 J Clin Aesthet Dermatol. 2022;15(12):33–37.
J Clin Aesthet Dermatol. 2022;15(12):33–37.
by Amany Ibrahim Mustafa, MD; Ahmed Said Kadah, MD; Eman Mohamed Fawzy, PhD;
Ghada Mohamed Mahmoud, MD
Dr. Mustafa is with the Department of Dermatology, Venereology, and Andrology and Faculty of Medicine at Benha University in Benha, Egypt. Dr. Kadah is with the Department of Dermatology, Venereology, and Andrology, and Faculty of Medicine at Al-Azhar University in Cairo, Egypt. Dr. Fawzy is with the Department of Laboratory Medicine at Mansoura Fever Hospital, in Mansoura, Egypt. Dr. Mahmoud is with the Department of Clinical and Chemical Pathology, Faculty of Medicine at Benha University in Benha, Egypt.
FUNDING: No funding was provided for this study.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. Post-adolescent acne is acne in patients aged older than 25 years. It is more common in women, suggesting an underlying hormonal imbalance. It has been postulated that insulin resistance (IR) may play a role in pathogenesis.
Objective. To explore the relationship between fetuin-A, IR, and post-adolescent acne.
Methods. Serum fetuin-A levels were assessed using an ELISA technique in 50 female patients with post-adolescent acne and 50 healthy controls, and IR was calculated using the Homeostasis Model Assessment of Insulin Resistance Index (HOMA-IR).
Results. Studied patients had significantly higher HOMA-IR indices and serum fetuin-A levels than control subjects (P=0.001 and <0.001, respectively) and they were significantly increased in patients with severe lesions (P<0.001).
Conclusion. We found that IR was more significantly prevalent among studied patients, especially those with more severe acne grades, and that could be attributed to higher serum fetuin-A levels. Fetuin -A might be a predictor for acne severity and associated metabolic comorbid conditions, such as IR. However, further large-scale studies will be needed.
Keywords: Post-adolescent acne, insulin resistance, fetuin-A
Acne vulgaris is commonly associated with adolescence. Acne over the age of 25 has traditionally been defined as post-adolescent acne, and it affects about 14 percent of women aged 25 to 50.1–5
The pathophysiology of post-adolescent acne remains unclear. The higher prevalence of acne in females with polycystic ovary syndrome (PCOS), a condition associated with insulin resistance (IR) and hyperandrogenism, lends credence to insulin’s role in acne development as both insulin and insulin-like growth factor-1 (IGF-1) levels peak in late puberty and then eventually decrease until the third decade, and acne usually clears up by this point. They have been shown to increase androgen bioavailability by inducing adrenal androgen synthesis and inhibiting sex hormone-binding globulin production. Female patients with post-adolescent acne have been found to have increased serum IGF-1 levels and may be insulin resistant even if they do not have overt PCOS. It was hypothesized that IR persists in those patients, contributing to acne development.7,8
Fetuin-A is a vital glycoprotein that promotes IR by functioning as an antagonist of the insulin receptor tyrosine kinase in the liver and skeletal muscle.9-12 High fetuin-A levels have been associated with a higher risk of type 2 diabetes (T2D) and metabolic syndrome in humans.13
There is limited research on the role of IR in post-adolescent acne pathogenesis. Thus, we evaluated serum fetuin-A level and IR in patients with post-adolescent acne to reveal the relationship between fetuin-A as a marker for IR and post-adolescent acne.
Methods
Studied population. This study included 50 female patients with post-adolescent acne and 50 healthy controls. The study was approved by the local ethics committee on research involving humans. Each subject provided informed consent before participation. Patients were excluded if they had diabetes, PCOS, or another systemic inflammatory disease, as well as a history of medications that affect insulin metabolism, oral retinoids, hormonal treatment in the previous three months, smoking, or thyroid abnormalities. Patients with obesity were also excluded. The Global Acne Grading System was used to evaluate acne severity.14
Laboratory investigations. After 6 to 8 hours of fasting, each participant provided a 3mL venous blood sample. Serum was extracted from clotted blood through centrifugation at 1,262g for 10 minutes, then aliquoted and stored at 20°C until further testing. An enzyme-linked immunosorbent assay (ELISA) was utilized to evaluate serum fetuin-A levels (BioVendor Laboratory Medicine, Brno, Czech Republic). The intra-assay and inter-assay coefficients of variation (CVs) were respectively 3.9–4.9% and 7.3–8.4%. Fasting insulin levels were determined using a diagnostic double-antibody sandwich ELISA kit (Cat # E29-072, IMMUNOSPEC, Canoga Park, California), and fasting blood glucose levels were estimated using a standard colorimetric method.
Insulin resistance was calculated according to the Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) Index as follows: [Fasting glucose (mmol/L) × Fasting serum insulin (mIU/mL)] ÷ 22.5.
Statistical tests. Data were analyzed using SPSS version.16 To analyze categorical data as numbers (percentages), the Chi-square test was utilized, and quantitative data as mean, SD, and median were analyzed by the Student’s “t” test, Kruskal-Wallis test, and Mann-Whitney U test, respectively. The correlation was assessed using Spearman’s correlation coefficient (r). The receiver operating characteristic (ROC) curve assessed serum fetuin A level and HOMA-IR index cut-off values that had the highest sensitivity and specificity in distinguishing patients from controls, predicting the severity, and scar formation among patients with post-adolescent acne. In our study, a p-value ≤ 0.05 was considered statistically significant.
Results
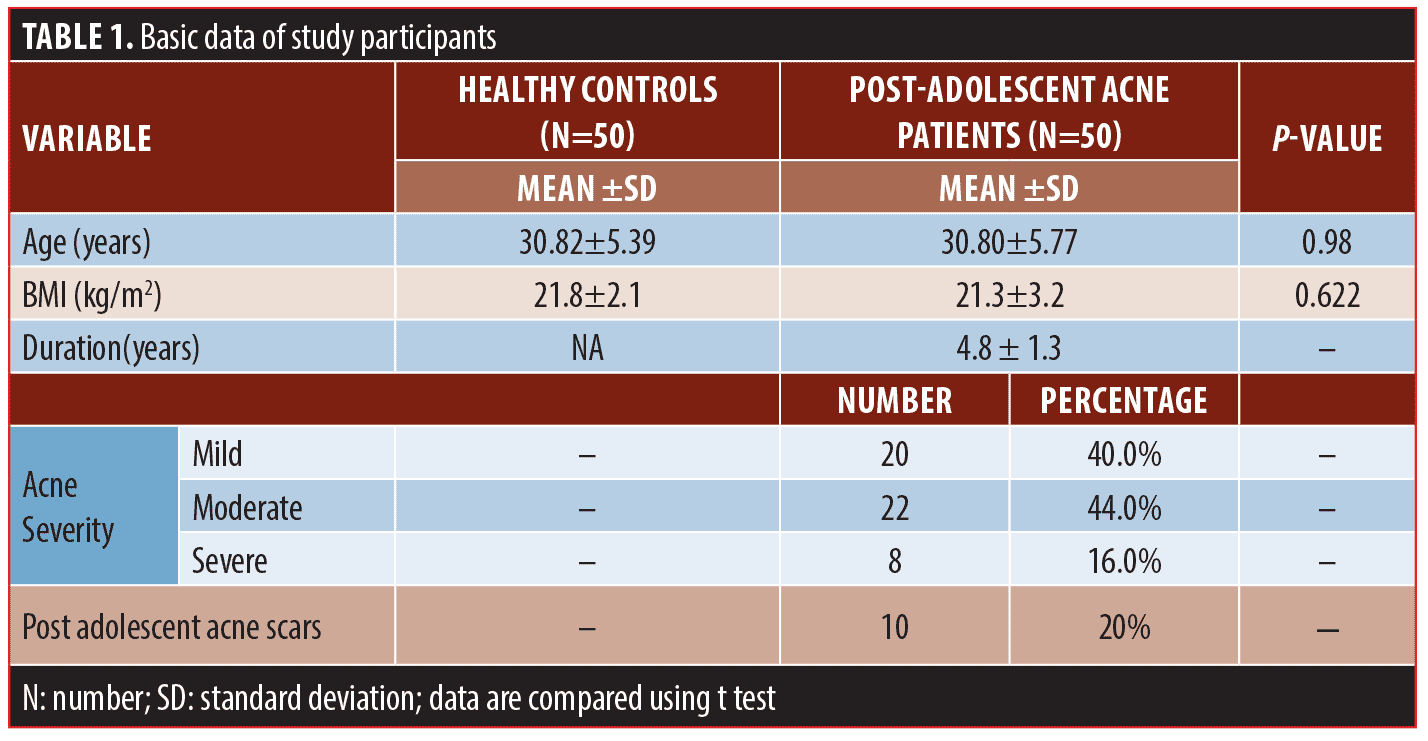
Subjects’ baseline information. This study included 50 female patients with post-adolescent acne; their mean age was 30.82 + 5.39 years. A control group of 50 healthy female participants of matched age and body mass index was also included. Other baseline data is included in Table 1. Serum fasting insulin levels and HOMA-IR indices were significantly higher in studied patients than in control subjects (P=0.0001 and 0.001, respectively) (Table 2 and Figure 1A).
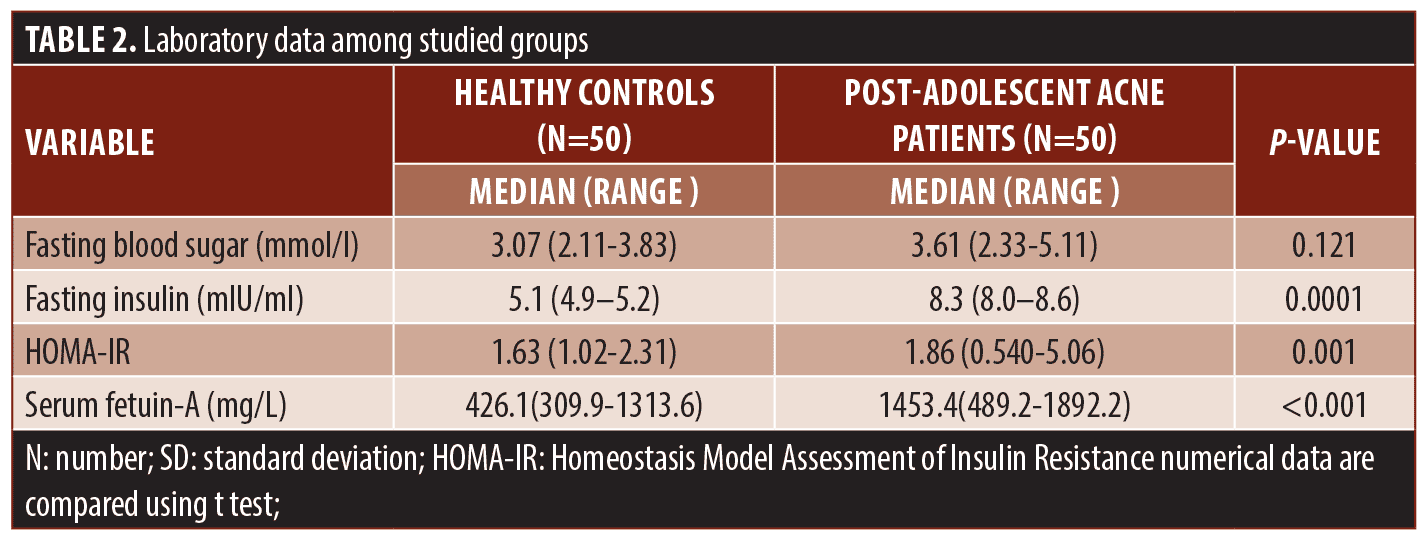
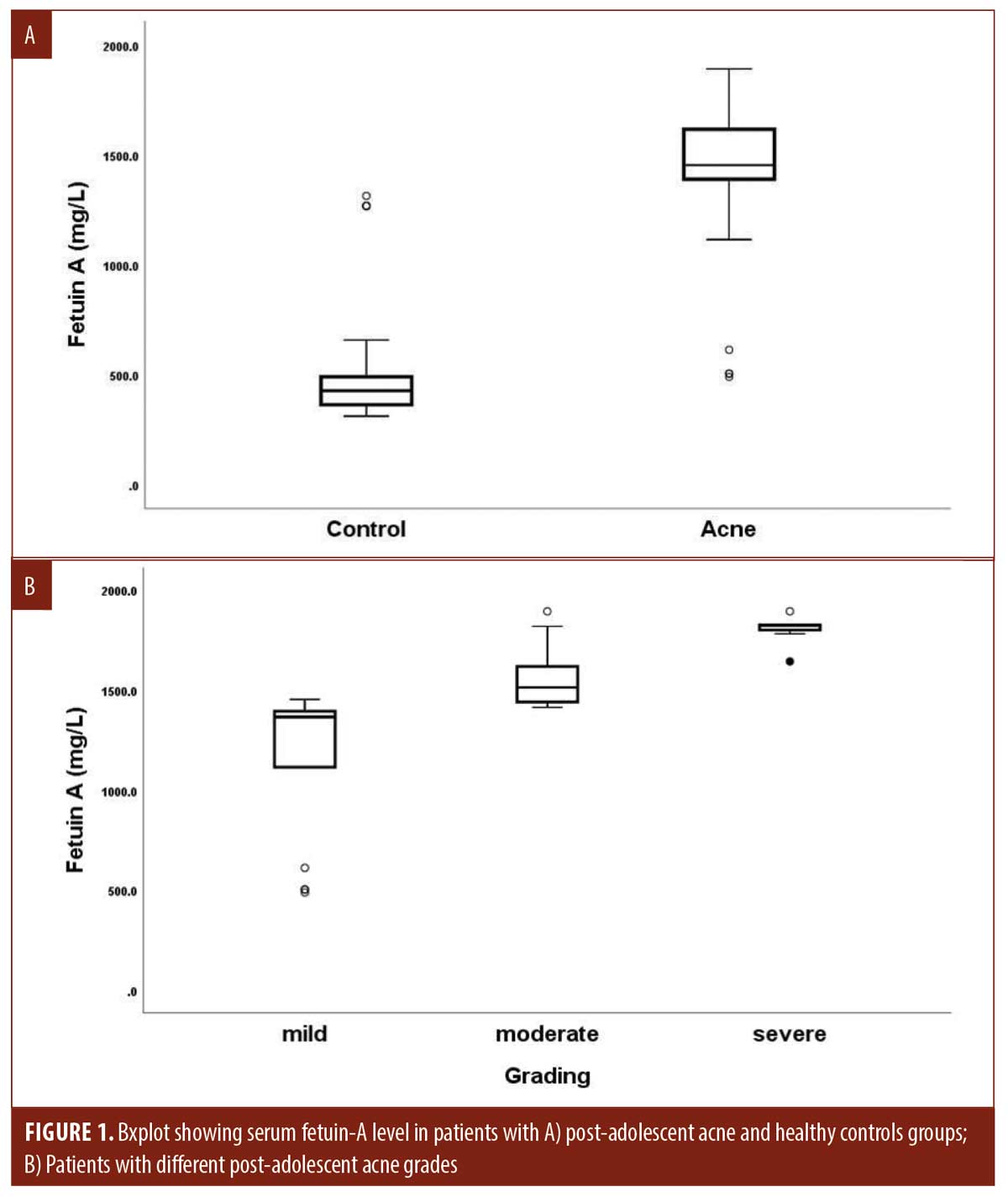
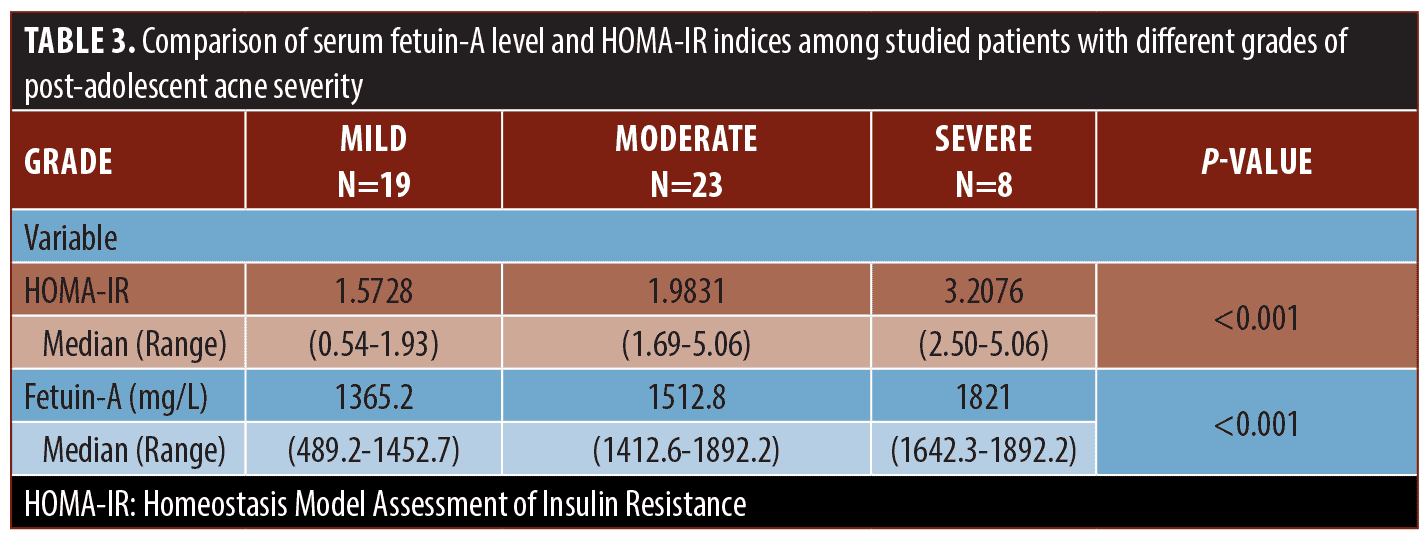
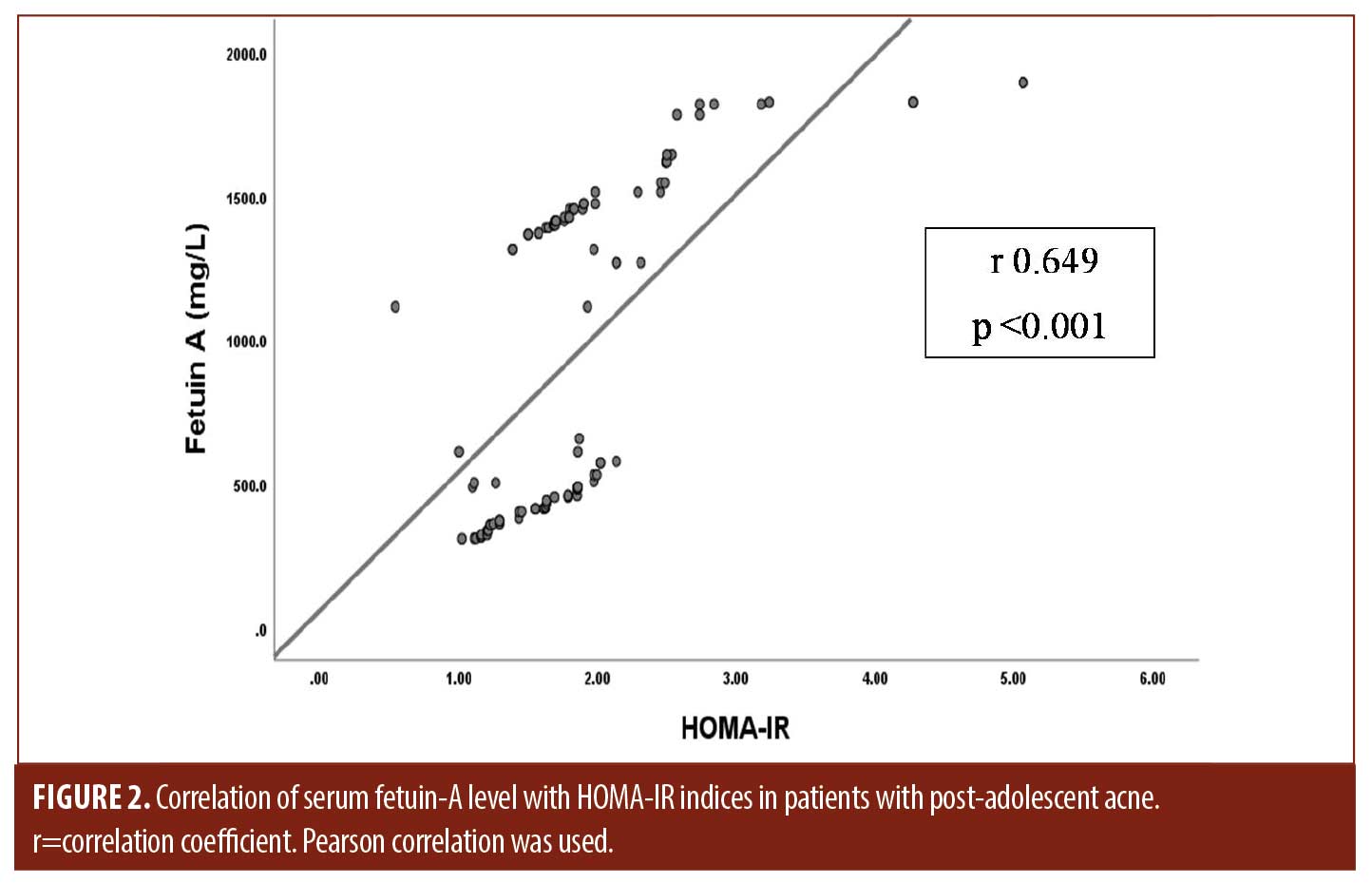
Serum fetuin A levels and clinical parameters. Serum fetuin-A levels in patients were significantly higher than in controls (P=0.001). Moreover, its levels increased significantly in patients with severe post-adolescent acne compared to those with mild and moderate disease (P<0.001) and were positively correlated with HOMA-IR indices among the studied patients (r 0.649, P<0.001) (Tables 2, 3 and Figures 1B, 2).
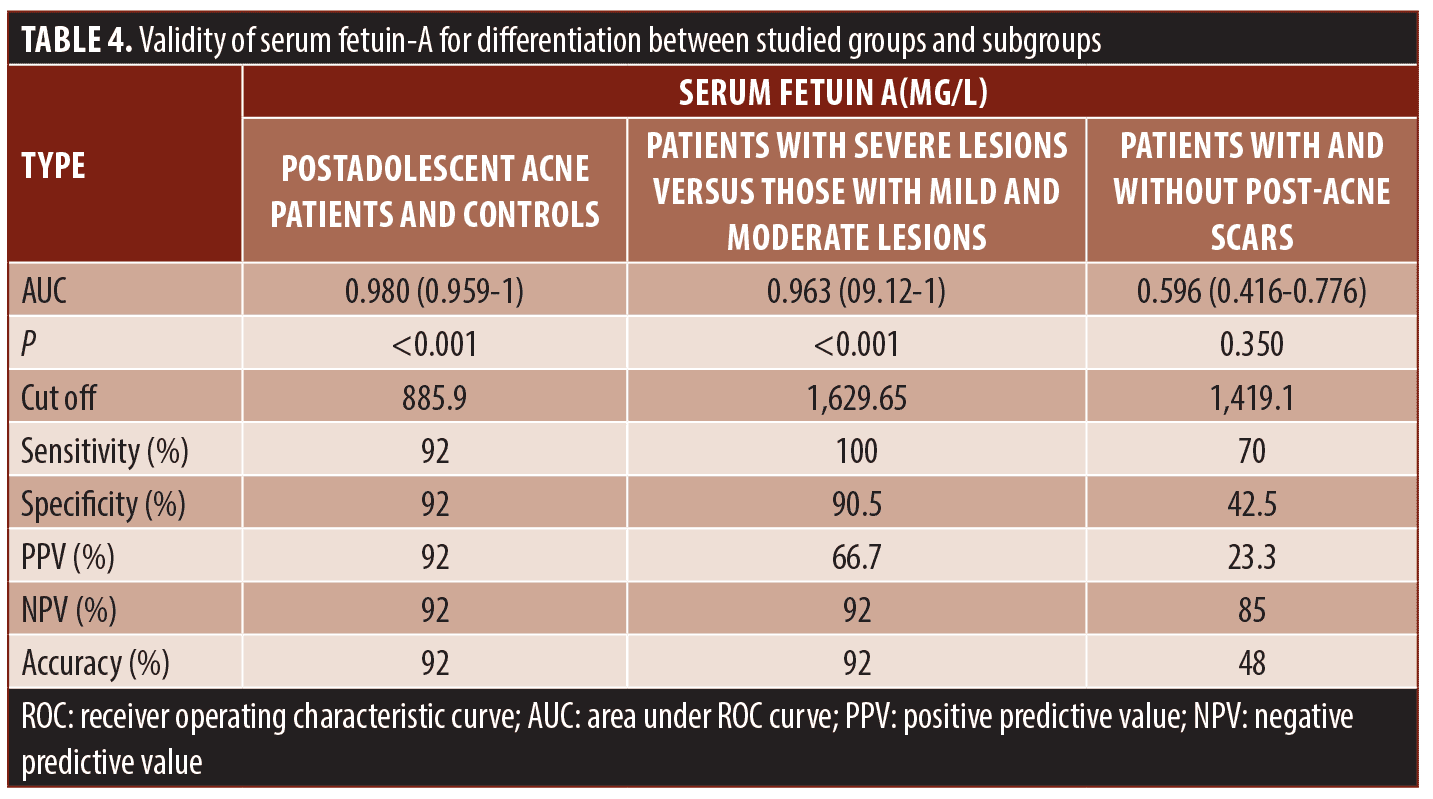
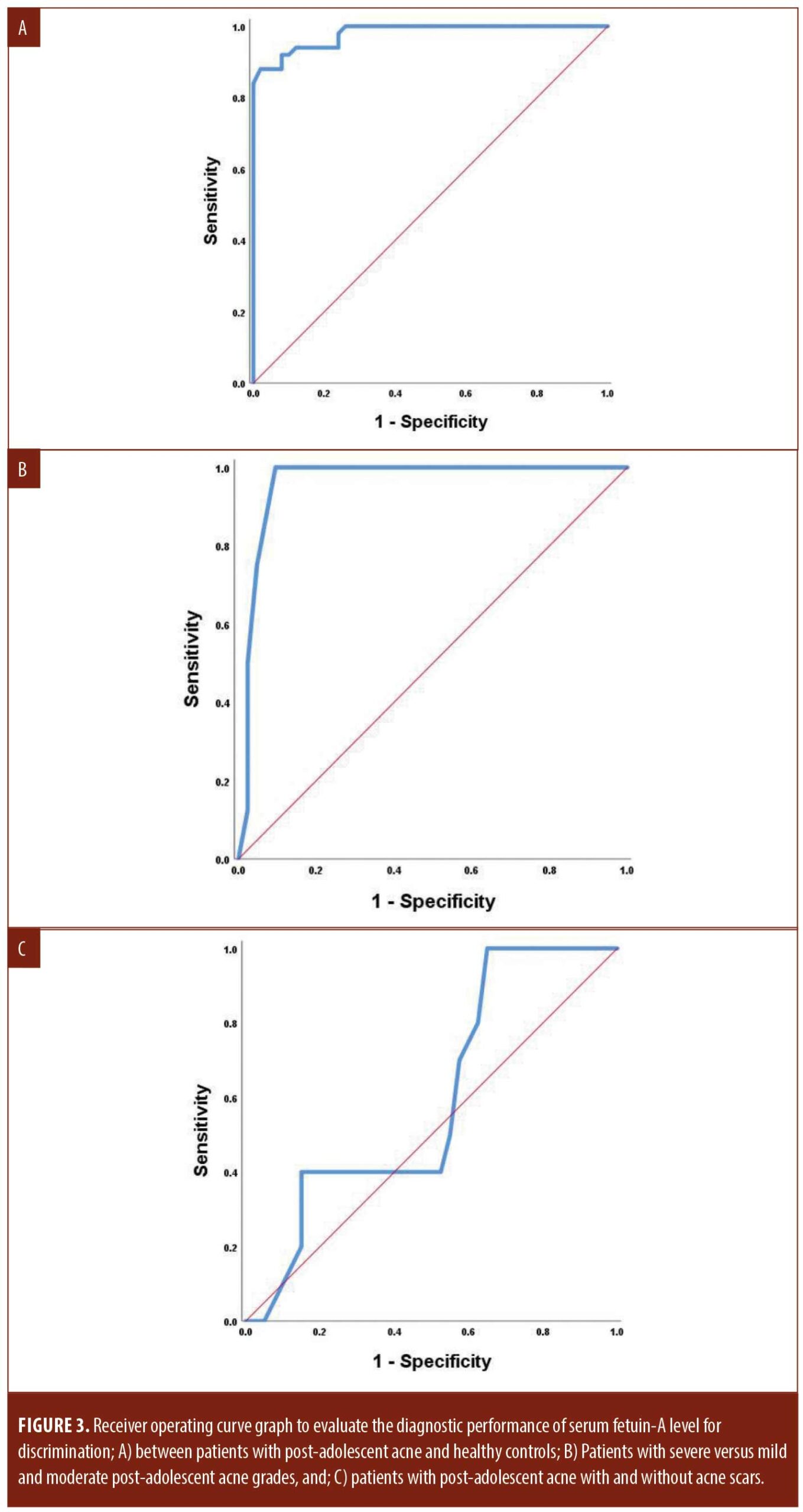
Regarding the diagnostic ability of serum fetuin-A for post-adolescent acne, high accuracy was detected (AUC=0.980) at the best cut-off value of 885.9mg/L with 92-percent sensitivity and 92-percent specificity. Moreover, serum fetuin-A showed high accuracy (AUC=0.963) at the best cut-off value of 1629.65mg/L with 100-percent sensitivity and 90.5-percent specificity in the prediction of acne severity. On the other hand, serum fetuin-A could not predict post-acne scar formation as it showed low accuracy (AUC=0.596). Other performance characteristics are shown in Table 4 and Figure 3.
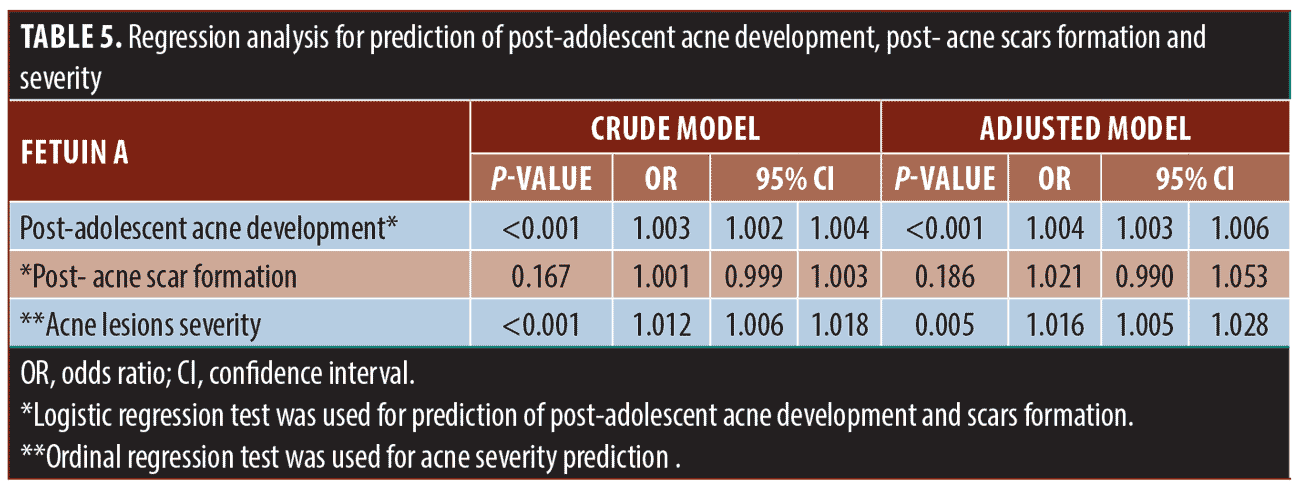
Serum fetuin-A concentration was significantly associated with the prediction of post-adolescent acne development, as well as more severe grades in both crude and adjusted model regression analyses after adjusting for other risk factors. On the other hand, it could not predict post-acne scar formation (Table 5).
Discussion
Post-adolescent acne is thought to share pathogenetic characteristics with adolescent acne. Its higher prevalence in women suggests that there are underlying hormonal imbalances. However, research has yielded contradictory results.15 Our results revealed that studied patients had significantly higher fasting insulin levels and HOMA-IR indices than controls, supporting the association between IR and post-adolescent acne pathogenesis and in agreement with research by Nagpal et al.16 However, Balta et al17 found no link between IR and acne development in adult patients. These differences could be attributed to sex differences, age, and the number of patients included in the study. Furthermore, regional differences in hormonal values may have existed.
Insulin resistance is a dysfunctional biologic response to either exogenous or endogenous insulin and is strongly linked to high insulin levels, as insulin promotes hepatic IGF-1 synthesis.18,19 High levels of insulin during fasting and/or post-prandial states decrease IGF-1 binding protein and increase IGF-1 bioavailability.20-22 Epidermal keratinocytes express insulin/IGF-1 receptors and hyperinsulinemia may result in increased basal keratinocyte proliferation within the pilosebaceous ducts and aberrant follicular corneocyte desquamation. Also, IGF-1 promotes androgen synthesis, androgen receptor signal transduction, sebaceous proliferation, and lipogenesis and, as a result, may aggravate acne.7 Polat and Ekflio23 reported significantly elevated IGF-1 levels in females with post-adolescent acne.
Our study results showed that serum fetuin-A concentration was significantly higher in studied patients than in control subjects. Moreover, its serum positively correlated with HOMA-IR indices among the studied patients (r 0.649, P<0.001). Fetuin-A is a glycoprotein that has a similar amino acid sequence to insulin receptor tyrosine kinase and can link to it, causing its inactivation (instead of activating, as in the case of insulin) and inhibition of hepatic and muscle insulin signaling, resulting in IR.24,25
Our results revealed that HOMA-IR indices and fetuin-A levels increased significantly as the patient’s acne severity increased, confirming the association between IR, fetuin-A, and post-adolescent acne severity. Early inflammatory mediators (TNF-α, ILs-1 and 6, and INF-γ) suppress fetuin-A, causing a robust inflammatory response and a surplus of later mediators (e.g., High-Mobility-Group-Protein B1), which induces hepatic fetuin-A production and acne severity.26
Serum fetuin-A levels showed high accuracy at best cut-off values with high sensitivity and specificity for diagnosis and prediction of post-adolescent acne severity. However, their levels could not predict the possibility of post-acne scar formation. High fetuin-A levels may help to reduce scar formation during wound healing, as it has unique pro-migratory properties and antagonizes transforming growth factor (TGF) and TGF-related proteins. There was a high potential for improving burn wound outcomes by promoting wound closure and influencing the inflammatory response and myofibroblast formation.27
Conclusion
Additional large-scale studies evaluating blood glucose levels and the glycemic index may be more conclusive. Insulin resistance was significantly more common in the studied patients, particularly those with severe lesions, which could be explained by elevated serum fetuin A levels that could be a reliable predictor of post-adolescent acne severity and associated metabolic comorbidities, alerting us to the onset of IR and diabetes.
References
- Goodman G. Acne – natural history, facts and myths. Aust Fam Physician. 2006; 35: 613–616.
- Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. J Am Acad Dermatol. 1999; 41: 577–580.
- Goulden V, Clark SM, Cunliffe WJ. Post-adolescent acne: a review of clinical features. Br J Dermatol. 1997; 136: 66–70.
- Collier CN, Harper JC, Cantrell WC, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008; 58: 56–59.
- Knaggs HE, Wood EJ, Rizer RL, et al. Post-adolescent acne. Int J Cosmet Sci. 2004; 26: 129–138.
- Melnik BC, Schmitz G. Role of insulin, insulin-like growth factor-1, hyperglycemic food and milk consumption in the pathogenesis of acne vulgaris. Exp Dermatol. 2009; 1: 1–9.
- Arora MK, Yadav A, Saini V. Role of hormones in acne vulgaris. Clin Biochem. 2011; 44: 1035–1040.
- Aizawa H, Niimura M. Mild insulin resistance during oral glucose tolerance test (OGTT) in women with acne. J Dermatol. 1996; 23: 526–529.
- Denecke B, Graber S, Schafer C, et al. Tissue distribution and activity testing suggest a similar but not identical function of fetuin-B. Biochem J. 2003; 376: 135–145.
- Briana DD, Boutsikou M, Gourgiotis D, et al. Alpha2-Hsglycoprotein in human pregnancies with normal and restricted fetal growth. J Matern Fetal Neonatal Med. 2008;21: 826–830.
- Mathews ST, Chellam N, Srinivas PR, et al. Alpha2-HSG, a specific inhibitor of insulin receptor auto phospho – rylation, interacts with the insulin receptor. Mol Cell Endocrinol. 2000; 164: 87–98.
- Siddiq A, Lepretre F, Hercberg S, et al. A syno nymous coding polymorphism in the alpha2- Heremans-schmid glycoprotein gene is associated with type 2 diabetes in French Caucasians. Diabetes. 2005; 54: 2477–2481.
- Ishibashi A, Ikeda Y, Ohguro T, et al. Serum fetuin-A is an independent marker of insulin resistance in Japanese men. J Atheroscler Thromb. 2010; 17: 925–933 .
- Ramli R, Malik AS, Hani AF, et al. Acne analysis, grading and computational assessment methods: an overview. Skin Res Technol. 2012;1–14.
- Özkur E, Demir D, Kıvanç Altunay İ, et al. Factors in the etiopathogenesis of post-adolescent female acne. Turkderm-Turk Arch Dermatol Venereol. 2021;55:119–124
- Nagpal M, De D, Handa S, et al. Insulin resistance and metabolic syndrome in young men with acne. JAMA Dermatol. 2016;152(4):399–404.
- Balta I, Ekiz O, Ozuguz P, et al. Insulin resistance in patients with post-adolescent acne. Int J Dermatol. 2015;54(6):662–666.
- Wilcox G. Insulin and insulin resistance. Clin Biochem Rev. 2005;26(2):19–39.
- Böni-Schnetzler M, Schmid C, Meier PJ, et al. Insulin regulates insulin-like growth factor I mRNA in rat hepatocytes. Am J Physiol. 1991;260:E846–E851
- Zouboulis CC, Jourdan E, Picardo M. Acne is an inflammatory disease and alterations of sebum composition initiate acne lesions. J Eur Acad Dermatol Venereol. 2014;28(5):527–532.
- Mualla Polat, Meral Ekflio¤lu*Serum Growth Hormone and Insulin-Like Growth Factor-1 Levels in Women with Postadolescent Acne. Türkderm. 72 2010; 44: 69–72
- Okita K, Iwahashi H, Kozawa J, et al. Homeostasis model assessment of insulin resistance for evaluating insulin sensitivity in patients with type 2 diabetes on insulin therapy. Endocr J. 2013; 60: 283–290
- Edmondson SR, Thumiger SP, Werther GA, et al. Epidermal homeostasis: the role of the growth hormone and insulin-like growth factor systems. Endocr Rev. 2003; 24:737–764.
- Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000; 85: 2402–2410
- Ix JH, Shlipak MG, Brandenburg VM, et al. Association between human fetuin-A and the metabolic syndrome: data from the Heart and Soul Study. Circulation. 2006; 113:1760–1767.
- Lebreton J, Joisel F, Raoult J, et al. Serum concentration of human alpha 2 HS glycoprotein during the inflammatory process: evidence that alpha 2 HS glycoprotein is a negative acutephase. J Clin Invest. 1979;64:1118–1129.
- Wang X-Q, S Hung B, Kempf M, et al. Fetuin-A promotes primary keratinocyte migration: Independent of epidermal growth factor receptor signalling. Exp Dermatol. 2009; 19:e289–292.

