J Clin Aesthet Dermatol. 2024;17(7):38–42.
J Clin Aesthet Dermatol. 2024;17(7):38–42.
by Zahra Saffarian, MD; Zahra Vasfi Marandi, MD; Safoura Shakoei, MD; Shahin Hamzelou, MD; and Sima Rafati, PhD
Drs. Saffarian and Shakoei are with the Dermatology Department of Imam Khomeini Hospital Complex and Razi Dermatology Hospital at Tehran University of Medical Science in Tehran, Iran. Dr. Marandi is with the Dermatology Department of Imam Khomeini Hospital Complex School of Medicine at Tehran University of Medical Science in Tehran, Iran. Dr. Hamzelou is with the Dermatology Department of Imam Khomeini Hospital Complex and Razi Dermatology Hospital at Tehran University of Medical Science in Tehran, Iran. Dr. Rafati is with the Department of Immunotherapy and Leishmania Vaccine Research at the Pasteur Institute of Iran in Tehran, Iran.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. Cutaneous leishmaniasis is endemic in Iran.
Objective. We sought to investigate the therapeutic outcomes and complications of treatment in patients with cutaneous leishmaniasis.
Methods. This case series enrolled patients with smear-proven cutaneous leishmaniasis who visited our center in Iran from 2018 to 2019.
Results. In total, 36 patients were treated with intralesional meglumine antimoniate, intramuscular meglumine antimoniate, sodium stibogluconate, and amphotericin B. Overall, this treatment was effective in 81.8 percent of patients. Relapse and treatment failure occurred in 6.1 percent and 12.1 percent of patients, respectively. Treatment with intralesional meglumine antimoniate, intramuscular meglumine antimoniate, sodium stibogluconate, and amphotericin B yielded a clearance rate of 80.8 percent, 92.3 percent, 75 percent, and 85.7 percent, respectively. Clearance was associated with a shorter time interval between injections of intralesional meglumine antimoniate (p=0.006) and relapse was associated with a longer time interval between injections (p=0.018). The average number of side effects per patient for intralesional meglumine antimoniate, sodium stibogluconate, intramuscular meglumine antimoniate, and amphotericin B was 0.62, 1.4, 1.6, and 2.8, respectively. The most common side effect of intralesional meglumine antimoniate, intramuscular meglumine antimoniate, and amphotericin B was local pain, arthralgia, and hypokalemia, respectively.
Limitations. Low sample size was the limitation of this study.
Conclusion. The cure rate of intramuscular meglumine antimoniate was higher than amphotericin B, which was higher than the cure rate of sodium stibogluconate. In patients treated with intralesional meglumine antimoniate, reducing the time interval between injections increased the clearance rate and decreased the rate of relapse.
Keywords. Cutaneous leishmaniasis, treatment outcome, adverse effects, pentavalent antimonials, amphotericin B
Introduction
Leishmaniasis is a group of protozoan diseases that is caused by many species of the Leishmania parasite. Approximately one billion people, in 98 countries and three territories, are at risk of the disease.1,2 Cutaneous leishmaniasis (CL) is endemic in Iran and is considered an important health problem. Old World cutaneous leishmaniasis in Iran is mostly caused by L. major (zoonotic CL), less frequently by L. tropica (anthroponotic CL), and scarcely by L. infantum which are rarely the causal agent of mucosal leishmaniasis.
Pentavalent antimonials (PA) (i.e., meglumine antimoniate or sodium stibogluconate) are used as the first-line treatment for CL in most countries. For non-complicated localized CL, intralesional (PA) is the first-choice treatment. Inadequate infiltration of the lesions is one of the most common causes of treatment failure with intralesional PA. Lately, there have been concerns about the emergence of drug resistance. Combination therapy may prevent the development of resistance to new drugs. Amphotericin B is an antifungal treatment that has been used in the case of mucocutaneous and visceral leishmaniasis, HIV co-infection, and resistance to PA and pentamidine. Many therapeutic modalities have been effective in the treatment of CL including cryotherapy, fluconazole, miltefosine, terbinafine, etc.3, 4
Many studies have reported the efficacy and adverse effects of PA. Nonetheless, there are no adequate studies in terms of evaluating the treatment of CL with amphotericin B in the Old World.5 This case series compares the efficacy and side effects of treatments used while evaluating intralesional (IL) meglumine antimoniate efficacy in terms of injections time interval and total injection volume. We report on 36 cases of CL treated with IL meglumine antimoniate, parenteral meglumine antimoniate, sodium stibogluconate, amphotericin B, along with other alternative therapies in Iran.
Methods
Ethics approval and consent to participate. This study of a cross-sectional case series was performed on 36 patients with CL at Imam Khomeini hospital in Tehran, Iran, from 2018 to 2019. Informed consent from all participants and the ethical committee approval was obtained from the ethical committee of Imam Khomeini Hospital Complex.
Study participants. The inclusion criteria were positive smear results for CL and receiving any kind of treatment at the dermatology ward and clinic of Imam Khomeini Hospital Complex. Patients with PCR results of both L. major and L. tropica entered the study. Exclusion criteria were pregnancy, lactation, and patients who did not indicate therapy.
The diagnosis was confirmed clinically and by smear. Leishmania species were identified by PCR test in some patients (not all patients underwent PCR test). The choice of treatment was based on the number, size, and location of lesions, sporotrichoid distribution, superinfection, duration of lesions, patient’s underlying disease, history of anti-Leishmania treatment, and Leishmania species.
Patients’ medical history was included as
follows: immune deficiency 1 (2.8%), AIDS 1 (2.8%), hepatitis 5 (13.9%), ischemic heart disease 3 (8.3%), hypertension 7 (19.4%), diabetes mellitus 9 (25%), and hyperlipidemia 10 (27.8%).
Patients had undergone previous treatments for CL including IL meglumine antimoniate in 9 (25%) cases, IM meglumine antimoniate in 6 (16.7%), cryotherapy in 1 (2.8%), topical amphotericin B in 2 (5.6%), miltefosine in 1 (2.8%), fluconazole in 6 (16.7%), and terbinafine in 2 (5.6%).
PCR test. One tape disc was placed on the infected region. The tape disc was detached and transferred into a sterile 1.5mL vial. DNA was isolated from collected tape strip disc samples by DNAeasy Blood & Tissue kit (QIAGEN, Germany). For species identification, the internal transcribed space 1 (ITS1) PCR assay was used with primers LITSR (5’CTGGATCATTTTCCGATG3’) and L5.8S (5’ TGATACCACTTATCGCACTT 3’) to amplify the ribosomal ITS1 region. The DNA amplification was performed in a 50μl reaction comprised of 0.5mM dNTP, 1.5mM MgCl2, 1U of Taq polymerase (Roche, Germany), 25pmol of each primer, and 10ng of DNA from each reference strain ( L. major, L. tropica) or 100-200ng DNA isolated from clinical samples. The cycling conditions were 94˚C for five minutes, followed by 35 cycles of 94˚C for 30 seconds, 53˚C for one minute, 72˚C for one minute, and a final extension of 72˚C for 15 minutes. The PCR products were subjected to RFLP analysis by digesting the amplified PCR product with 1U HaeIII enzyme (Roche) at 37˚C for two hours. The restriction fragments were analyzed on 2% agarose gel.6
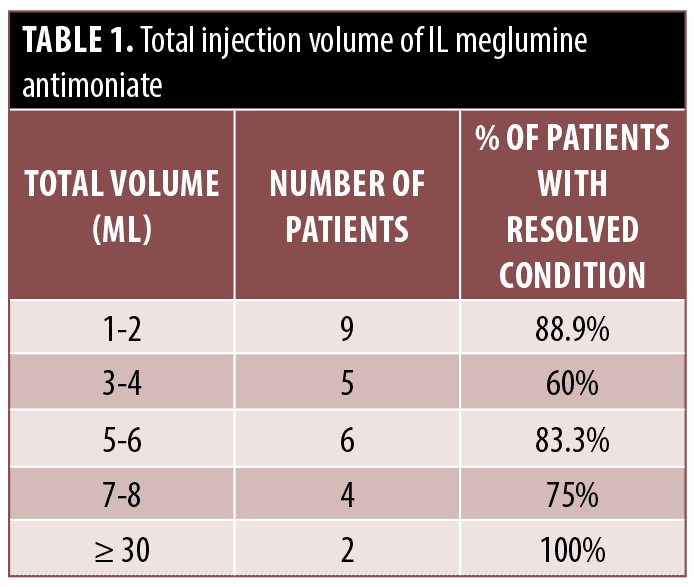
Treatment administration. IL meglumine antimoniate was administered for patients with less then five lesions and lesions less than 3cm in diameter, if the lesions were not located on the face, neck, or joints and the patient didn’t have sporotrichoid. Patients were treated with six different schedules: daily, alternate day, twice weekly, weekly, fortnightly, and monthly for a total of 1 to 32 times depending on the patient’s condition (Tables 1–2). Every session, patients received approximately 1.0mL/cm2 of meglumine antimoniate into each lesion until the lesion faded.
Patients with complicated lesions received systemic treatment as well i.e., IM glucantime®; Sanofi, Paris, France (meglumine antimoniate, 85mg Sb(V) Antimony 5+ /1ml i.e. 300mg glucantime/1mL) or IM pentostam®; Wellcome, London, UK (sodium stibogluconate, 100mg Sb(V)/1mL i.e. 330mg pentostam/1mL) at the dose of 20mg/kg/day Sb(V) for 20 days (75mg/kg/day glucantime or 66mg/kg/day pentostam). In case of lack of antimonials due to sanctions in Iran or treatment failure, patients received ambisome®; Gilead (at the dose of 3mg/kg on Days 1-5 and Day 10), miltefosine (weight > 45 kg: 50mg orally three times a day for 28 consecutive days), fluconazole (200mg orally daily for six weeks), terbinafine (125mg orally twice daily for 4 weeks), cryotherapy or topical amphotericin B (Sina-ampholeish, 0.4%, twice daily). Treatment options were chosen based on expert opinion (Supplemental Tables 1 to 6, available on the online version of this article).
In case of serious adverse effects and treatment failure, the treatment was replaced. Patients underwent examinations at baseline and daily including evaluation of complete blood cell count, electrolytes, liver, kidney, and pancreas function tests, fasting glycemia, and ECG to assess the adverse effects.
Data collection. The questionnaire was completed by physicians by interviewing patients and reviewing their charts covering demographic information of the patient, clinical presentation, medical history, Para-clinical data, received treatments, side effects, and outcome.
Patients were followed up at least three months after cessation of the treatment considering the cure definition of full re-epithelization and relapse was defined as the appearance of new lesions on the border of old scar after complete healing and treatment failure was defined as the absence of lesions improvement during the treatment. Three patients were lost to follow-up, however, their adverse events were reported but they were not accounted for in the efficacy calculation.
Statistical analysis. Statistical analyses were conducted using SPSS 26 software. Fisher’s exact test and Mann-Whitney U test were used to compare categorical and continuous variables, respectively with a confidence interval of 95 percent, and P<0.05 was considered significant. Bonferroni correction was used to account for multiple comparisons of treatment (P<0.008).
Results
Overall, 36 Old World CL patients were evaluated. 36.1 percent were male and 63.9 percent were female with a mean age of 50.11±16.62 years. Three cases were lost to follow-up.
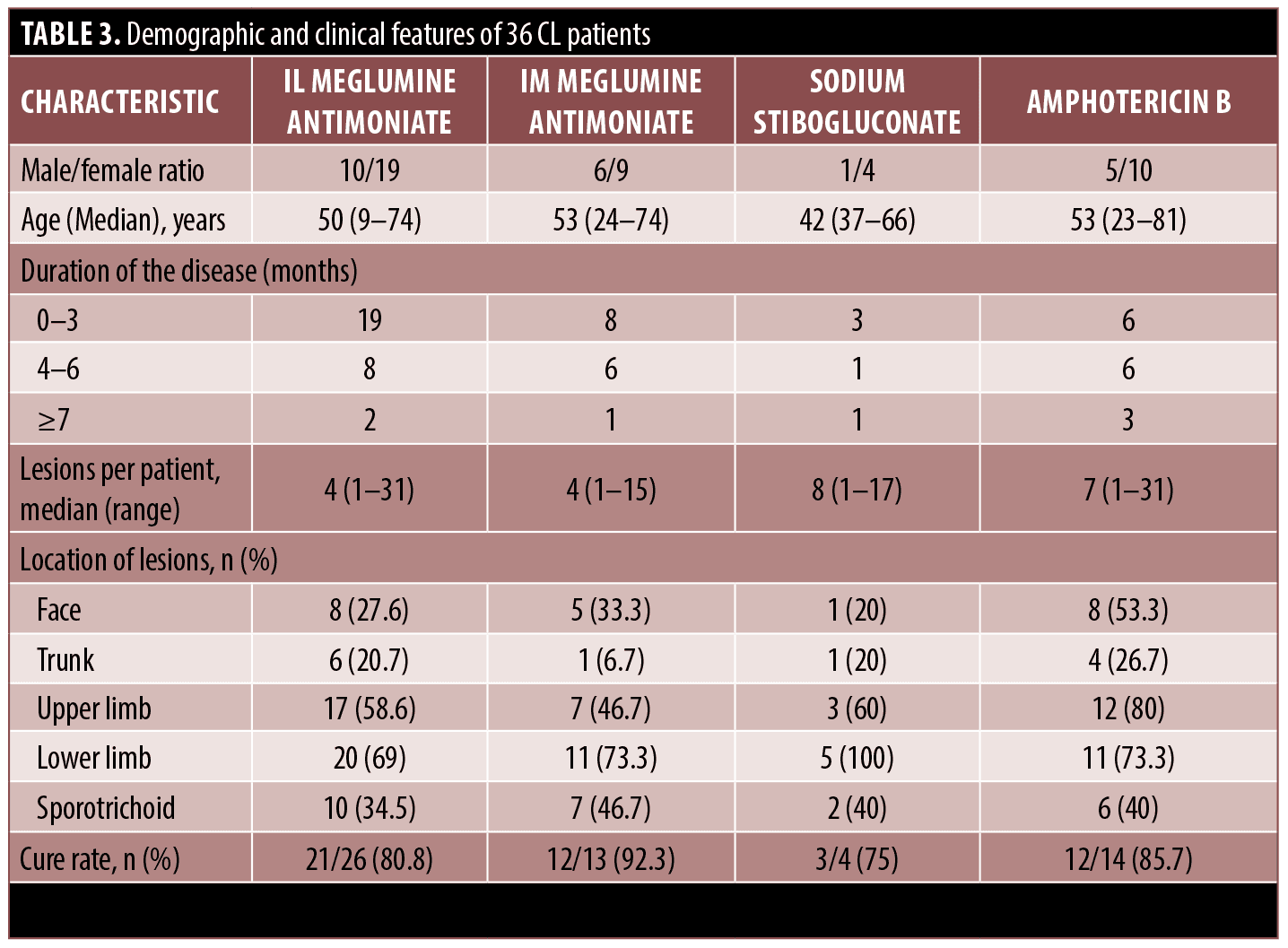
Clinical and paraclinical characteristics. Of 36 patients, 7 (19.4%) had lesions on the trunk, 11 on the face (30.6%), 12 (33.3 %) on the lower limb, and most commonly the upper limb 14 (38.9 %). The number of lesions ranged from 1 to 31 (mean=6.83 ± 6.73 median=5 IQR=6) (Table 3). Of all patients, 16 (44.4%) had superinfection, and 12 (33.3%) developed sporotrichoid. The diagnosis was established by smear in all patients. Histopathology was performed in three cases and was positive in 0 percent. The Leishmanin skin test was performed in 12 cases and was positive in 75 percent. Direct agglutination test (DAT) was performed in three cases and was positive in 66.7 percent. Identification of species was carried out by PCR in 17 patients, of which 11.8 percent were L. tropica (anthroponotic leishmaniasis) and 41.7 percent were L. major (zoonotic leishmaniasis). One patient was immunocompromised due to cutaneous psoriasis and psoriatic arthritis who was being treated with etanercept and methotrexate. This patient received IL and IM meglumine antimoniate in this center.
Treatment. IL meglumine antimoniate treatment was performed in 29 patients, intramuscular (IM) meglumine antimoniate in 15 patients, amphotericin B in 15 patients, and sodium stibogluconate in five patients. Most of the patients underwent multiple drug therapy. In one patient receiving amphotericin B, sodium stibogluconate was substituted because of adverse effects. Moreover, treatment with
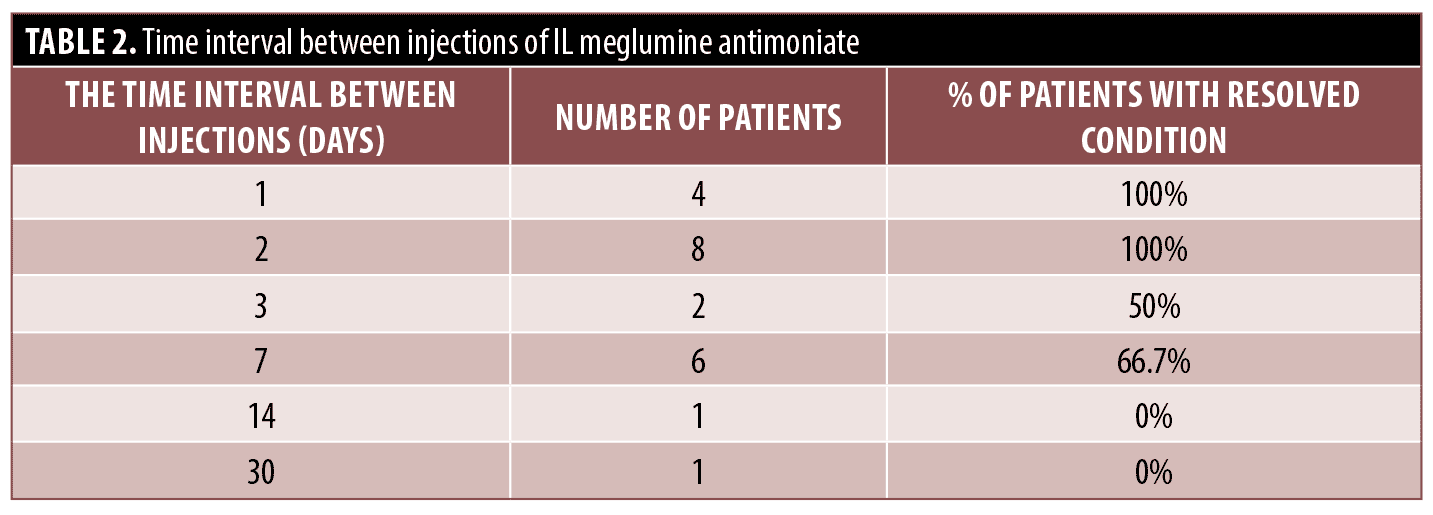
meglumine antimoniate was not completed in five patients; in two cases it was due to treatment failure in which amphotericin B was substituted, and the other three, in a case meglumine antimoniate was hold because of adverse effects and patient was healed spontaneously, another one was treated after receiving amphotericin B. In one patient, meglumine antimoniate was hold after showing adverse effects, and the case was lost to follow-up (Supplementary Tables 1–6). The mean total injection volume of IL meglumine antimoniate was 5.82 ± 7.45 cc (median=5 IQR=5) (Table 1) and the mean time interval between injections of IL meglumine antimoniate was 4.79 ± 6.24 days (median=2, IQR=5) (Table 2). Location of lesions at least three months after cessation of treatment was most commonly on the face in 3 (9.1%), followed by upper limb in 2 (6.1%), lower limb in 1 (3%), and trunk in 0 percent. The treatment failure rate regarding the location of the lesions was 22.2 percent on the face, 0 percent on the trunk, 5 percent on the upper limb, and 4.5 percent on the lower limb. Although there was no significant relationship between the location of lesions and treatment failure.
Data analysis revealed that overall, 27 (81.8 percent) were successfully treated, treatment failure occurred in four (12.1 percent) and relapse occurred in two (6.1 percent). The cure rate for patients treated with IL meglumine antimoniate was 80.8 percent (21/26), IM meglumine antimoniate 92.3 percent (12/13), amphotericin B 85.7 percent (12/14), and sodium stibogluconate 75 percent (3/4), yet there was no significant difference between cure rates of these treatments (P>0.008). Cure, treatment failure, and relapse rate were not significantly associated with IL meglumine antimoniate, IM meglumine antimoniate, amphotericin B, and sodium stibogluconate treatment. Moreover, cure, treatment failure, and relapse rate were not significantly associated with age, sex, Leishmania species, and total injection volume of IL meglumine antimoniate. Nonetheless, cure and relapse rates were found to be significantly associated with the time interval between injections of IL meglumine antimoniate as the interval between injections was significantly shorter in cured patients than non-cured patients (P=0.006), on the other hand, the interval between injections was significantly longer in relapsed patients than non-relapsed patients (P=0.018).
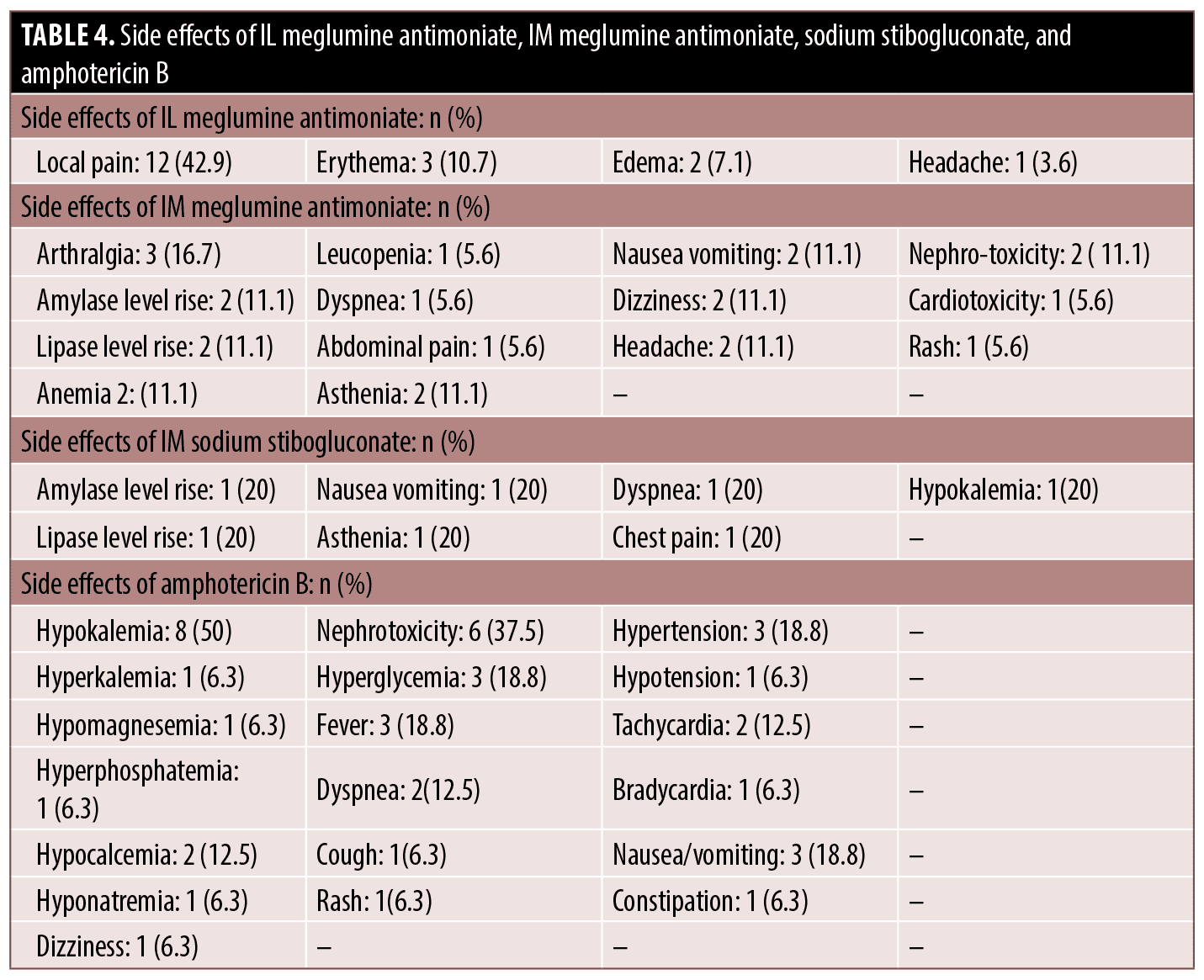
Adverse effects. The average number of side effects per patient in cases that received IL meglumine antimoniate was 0.62, sodium stibogluconate 1.4, IM meglumine antimoniate 1.6, and amphotericin B 2.8. The most commonly seen side effect in IL meglumine antimoniate, IM meglumine antimoniate, and amphotericin B was local pain, arthralgia, and hypokalemia, respectively (Table 4).
Supplemental Materials
Saffarian Supplemental Tables 1-6
Discussion
CL is a major health problem for people living in endemic areas and travelers visiting these regions. The emergence of drug resistance to antimonials is a serious concern that complicates the treatment of CL. Combination therapy may lead to decrease drug resistance, thus it is necessary to evaluate alternative treatment modalities. Numerous studies have illustrated the efficacy of antimonials, yet there is not enough evidence available about the treatment of CL with amphotericin B in the Old World.4,5
We describe 36 cases of CL in Iran that underwent treatment with IL meglumine antimoniate, IM meglumine antimoniate, sodium stibogluconate, and amphotericin B either as a single therapy or combination therapy. The highest rate of treatment failure among parts of the body was the face (22%). This study showed a total failure rate of 12.1 percent. This result is slightly lower than the failure rate of 22.6 percent reported by Mohammadzadeh et al7 and is slightly higher than the 3.5 percent failure rate by Karamian et al.8 PCR results of 17 patients were available of which 41.7 percent were L.major and 11.8 percent were L.tropica and there was no significant difference in the cure, relapse, and failure rate between L.major and L.tropica lesions. The failure rate of 17 patients infected with both L.major and L.tropica was 0 percent. The cure rate with IL meglumine antimoniate and IM meglumine antimoniate was 80.8 percent and 92.3 percent, respectively. Hodiamont et al5 pooled the results of 168 studies and the efficacy of IL meglumine antimoniate and IM meglumine antimoniate in the treatment of L.major lesions was calculated to be 86 percent and 69 percent and in the treatment of L.tropica lesions 76 percent and 46 percent, respectively. In this study cure rate of IM meglumine antimoniate was not significantly different from IM sodium stibogluconate (92.3% and 75% respectively), while according to a study by Yesilova et al.9 Cure rate with IL meglumine antimoniate was 82 percent compared to 67 percent with IL sodium stibogluconate and the difference was statistically significant. In this study, a cure rate of 85.7 percent was obtained for amphotericin B which concord with the cure rate of 84.6 percent by Solomon et al.10 The total relapse rate was 6.1 percent in this study (note that all two patients who had relapse were infected by L.major). Nonetheless, Sharifi et al11 reported a relapse rate of 18.1 percent by assessment of CL patients in a region endemic for anthroponotic CL.
This case series observed cure and relapse rates that were significantly related to the time interval between injections of IL meglumine antimoniate, as the cure was significantly associated with a shorter time interval (P-value=0.006), besides relapse was significantly associated with higher time interval (P-value=0.018). Mujtaba et al12 demonstrated that there was no significant difference in cure rate between weekly and fortnightly IL injections of meglumine antimoniate; 92 percent and 86 percent respectively.12 Another study conducted by Tallab et al13 reported that the clinical response (complete and partial cure) of IL sodium stibogluconate was 67 percent, 97 percent, and 91 percent for the daily, alternate day, and weekly schedules, respectively.
The average number of side effects per patient in cases that received IL meglumine antimoniate was 0.62, sodium stibogluconate 1.4, IM meglumine antimoniate 1.6, and amphotericin B 2.8. The most frequently seen side effect of IL meglumine antimoniate, IM meglumine antimoniate, and amphotericin B was local pain in 12 (42.9 percent), arthralgia in 3 (16.7 percent), and hypokalemia in 8 (50 percent). The most of adverse effects seen in CL patients in this study were similar to those in previous studies.4,12,14–19 Additionally, patients who received IM meglumine antimoniate showed dyspnea, rash, and asthenia. Patients who received sodium stibogluconate additionally showed dyspnea and chest pain simultaneously, asthenia and hypokalemia. Patients who received amphotericin B also showed hyperkalemia, hypocalcemia, hyperphosphatemia, hypomagnesemia, hyponatremia, cough, bradycardia, constipation, dizziness, and hyperglycemia.
Conclusion
The results observed in our patients in this case series suggests that reducing the time interval between injections of IL meglumine antimoniate leads to a higher cure rate, and increasing the time interval leads to a higher relapse rate in patients with CL. The cure rate of IM meglumine antimoniate was higher than amphotericin B, which was higher than the cure rate of sodium stibogluconate. Meanwhile, there was no significant difference in cure rate between IL and IM meglumine antimoniate, sodium stibogluconate, and amphotericin B. Further studies should be done to substantiate the results observed in this case series.
Acknowledgments
The authors would like to appreciate the constructive comments of Dr. Leila Sahebi, research development office, Imam Khomeini Hospital Complex, Tehran, Iran.
References:
- Reithinger R, Dujardin JC, Louzir H, Pirmez C, et al. Cutaneous leishmaniasis. Lancet Infect Dis. 2007;7(9):581–596.
- Alvar J, Vélez ID, Bern C, Herrero M, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7(5):e35671.
- Firooz A, Mortazavi H, Khamesipour A, Ghiasi M, et al. Old world cutaneous leishmaniasis in Iran: clinical variants and treatments. J Dermatolog Treat. 2021;32(7):673–683.
- González U, Pinart M, Reveiz L, Alvar J. Interventions for Old World cutaneous leishmaniasis. Cochrane Database Syst Rev. 2008(4):Cd005067.
- Hodiamont CJ, Kager PA, Bart A, et al. Species-directed therapy for leishmaniasis in returning travellers: a comprehensive guide. PLoS Negl Trop Dis. 2014;8(5):e2832.
- Taslimi Y, Sadeghipour P, Habibzadeh S, et al. A novel non-invasive diagnostic sampling technique for cutaneous leishmaniasis. PLoS Negl Trop Dis. 2017;11(7):e0005750.
- Mohammadzadeh M, Behnaz F, Golshan Z. Efficacy of glucantime for treatment of cutaneous leishmaniasis in Central Iran. J Infect Public Health. 2013;6(2):120–124.
- Karamian M, Bojd F, Salehabadi A, et al. Effectiveness of meglumine antimoniate against L. tropica in a recently emerged focus of cutaneous leishmaniasis in Birjand, eastern Islamic Republic of Iran. East Mediterr Health J. 2015;21(4):280–286.
- Yesilova Y, Surucu HA, Ardic N, et al. Meglumine antimoniate is more effective than sodium stibogluconate in the treatment of cutaneous leishmaniasis. J Dermatolog Treat. 2016;27(1):83–87.
- Solomon M, Pavlotsky F, Leshem E, et al. Liposomal amphotericin B treatment of cutaneous leishmaniasis due to Leishmania tropica. J Eur Acad Dermatol Venereol. 2011;25(8):973–977.
- Sharifi I, Fekri AR, Aflatoonian MR, et al. Leishmaniasis recidivans among school children in Bam, Southeast Iran, 1994–2006. Int J Dermatol. 2010;49(5):557–561.
- Mujtaba G, Khalid M. Weekly vs. fortnightly intralesional meglumine antimoniate in cutaneous leishmaniasis. Int J Dermatol. 1999;38(8):607–609.
- Tallab TM, Bahamdam KA, Mirdad S, et al. Cutaneous leishmaniasis: schedules for intralesional treatment with sodium stibogluconate. Int J Dermatol. 1996;35(8):594–597.
- Mahmoud Mohammadzadeha FB, Zahra Golshanb. Efficacy of glucantime for treatment of cutaneous leishmaniasis in Central Iran. J Infect Public Health. 2013;6(2):120–124.
- Neves DB, Caldas ED, Sampaio RN. Antimony in plasma and skin of patients with cutaneous leishmaniasis–relationship with side effects after treatment with meglumine antimoniate. Trop Med Int Health. 2009;14(12):1515–1522.
- Layegh P, Rajabi O, Jafari MR, et al. Efficacy of topical liposomal amphotericin B versus intralesional meglumine antimoniate (Glucantime) in the treatment of cutaneous leishmaniasis. J Parasitol Res. 2011;2011.
- Lee SA, Hasbun R. Therapy of cutaneous leishmaniasis. Int J Infect Dis. 2003;7(2):86–93.
- Meunier F, Prentice H, Ringden O. Liposomal amphotericin B (AmBisome): safety data from a phase II/III clinical trial. J. Antimicrob. Chemother. 1991;28(suppl_B):83–91.
- Ringden O, Andström E, Remberger M, Svahn B, et al. Safety of liposomal amphotericin B (AmBisome) in 187 transplant recipients treated with cyclosporin. Bone Marrow Transplant. 1994;14:S10–14.