 J Clin Aesthet Dermatol. 2023;16(7):35–41.
J Clin Aesthet Dermatol. 2023;16(7):35–41.
by Mehedi Sowlati, MD; Olguta-Anca Orzan, MD; Silviu Horia Morariu, MD;
Zlatka Etropolska, MD; Stefano Veraldi, MD; and Stefan Dimitrov, MD
Dr. Sowlati is with the County Clinical Emergency Hospital in Arad, Romania. Dr. Orzan is with Elias Emergency University Hospital in Romania. Dr. Morariu is with County Clinical Hospital of Mures in Romania. Dr. Etropolska is with the Ambulatory Practice for Primary Outpatient Medical Care SANA OOD in Bulgaria. Dr. Veraldi is with the Department of Pathophysiology and Transplantation at Università degli Studi, Foundation IRCCS Ca’ Granda Ospedale Maggiore Policlinico in Milan, Italy. Dr. Dimitrov is with the Medical center Prolet EOOD in Bulgaria.
FUNDING: The study was sponsored by Novinthetical Pharma SA in Lugano, Switzerland.
DISCLOSURES: All authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Objective. Atopic dermatitis (AD) management requires long-term use of drugs that come with side effects. Compounds such as xyloglucan (XG) and pea proteins (PP) are emerging alternatives to corticosteroids that have shown to restore skin barrier function in preclinical studies. This double-blind, parallel, randomized, placebo-controlled clinical trial investigated the efficacy and safety of XG and PP, in adult AD patients.
Methods. Fourty-two patients with AD were randomly assigned 1:1 to receive a XG+PP treatment or the vehicle without XG+PP twice/day for 14 consecutive days for assessment at baseline, Day 8 and Day 15; follow-up visit was 14 days after the end of treatment (Day 28). Efficacy was evaluated using the Scoring Atopic Dermatitis (SCORAD) index, AD severity index (ADSI) score and patient-oriented eczema measure (POEM). Safety and tolerability were monitored as the occurrence of Adverse Events (AEs).
Results. At baseline, both groups exclusively included moderate/severe AD cases. At Day 8, six patients treated with XG+PP displayed complete resolution of AD, while 15 patients had mild AD. At Day 28, 16 patients no longer had eczema, whereas five patients displayed mild AD. Notably, 21 patients in the vehicle group still displayed moderate/severe AD.
Conclusion. XG and PP promote rapid and long-lasting relief, supporting its use as a safe alternative to mainstay corticosteroid treatments for AD management. The study protocol has been registered in the ISRCTN registry (TN66879853).
Keywords. Atopic dermatitis, pea proteins, xyloglucan, POEM, ADSI
Atopic dermatitis (AD) is a chronic-relapsing disease that usually appears within the first two years of life. Its prevalence among children is 10 to 20 percent and it is estimated that 10 percent of these cases can last during adulthood.1
Acute AD is characterized by erythematous vesicles associated with excoriation and severe pruritus. Chronic AD is characterized by erythematous papules, plaques, lichenification, and pruritus.2
Skin exerts an important barrier function as the first defense mechanism against transepidermal water loss and external agents such as pathogens or physical agents.3–5 Structural abnormalities of the skin, alterations of the skin microbiota, immune dysregulation, and environmental factors all play important roles in the pathophysiology of the disease. Environmental factors such as lower mean temperatures, indoor heating, lower humidity, and lower UV index, can contribute to the increase of AD prevalence as well.6 Therefore, optimal management of AD requires a multifaceted approach, aimed at healing and protecting the skin barrier and addressing the inflammation involved in the disease.
Skin barrier abnormalities are associated with genetic mutations and impaired expression of the filaggrin gene, which encodes a structural protein essential for skin barrier formation. Individuals with atopic skin also display a loss of ceramides as well as antimicrobial peptides such as cathelicidins, which represent the first line of defense against infectious agents.7–9 These abnormalities lead to transepidermal water loss and to the penetration of microbes into the skin, notably S. aureus, which colonizes the skin in approximately 90 percent of AD patients.10–13 A disrupted skin barrier also facilitates the entry of allergens and irritants, leading to the further release of pro-inflammatory cytokines and chemokines by keratinocytes, and the activation of Th2, Th22 and Th17, during the acute phase of AD, and the activation of Th1, Th2 and Th22 during the chronic phase of AD.14,15
The alteration of skin barrier is also sustained by the local microbiome alteration, which further alters the epidermal lipid composition, establishing a self-perpetuating cycle.16,17
The initial step in AD management is patient education which is focused on appropriate skin care and bathing practices to get rid of crusts and mechanically eliminate bacterial contaminants in the case of bacterial superinfection.18 Other practices include the avoidance of triggering factors and the appropriate use of moisturizers and emollients.19 Topical treatments containing vehicle-type substances in addition to naturally derived non-pharmacological substances have gained much attention in the last years. These topical solutions act through several synergistic mechanism of action, including preserving barrier lipid content, preventing transepidermal water loss (TEWL), and moisturizing the skin, reducing inflammation, and reducing itch to improve atopic lesions. These topical solutions could be effective not only as maintenance therapy for AD, but also when used synergistically with anti-inflammatory pharmacological therapies to prevent flare-ups.20,21 When these practices are not effective, topical corticosteroids or calcineurin inhibitors are commonly prescribed.22 These agents are especially used in mild to moderate AD for the treatment of acute flares or as maintenance therapy and are often utilized with oral antihistamines.23 Mid-potency topical corticosteroid creams are among the most frequently used, but can lead to side effects such as bacterial skin infections, acne, thinning of the skin, loss of skin color and excessive hair growth.24 The treatment of moderate to severe AD can require the intake of systemic drugs such as biologics, immunomodulating agents, allergen immunotherapy, anti-IgE therapy or phototherapy.25 However, these therapies can induce several side effects in the long-term.26
Plant-based products have been studied for a long time as an alternative and complementary strategy for the treatment of skin diseases, including AD.27 Pea proteins (PP) appears to be the most hypoallergenic of all protein powders.28,29 They are derived from Pisum sativum, are particularly rich in glutamine, aspartic acid, arginine and lysine30 and have been studied for their ability to help attenuate inflammatory symptoms and microbial colonization.31 Xyloglucan (XG) is a non-ionic polysaccharide composed of a ß-(1,4)-D-glucan backbone. XG is an FDA-approved food additive, a stabilizing agent, and a pharmaceutical excipient. Known for its wound-healing properties, it can be used as a mucosae and tissue protectant, with restorative functions.32 In-vitro and in-vivo evidence conducted on human HaCaT keratinocytes and in a mouse model of AD respectively, recently highlighted the skin barrier protective effects of the topical application of PP and XG. In particular, the results showed that the topical application of XG and PP, applied in a combination product, was able to reduce tissue damage and erythema caused by AD, to preserve tight junction functionality and filaggrin, and to reduce S. aureus superinfection.33,34
Based on this evidence, the aim of the present double-blind, parallel, randomized, placebo-controlled, multicenter study is to evaluate the tolerability and efficacy of the topical application of PP and XG on a cohort of adults affected by AD.
Methods
Aim and design of the study. A double-blind, parallel, randomized, placebo-controlled, multicenter study investigating the efficacy and tolerability of a topical formulation consisting of xyloglucan and pea proteins versus placebo (i.e., the vehicle without the XG/PP), in adult patients with AD.
The study was performed by dermatologists from six accredited centers, approved to carry out a clinical study in Romania and Bulgaria between June 2019 and May 2020, according to the revised Declaration of Helsinki for biomedical research involving human subject, the rules of Good Clinical Practice (GCP) of the European Community CPMP (CPMP/ICH/135/195; ICH Topic E6) of UNI EN ISO 14155:2012. The study protocol (number CBSNOV2301) received full regulatory approval on 13/06/2019 and has been registered in the ISRCTN registry (TN66879853).
Study population. Fourty-two patients were recruited in the study, according to the following inclusion and exclusion criteria. Inclusion criteria was as follows:
- Age ≥ 18
- Affected by AD at the screening visit (any severity was allowed)
- Availability of clinical digital photography of affected and healthy skin.
- All the enrolled patients signed the informed consent. Exclusion criteria was as follows:
- Treatments within four weeks before the baseline with systemic corticosteroids, immunosuppressive or immunomodulating drugs and phototherapy
- Treatment with biologics
- Use of tanning booth/parlor (> 2 visits/week) within four weeks before baseline
- Major surgical procedures during the participation in the study
- Patients’ member of the investigational team or of his/her immediate family
- Pregnancy, breastfeeding, or plan to become pregnant or breastfeed
- Hypersensitivity to any of the ingredients used in the studied formulations.
Treatment and evaluations. The patients were randomly allocated to the natural formulation or vehicle group (1:1 randomization according to a computer-generated sequence). The treatment scheme consisted of two topical applications/day on the affected areas, for 14 days. Each patient received the necessary amount of product for 14 days at Baseline Visit. Patients underwent clinical evaluations at Day 0 (baseline), Day 8 and Day 15. Final follow-up visit was performed at Day 28. Since the study had an intention-to-treat design, only the patients that completed the treatment (“for-protocol” population) to reach the primary endpoints were considered.
The primary endpoint was to assess the efficacy of the investigated formulation in the amelioration of AD symptoms. Specifically, reduction in severity was evaluated via the reduction of subjective parameters by three well-established eczema evaluation tools: the ADSI (Atopic Dermatitis Severity Index), SCORAD (SCORing Atopic Dermatitis) and POEM (Patient Oriented Eczema Measure). Scores from each test were collected at the baseline (Day 0), Day 8, Day 15 and Day 28.
ADSI score is a tool used to measure the response to a topical treatment in patients with AD; the score ranges between 0 and 15: clear/almost clear (0 to < 2), mild (2 to < 6), moderate (6 to < 9), and severe (9–15).
SCORAD is a tool used to assess the extent and severity of eczema and to evaluate the efficacy of a treatment. It measures the extent (ranging from 0 to 100), the intensity (ranging from 0 to 18), and the patients’ subjective symptoms (ranging from 0 to 20). To assess eczema evolution, intensity, erythema, oedema/papulation, excoriations, lichenification, oozing/crusts and dryness were evaluated. The subjective symptoms evaluation was based on the daily monitoring of pruritus and sleeplessness, which are graded on a 10cm visual analogue scale.
POEM was used for monitoring atopic eczema severity based on the following score: clear/almost clear (0–2), mild eczema (3–7), moderate eczema (8–16), severe eczema (17–24), and very severe eczema (25–28).
The safety and tolerability of the treatment were evaluated by monitoring the occurrence of AEs, in the two study groups. For example, patients were asked about stinging, burning, and pain at the application sites and regarding any other possible adverse event shown (both related and not related to the formulation).
Per study protocol, all subjects that experienced a serious adverse event or underwent surgery during the study were withdrawn.
Statistical analysis. To determine the sample size, the effect size and the standard deviation were obtained from data published in previous studies. The mean difference and standard deviation were 10 and 11, respectively. Thus, a sample size of 21 patients for each group was calculated using an 80 percent power, a 5% percent significance level and a 10 percent dropout rate.
Continuous data were expressed as mean, standard deviation, median, minimum, maximum and number of observations, unless differently stated. Categorical data are summarized as the number of patients providing data at a relevant time point (n), frequency counts and percentages.
For the analysis, the SPSS IBM software was used.
Results
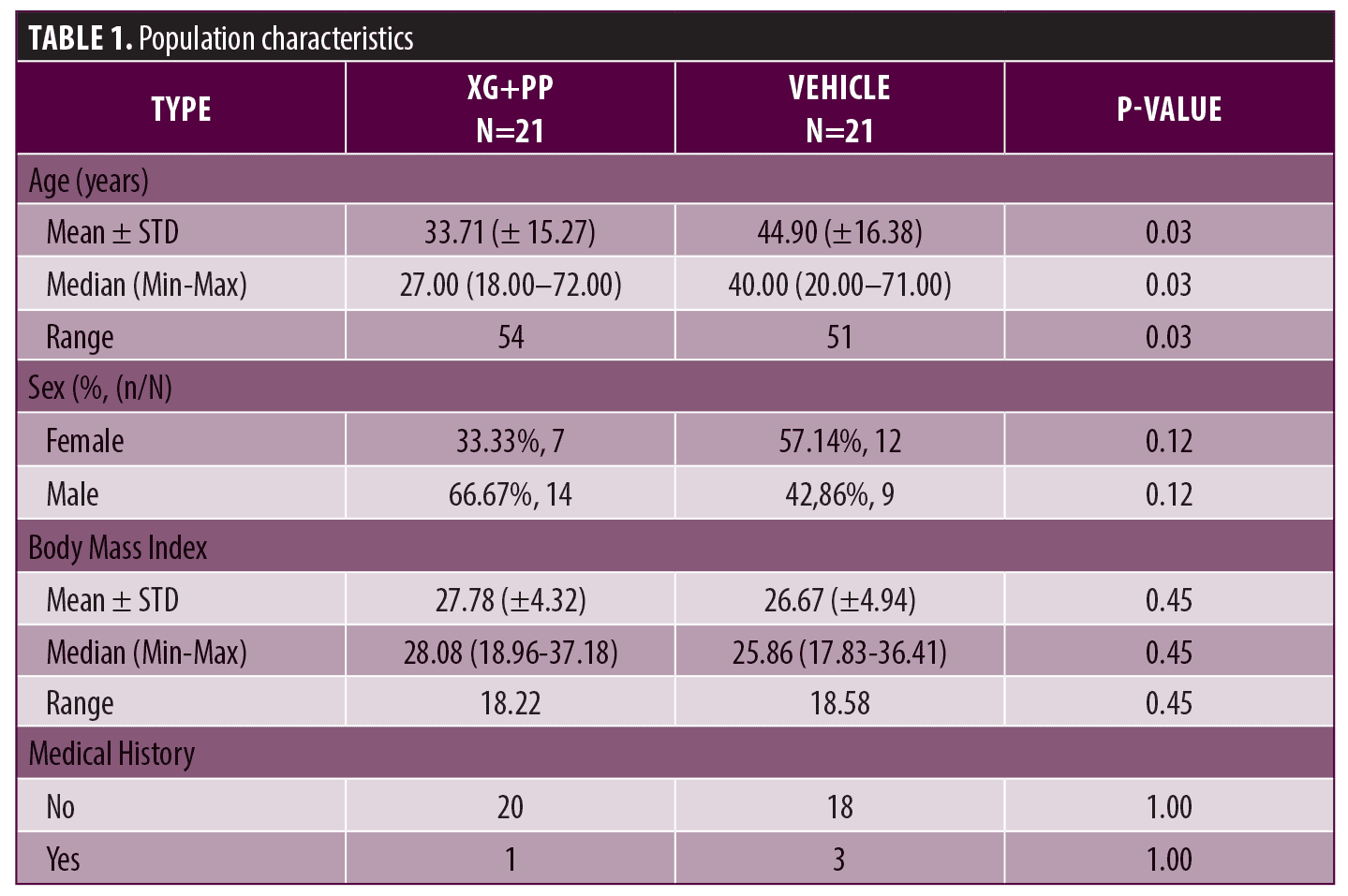
Population characteristics. Fourty-two patients with AD were enrolled in the study and randomly allocated (1:1) to the XG+PP or vehicle group. The sex distribution among the two groups was not statistically significantly different (p=0.12): in the XG+PP group, there were 14 males and seven females; while in the vehicle group, there were nine males and 12 females. The mean age was 33.71 ± 15.27 and 44.90 ± 16.38 in the XG+PP treatment and vehicle group, respectively. The average age in the two groups was statistically different (p=0.03). Detailed population characteristics are reported in Table 1.
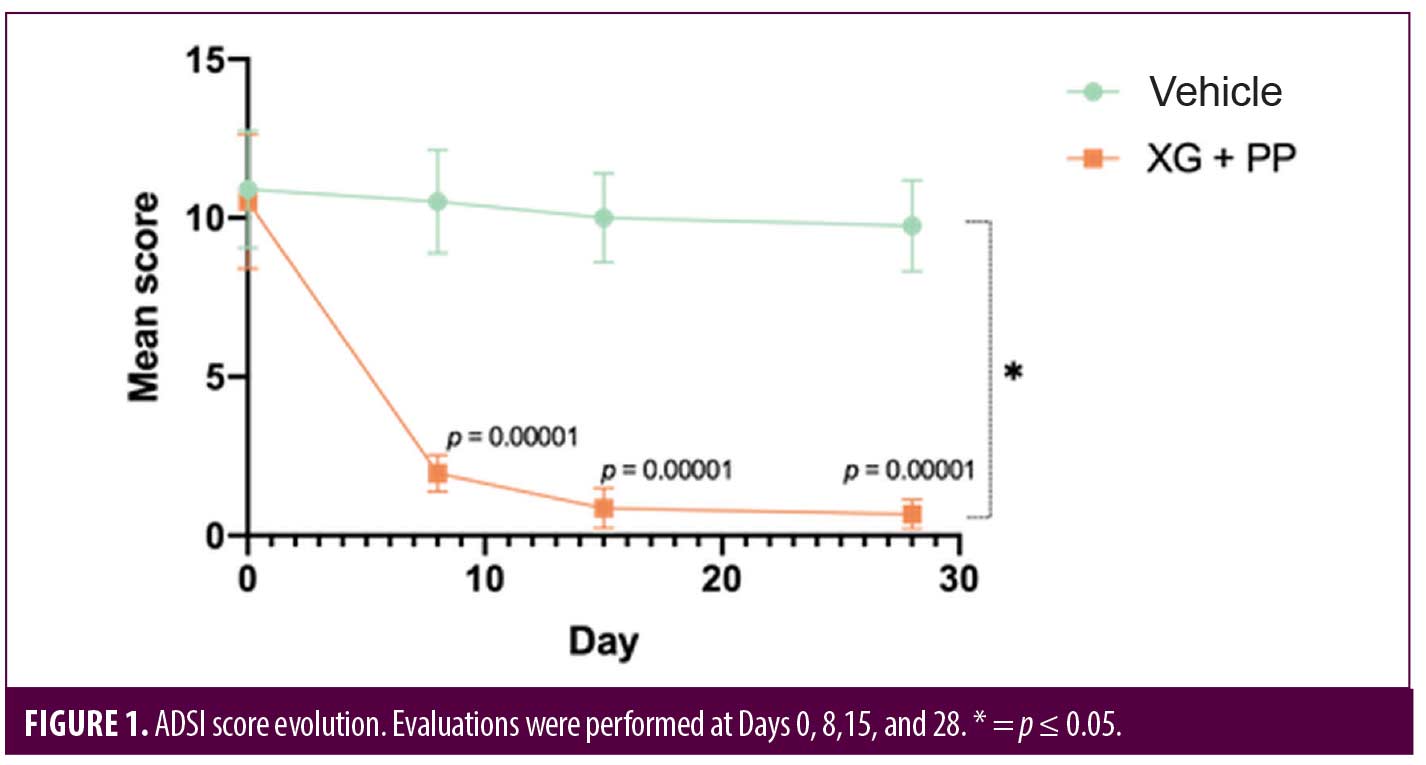
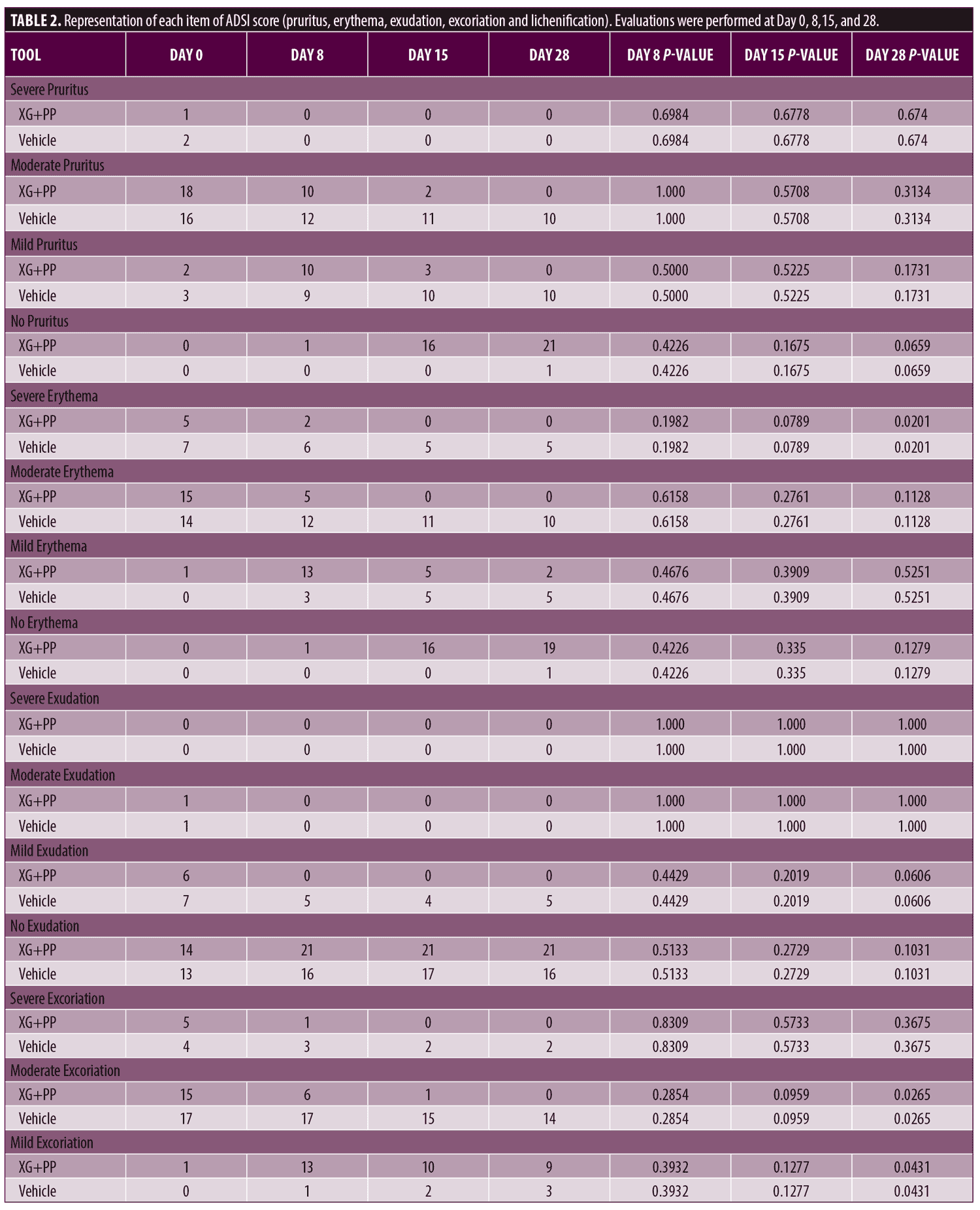
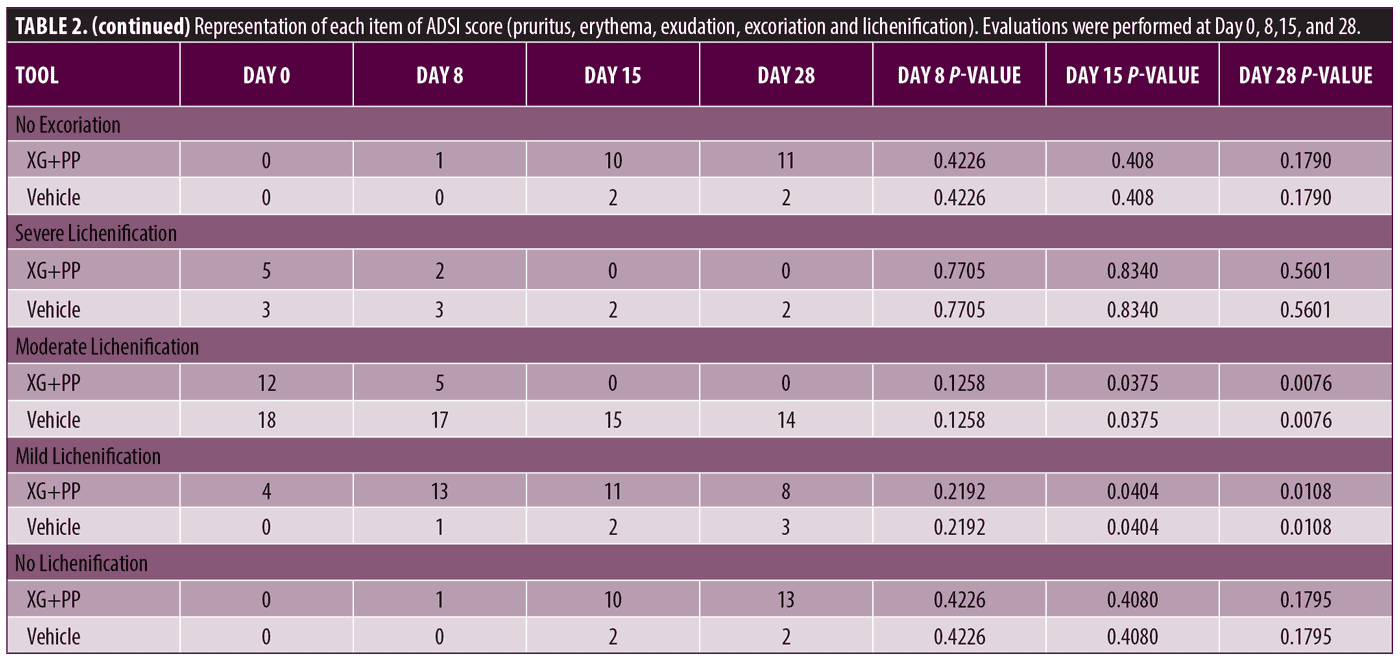
Primary endpoints. To evaluate the efficacy of the treatment, ADSI score, SCORAD and POEM were used. The results showed that the mean of ADSI score at the baseline was not statistically different among the two trial arms (10.52 and 10.90 in the XG+PP and vehicle group, respectively). As shown in Figure 1, at Day 8, the mean score registered in the XG+PP group was strongly reduced (1.95), compared to that observed in the vehicle group (10.52) (p=0.00001). At the end of the trial (Day 28), the results indicate that there was a significant difference in the reduction in ADSI scores which indicates the severity of the AD associated symptoms between the study arms, notably with XG+PP showing the ability to alleviate the symptoms with superior efficacy (p=0.028). A statistically significant difference between the baseline and follow-up, among the two arms was observed for severe erythema (Day 28), mild and moderate excoriation (Day 28), and lichenification (Day 15 and Day 28). Detailed results for each issue evaluated in ADSI score are reported in Table 2.
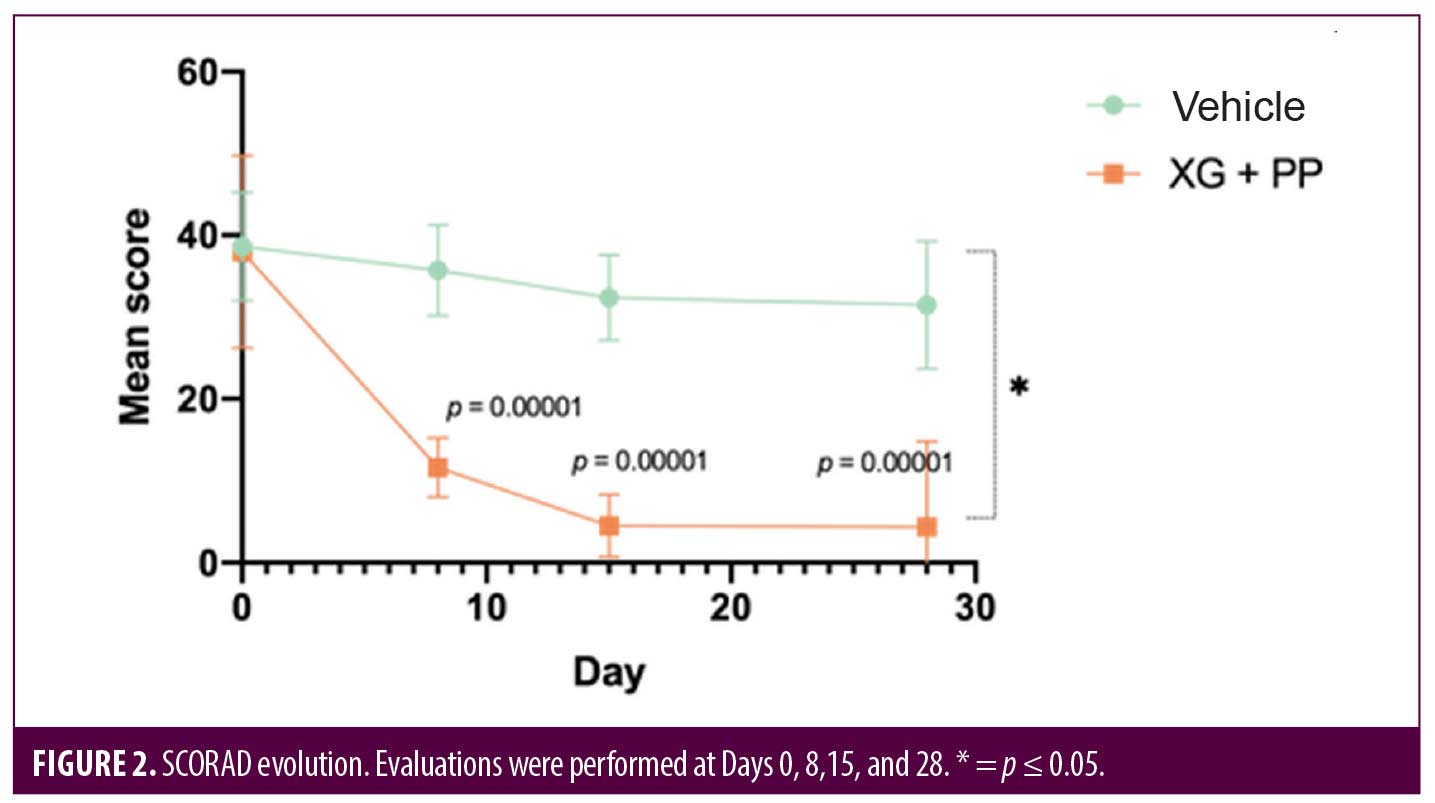
Eczema severity and clinical response were also measured by using the SCORAD index. At baseline, the index was 38.62 and 37.95 in the vehicle and XG+PP group, respectively. The difference of SCORAD index between the two arms was already statistically significant at Day 8 (p=0.00001) and the trend was maintained up to the follow-up visit at Day 28 (p=0.049) (Figure 2). Indeed, in the group treated with XG+PP, the SCORAD index progressively decreased, reaching at Day 28 an average value of 4.39 compared to an index of 31.47 which was observed in the vehicle group.
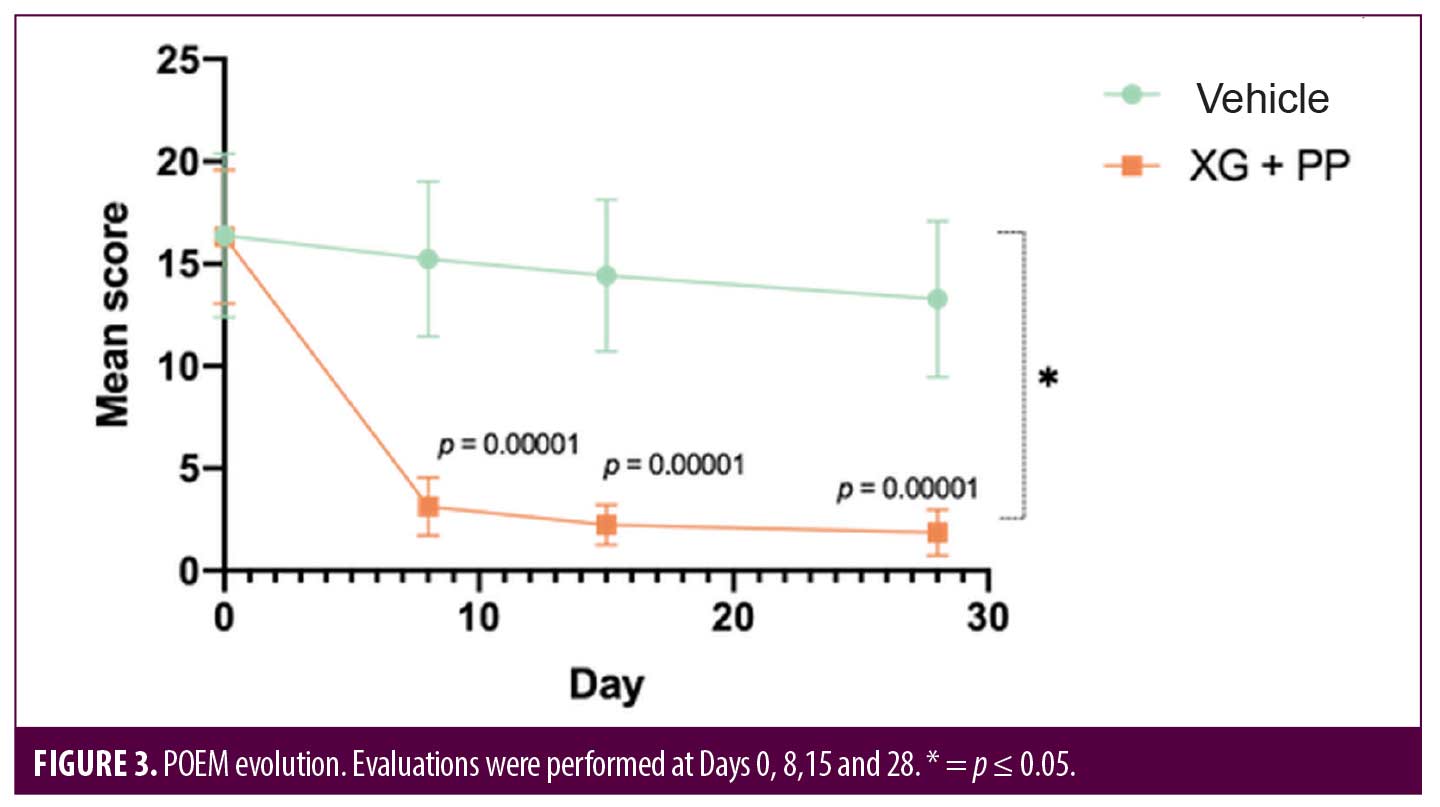
A patient-oriented clinical tool to evaluate some indicators related to the quality of life and resulting disease severity is the POEM score. At the baseline, the POEM score was 16.33 and 16.38 in the XG+PP and vehicle groups, respectively. At baseline, 14 and 13 patients exhibited moderate eczema in the XG+PP and vehicle groups, respectively, while 7 and 8 patients exhibited severe eczema in the XG+PP and vehicle groups, respectively. At Day 8, the mean POEM score was significantly reduced (3.14) in the treatment arm compared to baseline (16.33) (p=0.00001), while no significant reduction was observed in the vehicle arm (p=0.3603). In XG+PP group, mean POEM score continued to decrease up to the follow-up (1.86; p=0.028; Day 28) and all cases of moderate and severe eczema were completely resolved. As reported for the other clinical evaluations, there is a statistically significant difference between the evolution of the POEM score in the two groups (p=0.041) (Figure 3).
Secondary endpoint. To evaluate the safety and tolerability of the treatments, the Safety Analysis Set included all the patients that received at least one application of the formulations. The results showed no adverse events or disease progression during the study.
Discussion
This double-blind, parallel, randomized, placebo-controlled clinical trial investigated the efficacy and tolerability of a product consisting of XG, PP, chemical emollients, and skin conditioners, in adult patients with AD. The formulation under investigation was fragrance free and free of lanolin; it contains 1% of propylene glycol but no other known sensitizers. In addition, biocompatibility studies had confirmed the formula was non-sensitizing, non-irritant and non-cytotoxic.
The results obtained during this study suggest that the twice daily application of XG and PP significantly improves AD severity in an adult population, as early as already after eight days of treatment. Thus, considering the strong impact that AD symptoms have on patients’ quality of life, the rapid and lasting effect promoted by XG and PP– since results were maintained for 14 days after last application– appears to be extremely promising for the improvement of patients’ quality of life. Moreover, given the possible contraindications associated with the use of topical and oral corticosteroids, the results obtained in the study could pave the way for the topical use of XG and PP as an alternative safe and effective treatment to ameliorate AD symptoms.
One of the difficulties in the clinical evaluation of AD therapies is the lack of a gold standard for the evaluation of the severity of this pathology.35 The results obtained in this study can be considered reliable, as the use of scoring such as SCORAD is well accepted as a reliable tool for the evaluation of dermatitis severity. Moreover, the evaluation performed is further strengthen by the parallel use of ADSI and of POEM, which is recommended as the preferred assessment of AD symptoms as it focuses on the illness experienced by the patient.35 The combination of three well-established eczema evaluation tools increases the reliability of results, as opposed to utilizing only one index.
To better validate the efficacy of the formulation, it would be useful to evaluate a long-term therapy and to monitor the patients with a longer follow-up to evaluate the symptoms recurrence.36,37
It is of particular relevance that the positive effects promoted by XG and PP topical formulation were benefited by patients with severe cases of eczema, and there were a significant improvement between Day 8 to Day 15 compared to the vehicle after eight days of application, suggesting a potential beneficial effect in complicated AD cases. Interestingly, preclinical studies showed that the efficacy of the tested formulation goes beyond the symptomatic effects, thanks to the ability of XG and PP to restore the skin barrier function.33,34 Histopathological data can be useful to confirm this result. In-vitro and in-vivo studies demonstrated that XG and PP can counteract S. aureus infection and preserves the functionality of mucosal tight junctions and filaggrin expression, thus avoiding the loss of skin barrier function.33,34 However, human histological data would be needed to further confirm the ability to restore the barrier function (e.g., by quantifying filaggrin or measuring TEWL).
Considering its excellent safety profile and robust efficacy, topical XG and PP could represent an interesting option as non-steroidal pro-active treatment. Further clinical studies are needed to demonstrate the efficacy of this product in the prevention of flare-ups and the establishment of long-term disease control.
Moreover, while systemic absorption was not evaluated in this trial, oral utilization of XG and PP in other clinical trials demonstrated no systemic adverse events during six months of treatment.38,39
To date, while aloe vera40 or Cardiospermum halicacabum41 based creams for the most part do exhibit a good efficacy profile, clinical trials assessing the efficacy and safety of topical phytotherapy for AD treatment generally remain poor. In one randomized-controlled study, while no serious adverse effects were recorded with the use of aloe vera, 55 percent of subjects reported drying up of the skin on test areas.42 Other studies indicated that a prolonged use of topical aloe-vera based products could lead to unwanted side effects such as urticaria,43 contact dermatitis,44–46 and widespread dermatitis.47 However, a more in-depth study of natural-based products for the treatment of AD is now necessary to overcome the limitations of alternative treatments, improving the patients’ quality of life. For example, the current guidelines (ISO 14155:2020. Clinical investigation of medical devices for human subjects – Good clinical practice) do not mention plant-based products for the treatment of AD; however, the preliminary data obtained by these products appear to be encouraging. It is important to mention that not 100 percent of moderate and severe cases were completely resolved. However, this study is significant as demonstrated that, credit to the use of a topical formulation based on XG and PP, the AD severity of patients can reduce. Other limitations of the study include the fact that it was not possible to collect some information such as the number of years participants have had eczema, other atopic comorbidities, and the percentage of participants previously treated with systemic agents and/or topical corticosteroids.
Conclusion
This double-blind, parallel, randomized, placebo-controlled clinical trial showed that a XG+PP -based formulation can represent an alternative treatment for mild to moderate AD in adult patients.
References
- Weidinger S, Beck LA, Bieber T, et al. Atopic dermatitis. Nat Rev Dis Primers. 2018;4:1.
- Furue M, Ulzii D, Vu YH, et al. Pathogenesis of Atopic Dermatitis: Current Paradigm. Iran J Immunol. 2019;16:97–107.
- Guttman-Yassky E, Waldman A, Ahluwalia J, et al. Atopic dermatitis: pathogenesis. Semin Cutan Med Surg. 2017;36:100–103.
- Li Q, Fang H, Dang E, et al. The role of ceramides in skin homeostasis and inflammatory skin diseases. J Dermatol Sci. 2020;97:2–8.
- van Smeden J, Bouwstra JA. Stratum Corneum Lipids: Their Role for the Skin Barrier Function in Healthy Subjects and Atopic Dermatitis Patients. Curr Probl Dermatol. 2016;49:8–26.
- Kapur S, Watson W, Carr S. Atopic dermatitis. Allergy Asthma Clin Immunol. 2018;14(Suppl 2):52.
- Chen YE, Fischbach MA, Belkaid Y. Skin microbiota-host interactions [published correction appears in Nature. 2018 Mar 21;555(7697):543]. Nature. 2018;553:427–436.
- Chiesa Fuxench ZC. Atopic Dermatitis: Disease Background and Risk Factors. Adv Exp Med Biol. 2017;1027:11–19.
- Jang H, Matsuda A, Jung K, et al. Skin pH Is the Master Switch of Kallikrein 5-Mediated Skin Barrier Destruction in a Murine Atopic Dermatitis Model. J Invest Dermatol. 2016;136:127–135.
- Palmer CN, Irvine AD, Terron-Kwiatkowski A, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38:441–446.
- Iwamoto K, Moriwaki M, Miyake R, et al. Staphylococcus aureus in atopic dermatitis: Strain-specific cell wall proteins and skin immunity. Allergol Int. 2019;68:309–315.
- Geoghegan JA, Irvine AD, Foster TJ. Staphylococcus aureus and Atopic Dermatitis: A Complex and Evolving Relationship. Trends Microbiol. 2018;26:484–497.
- Li S, Villarreal M, Stewart S, et al. Altered composition of epidermal lipids correlates with Staphylococcus aureus colonization status in atopic dermatitis. Br J Dermatol. 2017;177:e125–e127.
- Yang G, Seok JK, Kang HC, et al. Skin Barrier Abnormalities and Immune Dysfunction in Atopic Dermatitis. Int J Mol Sci. 2020;21:2867.
- Turner MJ, Travers JB, Kaplan MH. T helper cell subsets in the development of atopic dermatitis. J Drugs Dermatol. 2012;11:1174–1178.
- Li W, Yosipovitch G. The Role of the Microbiome and Microbiome-Derived Metabolites in Atopic Dermatitis and Non-Histaminergic Itch. Am J Clin Dermatol. 2020;21(Suppl 1):44–50.
- Park K, Lee S, Lee YM. Sphingolipids and antimicrobial peptides: function and roles in atopic dermatitis. Biomol Ther (Seoul). 2013;21:251–257.
- Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I [published correction appears in J Eur Acad Dermatol Venereol. 2019 Jul;33:1436]. J Eur Acad Dermatol Venereol. 2018;32:657–682.
- Vakharia PP, Silverberg JI. Adult-Onset Atopic Dermatitis: Characteristics and Management. Am J Clin Dermatol. 2019;20:771–779.
- Araviiskaia E, Pincelli C, Sparavigna A, et al. The Role of a Novel Generation of Emollients, ‘Emollients Plus’, in Atopic Dermatitis. Clin Cosmet Investig Dermatol. 2022 Dec 14;15:2705–2719.
- Wollenberg A, Kinberger M, Arents B, et al. European guideline (EuroGuiDerm) on atopic eczema – part II: non-systemic treatments and treatment recommendations for special AE patient populations. J Eur Acad Dermatol Venereol. 2022 Nov;36:1904–1926.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116–132.
- Sidbury R, Tom WL, Bergman JN, et al. Guidelines of care for the management of atopic dermatitis: Section 4. Prevention of disease flares and use of adjunctive therapies and approaches. J Am Acad Dermatol. 2014;71:1218–1233.
- Dhar S, Seth J, Parikh D. Systemic side-effects of topical corticosteroids. Indian J Dermatol. 2014;59:460–464.
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327–349.
- Siegels D, Heratizadeh A, Abraham S, et al. Systemic treatments in the management of atopic dermatitis: A systematic review and meta-analysis. Allergy. 2021;76:1053–1076.
- Hussain Z, Thu HE, Shuid AN, et al. Phytotherapeutic potential of natural herbal medicines for the treatment of mild-to-severe atopic dermatitis: A review of human clinical studies. Biomed Pharmacother. 2017;93:596–608.
- Zhao H, Shen C, Wu Z, et al. Comparison of wheat, soybean, rice, and pea protein properties for effective applications in food products. J Food Biochem. 2020;44:e13157.
- Ge J, Sun CX, Corke H, et al. The health benefits, functional properties, modifications, and applications of pea (Pisum sativum L.) protein: Current status, challenges, and perspectives. Compr Rev Food Sci Food Saf. 2020;19:1835–1876.
- Dahl WJ, Foster LM, Tyler RT. Review of the health benefits of peas (Pisum sativum L.). Br J Nutr. 2012;108 Suppl 1:S3–S10.
- Ndiaye F, Vuong T, Duarte J, et al. Anti-oxidant, anti-inflammatory and immunomodulating properties of an enzymatic protein hydrolysate from yellow field pea seeds. Eur J Nutr. 2012;51:29–37.
- Ajovalasit A, Redondo-Gómez C, Sabatino MA, et al. Carboxylated-xyloglucan and peptide amphiphile co-assembly in wound healing. Regen Biomater. 2021;8:rbab040.
- Campolo M, Lanza M, Filippone A, et al. Evaluation of a Product Containing Xyloglucan and Pea Protein on Skin Barrier Permeability. Skin Pharmacol Physiol. 2020;33:231–236.
- Campolo M, Casili G, Paterniti I, et al. Effect of a Product Containing Xyloglucan and Pea Protein on a Murine Model of Atopic Dermatitis. Int J Mol Sci. 2020 May 19;21(10):3596.
- Chopra R, Silverberg JI. Assessing the severity of atopic dermatitis in clinical trials and practice. Clin Dermatol. 2018;36:606–615.
- de Los Rios CC, Falcón BS, Arguelles-Arias F, et al. Long-term safety and efficacy study of a medical device containing xyloglucan, pea protein reticulated with tannins and xylo-oligosaccharides, in patients with diarrhoea-predominant irritable bowel syndrome. Therap Adv Gastroenterol. 2021;14:17562848211020570.
- Chan S, Cornelius V, Cro S, et al. Treatment Effect of Omalizumab on Severe Pediatric Atopic Dermatitis: The ADAPT Randomized Clinical Trial. JAMA Pediatr. 2020;174:29–37.
- Trifan A, Burta O, Tiuca N, et al. Efficacy and safety of Gelsectan for diarrhoea-predominant irritable bowel syndrome: A randomised, crossover clinical trial. United European Gastroenterol J. 2019;7:1093–1101.
- Bellini M, Berti G, Bonfrate L, et al. Use of GELSECTAN® in Patients with Irritable Bowel Syndrome (IBS): an Italian Experience. Patient Prefer Adherence. 2021;15:1763–1774.
- Panahi Y, Rastgar N, Zamani A, et al. Comparing the Therapeutic Effects of Aloe vera and Olive Oil Combination Cream versus Topical Betamethasone for Atopic Dermatitis: A Randomized Double-blind Clinical Trial. J Pharmacopuncture. 2020;23:173-178.
- Fai D, Fai C, Di Vito M, et al. Cardiospermum halicacabum in atopic dermatitis: Clinical evidence based on phytotherapic approach. Dermatol Ther. 2020;33:e14519.
- Paulsen E, Korsholm L, Brandrup F. A double-blind, placebo-controlled study of a commercial Aloe vera gel in the treatment of slight to moderate psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2005;19:326–331.
- Morrow DM, Rapaport MJ, Strick RA. Hypersensitivity to aloe. Arch Dermatol. 1980;116:1064–1065.
- Shoji A. Contact dermatitis to Aloe arborescens. Contact Dermatitis.1982;8:164–167.
- Nakamura T, Kotajima S. Contact dermatitis from aloe arborescens. Contact Dermatitis. 1984;11:51.
- Savchak VI. Ostryĭ bulleznyĭ allergichekiĭ dermatit ot mestnogo primenenniia list’ev aloé [Acute bullous allergic dermatitis due to local application of aloe leaves]. Vestn Dermatol Venerol. 1977;(12):44–45.
- Hogan DJ. Widespread dermatitis after topical treatment of chronic leg ulcers and stasis dermatitis. CMAJ.1988;138:336–338.

