumulative irritation score for each subject and study material was calculated by summing individual scores from each of the nine evaluation days in the induction phase. Cumulative irritancy was quantified by means of the cumulative irritancy index (CII), defined as the mean of irritation scores received during the induction phase.
The CII (mean irritation score) was tested pairwise for product differences using Fisher’s protected least significant differences (LSD) in the context of a two-way analysis of variance (ANOVA) including main effects of subject and product, without interaction. If a maximum score of three points or greater was achieved for any test site, the cumulative irritancy score was calculated as the maximum score of three points for that individual site for the remainder of the induction phase.
Study B: 21-day cumulative irritation. Cumulative irritancy patch evaluation is a modified primary irritancy test, which can detect irritants that require multiple applications to cause a skin reaction. These reactions are the result of direct damage to the epidermal cells, and no immunologic (allergic) mechanism is involved. This procedure can detect so-called “fatiguing substances,” which are mild irritants that cause more strongly positive reactions with successive multiple skin exposures.
Here, a single-center, controlled, evaluator-blinded, within-subject randomized assessment in healthy adult volunteers to evaluate the irritation potential of HP/TAZ lotion, vehicle, tazarotene 0.05% cream (Tazorac®; Allergan, Dublin, Ireland), and both positive (0.2% sodium lauryl sulfate [SLS; w/v in deionized water]) and negative (0.9% saline) controls, following daily semiocclusive patch applications, was conducted.
The test formulations (approximately 0.2mL) were applied to semiocclusive patches (2cm×2cm Webril® patch pad; Medline Industries, Inc., Northfield, Illinois) on the same spot every day across the upper back and secured with hypoallergenic porous tape, as needed. A total of 21 applications of test formulation were made over a period of 22 days. Patches were removed every 24±2 hours after application and the test sites were evaluated.
If the skin was not disrupted (skin irritation score ?2), identical treatments and patches were reapplied to the same test sites using the procedures above. Separate individuals were responsible for the test sites (blinded) and patch application/removal (unblinded). If a subject scored three points for two consecutive visits, the corresponding test formulation was discontinued and the last score was carried forward. Each test site was evaluated for signs of irritation, pruritus, burning, stinging, and tape reaction.
The mean (i.e., mean of the observed scores on Days 2–22) and total cumulative irritancy scores were calculated as above. These parameters were tested pairwise for product differences using Fisher’s protected LSD in the context of a two-way ANOVA, including main effects of subject and test formulation, without interaction. Pairwise differences were tested only if the null hypothesis of a common mean score for all products was rejected at the five-percent level. A normalized total score for each patch was calculated by summing the total irritation scores for each subject, dividing by the number of subjects, and multiplying by a factor of 10 (where 0–49=no significant irritation, 50–199=slightly irritating, 200–449=moderately irritating, and 450–630=highly irritating).19
Results
Study A: contact sensitization. Overall, 244 subjects were enrolled and randomized, with 208 (85.2%) of subjects completing the study. Main reasons for study discontinuation were protocol violation (n=21, 8.6%), subject withdrawal (n=7, 2.9%) and lost to follow-up (n=6, 2.5%). Mean age (standard deviation [SD]) was 50.2 (12.61) years with 67.6 percent being women (n=165). The majority of subjects were African-American (71.7%, n=175), with Fitzpatrick skin Type V or VI (63.1%, n=154) (Table 2).
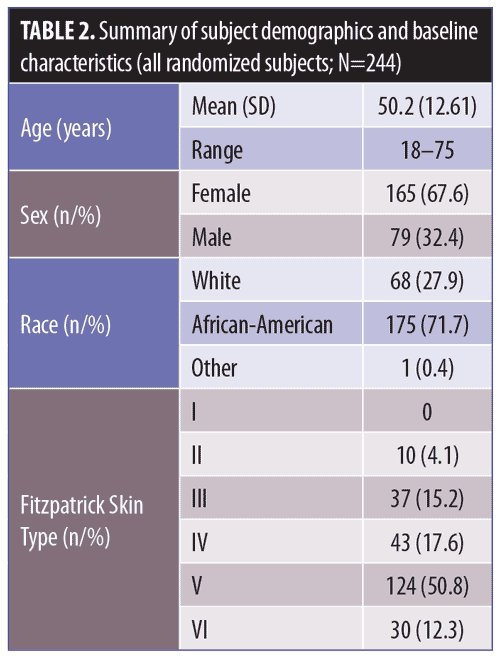
Twelve subjects out of the 220 who completed the induction phase had the necessary nine applications of test formulation, but did not complete the subsequent challenge evaluations during the challenge phase.
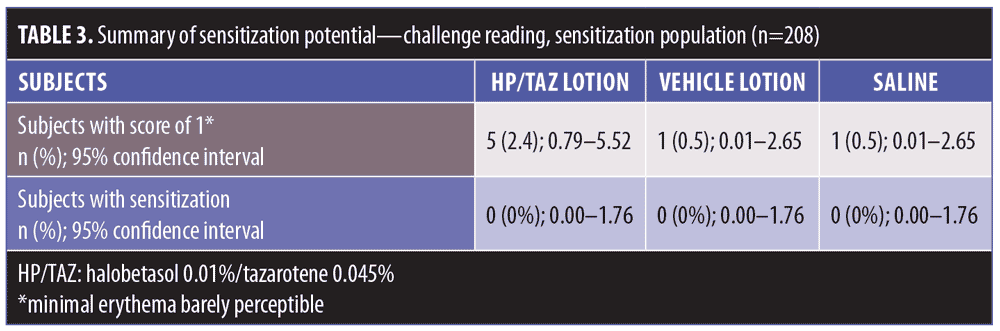
The determination of cutaneous sensitization potential was based on the recurrence of a skin response at rechallenge equivalent to or more severe than that observed during the challenge phase. No subjects were classified as showing signs indicative of sensitization to any of the test formulations or control. During the challenge phase, five (2.4%), three (1.4%) and one (0.5%) subjects had scores of one point (HP/TAZ, vehicle, and saline, respectively) (Table 3). No subject had a score of more than one point at any of the time points. No subjects were rechallenged.
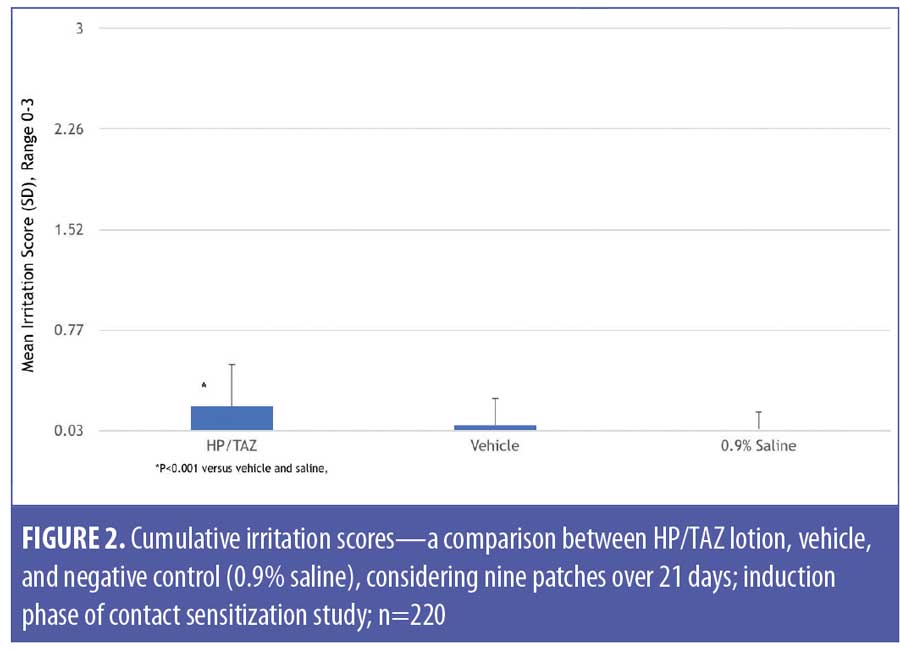
The 220 subjects who completed the induction phase were included in the cumulative irritancy population. HP/TAZ lotion exhibited a low level of irritation, although the results were significantly (p<0.0001) higher than those of the vehicle and saline control (Table 4). Mean cumulative irritation scores (CII) were 0.21 (HP/TAZ), 0.07 (vehicle), and 0.04 (saline) (Figure 2), while total irritation scores were 1.85 (HP/TAZ), 0.62 (vehicle), and 0.33 (saline).
Overall, there were 43 treatment-emergent AEs; none were treatment-related. Three subjects (1.2%) reported serious AEs (lower abdominal pain, dehydration and vomiting; pyelonephritis; colitis) and one subject reported an AE leading to study withdrawal (acneiform eruption).
Study B: 21-day cumulative irritation. Overall, 52 subjects were screened and 40 randomized; 36 (90.0%) subjects completed the study, with four subjects withdrawing. Mean age (SD) was 51.2 (14.08) years with 80.0 percent being female (n=32). The majority of subjects were African-American (65.0%, n=26), with Fitzpatrick skin Type IV or V (77.5%, n=31)
(Table 5).
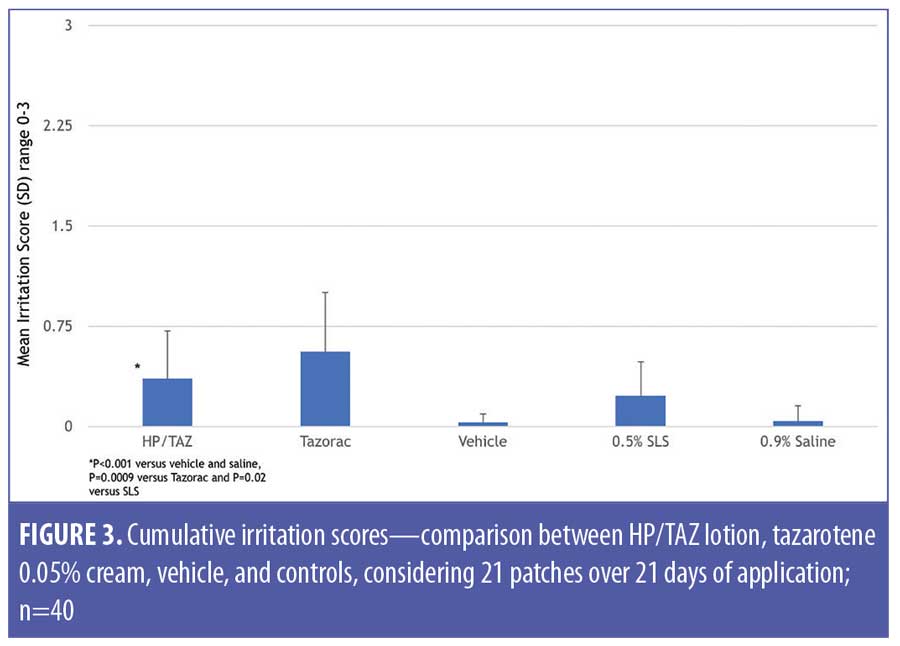
HP/TAZ lotion exhibited a low level of irritation, although results were significantly higher than those seen with the vehicle (Table 6). HP/TAZ lotion was significantly less irritating than tazarotene 0.05% cream (p=0.0009). Mean cumulative irritation scores were 0.36 (HP/TAZ, p=0.02 vs. SLS and p<0.00001 vs. vehicle and saline), 0.56 (tazarotene, p<0.00001 vs. SLS and saline), 0.03 (vehicle, p<0.00001 vs. tazarotene and 0.0012 vs. SLS), 0.23 (SLS, p=0.002 vs. saline), and 0.04 (saline) (Figure 3). Additionally, mean total irritation scores were 7.55 (HP/TAZ, p=0.0013 vs.tazarotene), 11.60 (tazarotene), 0.63 (vehicle), 4.73 (SLS), and 0.88 (saline). Normalized total scores were 76 points and 116 points for HP/TAZ lotion and tazarotene, respectively, (slightly irritating) and six points for vehicle (no significant irritation).
Overall, two subjects reported four treatment-emergent AEs (respiratory, thoracic, mediastinal disorder [i.e., cough]; tooth extraction and tooth repair), with none being treatment-related. No subjects reported serious AEs or AE leading to study withdrawal.
Discussion
HP/TAZ lotion has been shown to be more effective than its individual active ingredients or vehicle in the treatment of moderate-to-severe plaque psoriasis and is well-tolerated.18
Substances that come into contact with human skin need to be evaluated for their propensity to irritate and/or sensitize the skin. Topical retinoids have been shown to cause local irritation, which is manifested as erythema and peeling.20,21 Consequently, our findings that tazarotene 0.05% cream was associated with skin irritation was anticipated. Tazarotene gel (0.1% and 0.05%) and cream (0.1% and 0.05%) have both been shown to be more irritating than adapalene 0.1% cream/gel22 and adapalene 0.1% gel/solution.23 Furthermore, tazarotene 0.1% cream was shown to be more irritating than tazarotene 0.05% cream.22 Tazarotene 0.1% foam has also demonstrated the potential to induce skin irritation, with a low potential for contact sensitization reactions.24
Local skin reactions, such as skin atrophy, telangiectasia, erythema, and folliculitis, might occur with topical halobetasol.6 However, we are not aware of any studies that have investigated cumulative irritant potential. Contact allergy to TCS is a potential problem, although the diagnosis might not be obvious to clinicians, and evaluation can be complex.25
One potential benefit in developing fixed combinations is the ability to formulate effective products with lower concentrations of active ingredients. HP/TAZ lotion contains only 0.1% halobetasol propionate and 0.045% tazarotene. The combination has been shown to be significantly more effective than tazarotene 0.05% cream and as effective as halobetasol 0.5% cream in treating moderate-to-severe psoriasis.18 As shown above, HP/TAZ lotion was significantly less irritating than tazarotene 0.05% cream. In addition, HP/TAZ lotion did not exhibit any contact sensitization potential.
While it has been shown that lower concentrations of tazarotene are associated with less cutaneous irritation, skin sensitivity and vehicle formulation can also affect tolerability.10 As the concentration of tazarotene in HP/TAZ lotion is very similar (0.045%) to the commercially available formulations of tazarotene (0.05%), the benefits seen, in terms of less irritation, might be a result of the lotion formulation and/or the combination with halobetasol propionate.
Conclusion
HP/TAZ lotion is an effective, well-tolerated treatment for moderate-to-severe plaque psoriasis. HP/TAZ lotion did not cause contact sensitization, exhibited a low level of irritation, and was significantly less irritating than Tazorac® cream.
Acknowledgments
We thank Brian Bulley, MSc (Konic Ltd., Lindfield, United Kingdom) for assistance with the preparation of the manuscript. Ortho Dermatologics funded Konic’s activities pertaining to this manuscript.
References
- Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133(2):377–385.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60(4):643–659.
- Levin E, Gupta R, Butler D, et al. Topical steroid risk analysis: differentiating between physiologic and pathologic adrenal suppression. J Dermatolog Treat. 2014;25(6):501–506.
- Lam LH, Sugarman JL. Adrenal suppression with chronic topical corticosteroid use in psoriasis patients. J Drugs Dermatol. 2016;15(8):945–948.
- Butani L. Corticosteroid-induced hypersensitivity reactions. Ann Allergy Asthma Immunol. 2002;89(5):439–445.
- Ultravate® (halobetasol propionate) Cream, 0.05% and (halobetasol propionate) Ointment 0.05% [US Prescribing Information]. Jacksonville, FL: Ranbaxy; 2015.
- Goldberg B, Hartdegen R, Presbury D, et al. A double blind multicenter comparison of 0.05% halobetasol propionate ointment and 0.05% clobetasol propionate ointment in patients with chronic localized plaque psoriasis. J Am Acad Dermatol. 1991;25(6):1145–1148.
- Mensing H, Korsukewitz G, Yawalkar S. A double blind multicenter comparison of 0.05% halobetasol propionate ointment and 0.05% betamethasone dipropionate ointment in chronic plaque psoriasis. J Am Acad Dermatol. 1991;25(6 Pt 2):1166–1169.
- Blum G, Yawalkar S. A comparative multicenter double blind trial of 0.05% halobetasol propionate ointment and 0.1% betamethasone valerate ointment in the treatment of patients with chronic localized plaque psoriasis. J Am Acad Dermatol. 1991;25(6 Pt 2):1153–1156.
- Rivera AM, Hsu S. Topical halobetasol propionate in the treatment of plaque psoriasis. Am J Clin Dermatol. 2005;6(5):311–316.
- Tazorac® (tazarotene) Cream, 0.05% and 0.1% [US Prescribing Information]. Irvine, CA: Amgen; 2013.
- Guenther LC. Optimizing treatment with topical tazarotene. Am J Clin Dermatol. 2003;4(3):
197–202. - Weinstein GD, Krueger GG, Lowe NJ, et al. Tazarotene gel, a new retinoid, for topical therapy of psoriasis: vehicle-controlled study of safety, efficacy, and duration of therapeutic effect. J Am Acad Dermatol. 1997;37(1):85–92.
- Weinstein GD, Koo JYM, Krueger GG, et al. Tazarotene cream in the treatment of psoriasis: Two multicenter, double-blind, randomized, vehicle-controlled studies of the safety and efficacy of tazarotene creams 0.05% and 0.1% applied once daily for 12 weeks. J Am Acad Dermatol. 2003;48(5):760–767.
- Koo JY, Martin D. Investigator-masked comparison of tazarotene gel q.d. plus mometasone furoate cream q.d. vs mometasone furoate cream b.i.d. in the treatment of plaque psoriasis. Int J Dermatol. 2001;40(3):210–212.
- Lebwohl MG, Breneman DL, Goffe BS, et al. Tazarotene 0.1% gel plus corticosteroid cream in the treatment of plaque psoriasis. J Am Acad Dermatol. 1998;39(4 Pt 1):590–596.
- Kaidbey K, Kopper SC, Sefton J, et al. A pilot study to determine the effect of tazarotene gel 0.1% on steroid-induced epidermal atrophy. Int J Dermatol. 2001;40(7):468–471.
- Sugarman JL, Stein Gold L, Lebwohl MG, et al. A phase 2, multicenter, double-blind, randomized, vehicle controlled clinical study to assess the safety and efficacy of a halobetasol/tazarotene fixed combination in the treatment of plaque psoriasis. J Drugs Dermatol. 2017;16(3):194–201.
- Berger RS, Bowman JP. A reappraisal of the 21-day cumulative irritation test in man. J Toxicol Cut and Ocular Toxicol. 1982;1(2):109–115.
- Leyden J, Grove G, Zerweck C. Facial tolerability of topical retinoid therapy. J Drugs Dermatol. 2004;3(6):641–651.
- Kim BH, Lee YS, Kang KS. The mechanism of retinol-induced irritation and its application to anti-irritant development. Toxicol Lett. 2003;146(1):65–73.
- Dosik JS, Homer K, Arsonnaud S. Cumulative irritation potential of adapalene 0.1% cream and gel compared with tazarotene cream 0.05% and 0.1%. Cutis. 2005;75(5):289–293.
- Greenspan A, Loesche C, Vendetti N, et al. Cumulative irritation comparison of adapalene gel and solution with 2 tazarotene gels and 3 tretinoin formulations. Cutis. 2003;72(1):76–81.
- Berg JE, Bowman JP, Alio Saenz AB. Cumulative irritation potential and contact sensitization potential of tazarotene foam 0.1% in 2 phase 1 patch studies. Cutis. 2012;90(4):206–211.
- Foti C, Calogiuri G, Cassano C, et al. Contact allergy to topical corticosteroids: update and review on cross-sensitization. Recent Pat Inflamm Allergy Drug Discov. 2009;3(1):33–39.

