J Clin Aesthet Dermatol. 2023;16(9):46–51.
J Clin Aesthet Dermatol. 2023;16(9):46–51.
by Cemre Busra Turk, MD; Melis Baykara Ulusan, MD; Yusuf Mert Döş, MD; Vildan Manav Baş, MD; Ebru Sarikaya Tellal, MD; and Ayse Esra Koku Aksu, MD
Dr. Turk is with the Wellman Center for Photomedicine at Massachusetts General Hospital in Boston, Massachusetts. Additionally, Dr. Turk is with the Department of Dermatology at Harvard Medical School in Boston, Massachusetts. Dr. Baykara Ulusan is with the University of Health Sciences Istanbul Training and Research Hospital’s Radiology Clinic in Istanbul, Turkey. Drs. Döş, Manav Baş, Sarıkaya Tellal, and Koku Aksu are with the University of Health Sciences Istanbul Training and Research Hospital’s Dermatology Clinic in Istanbul, Turkey.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. Although the effects of oral isotretinoin (OI) on acne vulgaris and preventing further acne scars have been well-documented, the specific impact of OI alone on pre-existing atrophic acne scars (AAS) remains unclear. No clinical study has objectively evaluated the effect of OI on AAS yet.
Objective. We sought to investigate the OI effect on AAS quantitatively and reliably by shear-wave elastography (SWE).
Methods. This work is a single-center, prospective and observational study. Thirty patients with moderate and severe acne vulgaris accompanied by AAS were included. We started the OI with a standard dose regime. On Days 0 and 90 of treatment, patients’ global acne grading system (GAGS) and the Goodman and Baron’s Qualitative Global Scar Rating System (GSRS) were evaluated. The dermal thickness, subcutaneous tissue thickness, scar size, and scar and subcutaneous tissue’s elastic modules were measured on both cheeks of each patient by SWE.
Results. The improvement in GSRS stages and GAGS scores in 90 days were statistically significant (respectively; p=0.029, <0.001). Scar size and dermal thickness decreased, while the subcutaneous tissue thickness and the elastic modulus of scar and subcutaneous tissue increased in bilateral cheeks. The thickness changes in the right side dermis, and subcutaneous tissue on both sides were noteworthy (p<0.05).
Conclusion. Besides its well-known effect on acne vulgaris, OI also could be an effective treatment option for reducing scar size and severity while improving skin elasticity. SWE may help follow skin and scar properties.
Keywords. Shear-wave elastography, non-invasive imaging, atrophic acne scars, oral isotretinoin, skin elasticity, dermal thickness, scar therapy
Acne vulgaris is a multi-factorial, chronic, inflammatory disease of the pilosebaceous unit.1 Acne scars are common complications of acne vulgaris, with 95 percent of inflammatory acne cases leading to scarring.2,3 The most frequent type of acne scarring is the atrophic acne scar, characterized by a loss of collagen and elastin in the skin.4
Preventing scar formation and effectively treating early inflammatory acne are critical steps for scar management.5,6 Oral isotretinoin (OI) is the first option to treat severe papulopustular and nodular acne6,7 by reducing existing inflammation and the risk of scarring. Until now, it has been suggested that incorporating adjunctive therapies alongside OI may improve atrophic acne scarring.8,9 Low-dose OI has been shown to be effective in improving skin quality and elasticity in photoaging.10 Furthermore, OI rearranges the extracellular matrix, increases the density and thickness of collagen, and thickens the dermis while reducing the number of elastic fibers.11 Similarly, retinoids can contribute to skin elasticity by increasing and regulating dermal collagen.12
Shear-wave elastography (SWE) is real-time ultrasonographic elastography. It provides reliable biomechanical data on the natural elasticity and quality of tissues using acoustic radiofrequency impulses.13 It has been used to examine superficial or deeper solid organs for years.14 However, it is relatively new for dermatology. SWE has become an effective and reliable method for measuring skin thickness and elasticity.15
Although the effects of OI on acne vulgaris and preventing further acne scars have been well-documented, the specific impact of OI alone on pre-existing atrophic acne scar is still unclear. To the best of our knowledge, there is no clinical study that has objectively evaluated the impact of OI on atrophic acne scarring. Therefore, we aimed to investigate the OI effects on atrophic acne scar size, skin layer thickness, and elasticity by SWE measurements.
Methods
Study design and patient selection. This was a single-center, prospective, observational study evaluating changes in acne scarring clinical severity, skin layer thickness, and skin elasticity with OI treatment. Ethical approval was obtained from the Clinical Research Ethics Committee of the University of Health Sciences (UHS), Istanbul Training and Research Hospital (TRH) on 03.11.2022, numbered 98-2011-KAEK-50.
Thirty patients agreed to participate in the study and signed written consent. Included patients were between 18 and 65 years of age and had facial atrophic acne scarring with moderate to severe acne vulgaris.
Potential participants were excluded if they met any of the following criteria:
- Taking any systemic medication at the time of the study
- Cigarette smokers
- Used isotretinoin within the year prior to the study
- Used any other topical medication within two months of the study
- Had a personal or family history of keloidal or hypertrophic scarring
- Had any known chronic disease or any dermatological pathology besides acne on the facial area
- Hypersensitivity to isotretinoin
- Unrealistic expectations of treatment results
- Patients who had received or were planning to receive a blood transfusion within one month of treatment or during treatment
- Pregnant patients
- Lactating patients
- Patients who were able to bear children but who were unable to adhere to a method of contraception throughout the treatment period
Treatment regimen. We noted the age and sex of participants. Then, we started the OI at 0.5mg/kg/day and planned to evaluate all participants on Days 0, 30, 60, and 90. We adjusted OI dosage based on patient weight. We also arranged a simple skincare routine, including a gel cleanser, moisturizer, and sunscreen, for all patients during each interview.
Assessment. Clinical scoring and grading.
Two dermatologists evaluated clinical changes in acne vulgaris and atrophic acne scar with treatment over 90 days. We used Global Acne Grading System (GAGS) to evaluate the acne vulgaris severity. In the GAGS, six anatomical locations, including the face, the upper halves of the front and back sides of the trunk, are evaluated. For each region, a factor is determined that reflects the surface area, distribution, and density of pilosebaceous units. Additionally, there is a coefficient indicating the severity of different lesion types. The local score is determined by multiplying the factor of each anatomical region by the coefficient of the most severe lesion in that area. By summing up the local scores, a global score is calculated. The score range of GAGS is 0 to 44. The clinical equivalents of the scores are; 0=no lesion, 1–18= mild, 19–30= moderate, 31–38= severe, >39= very severe acne vulgaris.16
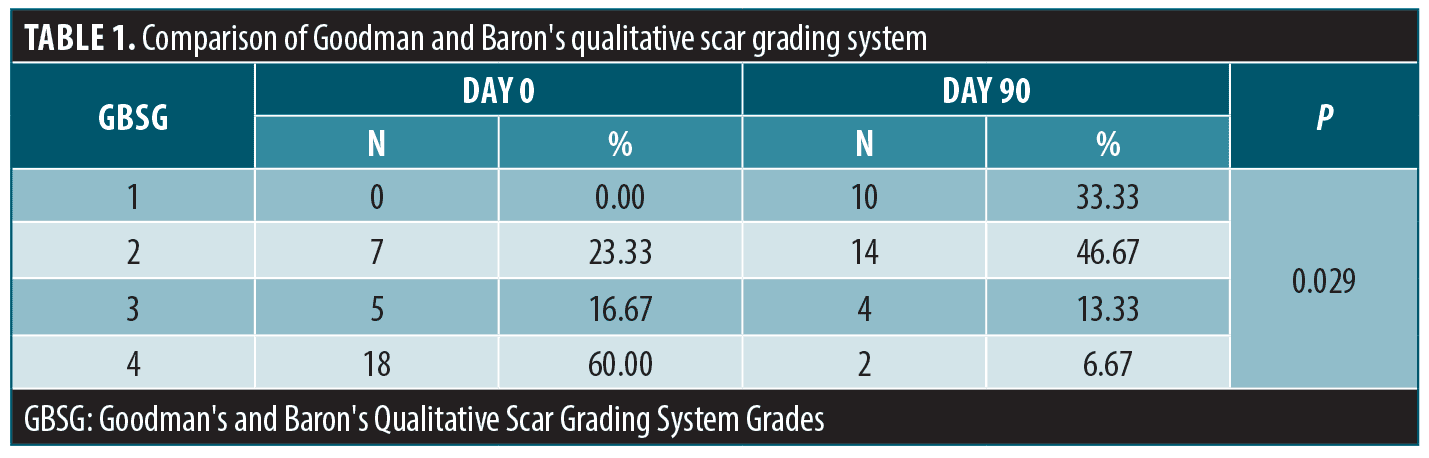
We evaluated the atrophic acne scar degrees by Goodman’s & Baron’s Qualitative Scar Grading System (GBSG) with four grades. Grade 1 states the macular scars. Grades 2, 3, and 4 represent mild, moderate, and severe atrophic acne scars, respectively. Changes in atrophic acne scar severity were followed by GBSG grade individually and by mean GBSG grade for all patients.17
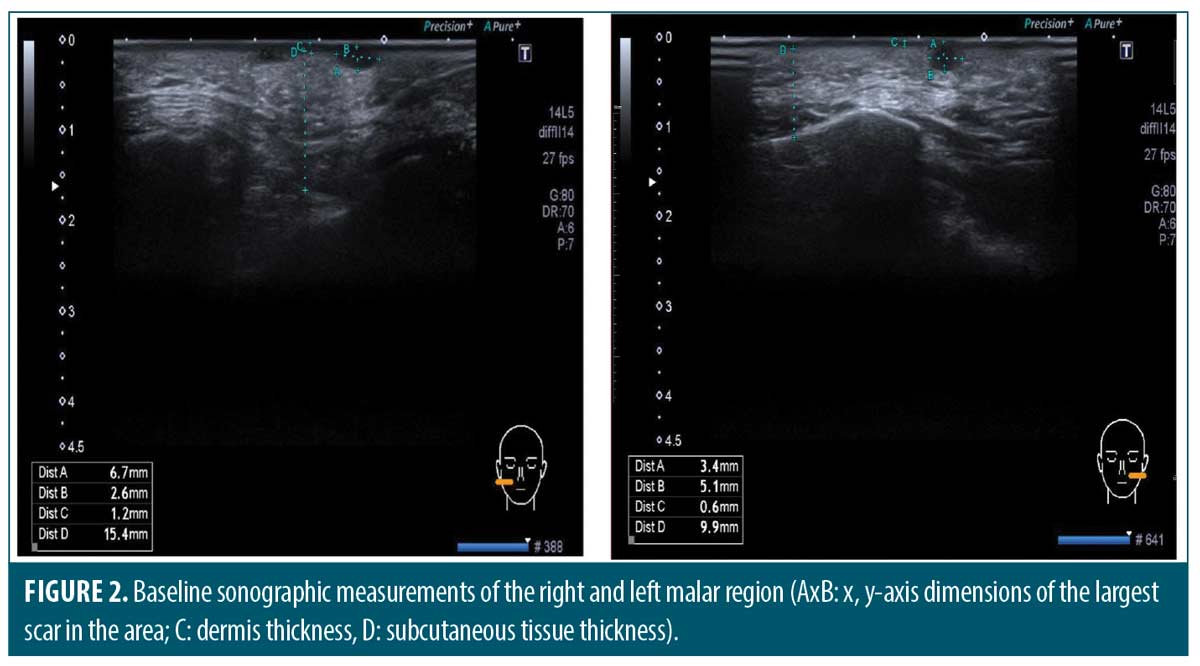
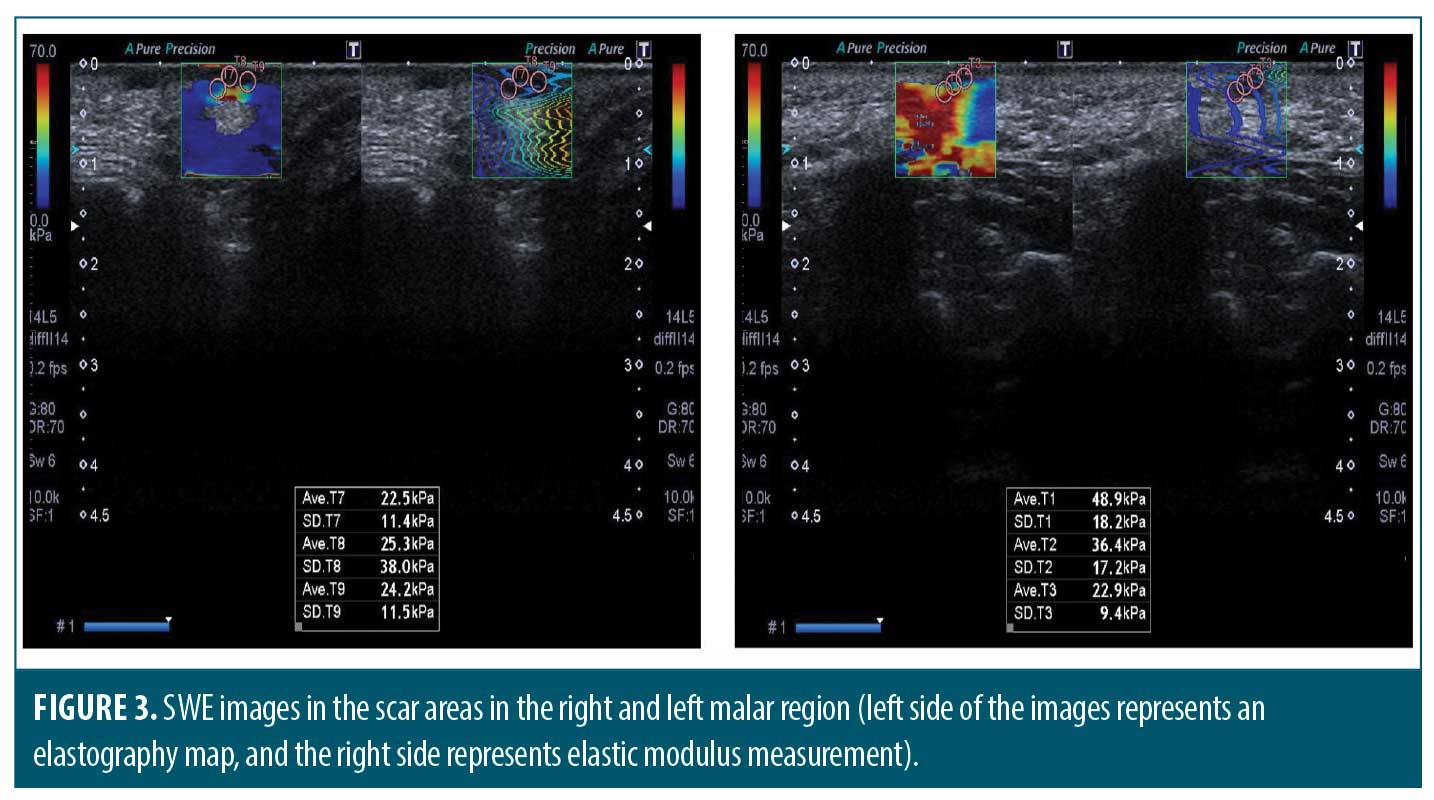
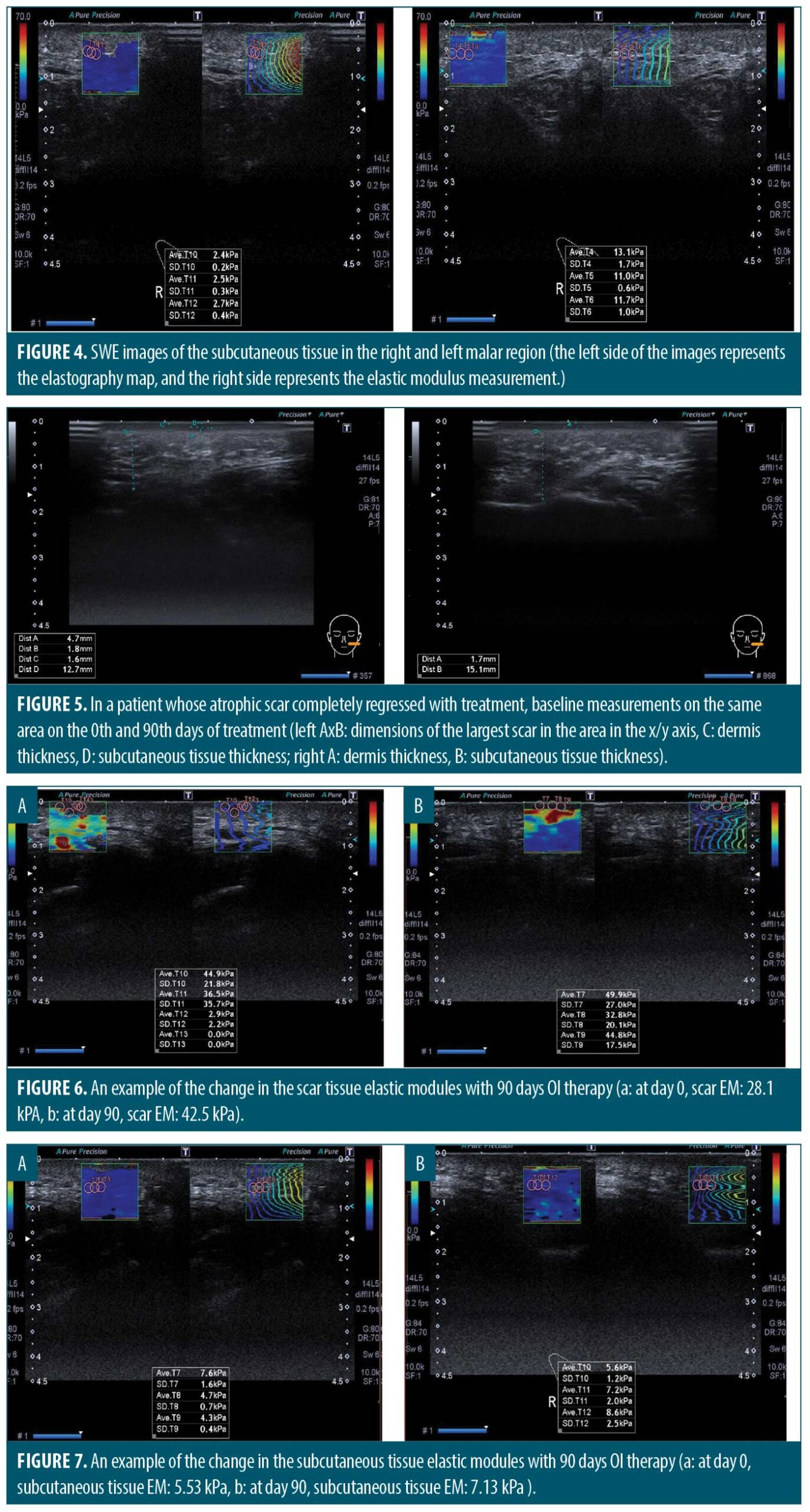
Measuring the thickness and elasticity of the skin and atrophic acne scars. We noted the most significant atrophic acne scar on each patient’s left and right cheek at the first visit. All measurements were performed on the same scar by the same experienced radiology specialist on Days 0 and 90. Ultrasound and 2D SWE were performed with Aplio 500 ultrasound device (Toshiba Medical Systems Corporation, Tochigi, Japan) using a PLT-805AT linear probe operating at 6.2–12 MHz frequency on appropriate longitudinal and transverse axes (Figure 1). Dermal thickness, subcutaneous tissue thickness, and scar size were measured on ultrasound imaging (Figure 2). The mean elastic modulus (MEM) of the scar and subcutaneous tissue was calculated as recorded in the “one-shot scan” mode on SWE using six different regions of interest with a diameter of 2mm per cheek (Figures 3 and 4).
Statistical analysis. An analysis was performed with the SPSS (Statistical Package for Social Science) version 23.0 program. The results were evaluated within the 95 percent confidence interval, and p<0.05 values were considered statistically significant.
Results
All participants (N=30) completed the study. The sample consisted of 30 individuals (21 females [70%] and 9 males [30%]). The age range of participants was 18 to 36 years, with a mean age 21.23±3.90.
Clinical evaluation. The GAGS range was 21–42 (mean: 34.60 ±6.65) on Day 0 and 7–39 (mean: 22.93 ±7.54) on Day 90. The mean GAGS significantly improved with treatment (p<0.001).
Patients’ GBSG grades on Day 0 and Day 90 are listed in Table 1. Mean GBSG was 3.36 on day 0 and 1.93 on Day 90. The improvement in GBSG grades was statistically significant (p:0.02).
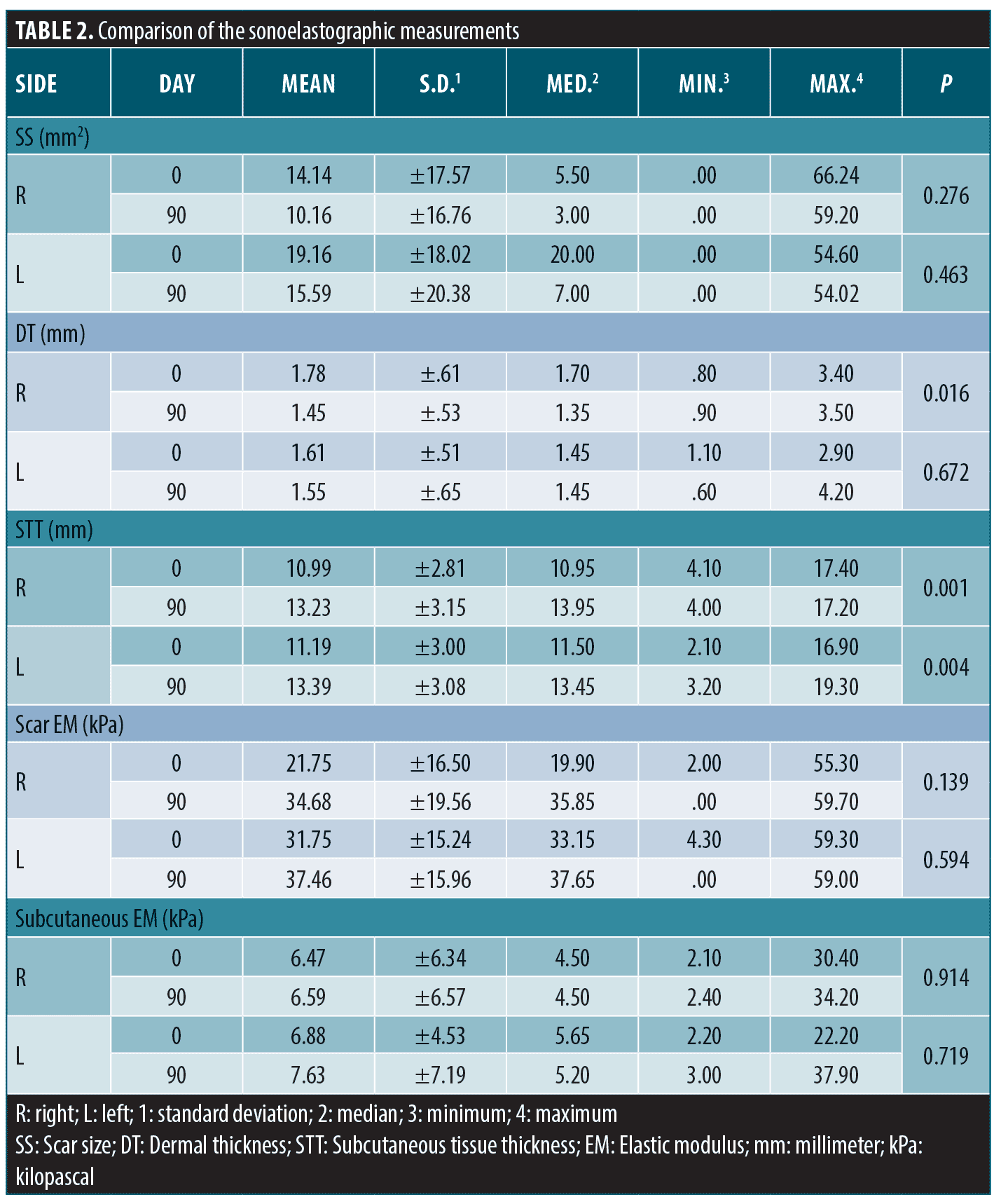
Sonoelastographic Evaluation. Measured scar size, thicknesses of the dermis and subcutaneous tissue, and the MEMs of the scar and subcutaneous tissue on Days 0 and 90 were as in Table 2. While scar size and dermal thickness bilaterally decreased, subcutaneous tissue thickness and the MEMs of scar and subcutaneous tissue increased with treatment (Figure 5–7).
Discussion
Atrophic acne scars are dermal fibrotic tissues characterized by epidermal and dermal atrophy, resulting from the natural wound healing after acne vulgaris. The main reasons for atrophy are enzymatic subcutaneous adipose tissue degradation and dermal collagen loss due to matrix metalloproteinases (MMPs).18–20 Clinically, they are presented as skin depressions and become more prominent with increasing scar stiffness and dermal collagen loss.21 Therefore, the induction of collagen production is crucial for their treatment.22–24
Unlike other medications, OI not only treats acne lesions but also suppresses MMP-9 and 13, thereby reducing tissue damage. Topical retinoids are known to aid in treating atrophic acne scars and induce collagen production.25-27 However, there is a lack of data objectively measuring the OI effect on atrophic acne scars in the literature. Therefore, in this study, we investigated the changes in atrophic acne scar with OI in 90 days by SWE.
We determined a significant improvement in the clinical severity of atrophic scars. They improved to macules in 33.3 percent of the patients. The mean GBSG improvement was 42.5 percent. It has been shown that many treatment modalities can significantly affect GBSG grades.23,28–32 One of the best options is the combination of microneedling and fractional radiofrequency for 36 weeks (mean improvement in GBSG of 52.3%).28 One of the weakest options is subcision for four sessions (mean improvement in GBSG of 8.3%).30 In another study, one session of autologous adipose tissue-derivated adult stem cells (AT-ASC) and three sessions of fractional CO2 laser were applied to each side of the face of 10 patients. There was a significant improvement in GBSG on both sides. The mean improvement in GBSG was 42.5 percent on the AT-ASC side.23 This improvement rate is similar to ours. The effects of topical retinoids on GBSG are controversial. Loss et al26 found that 0.3% adapalene gel application, once a day for the first four weeks and twice daily for the next 20 weeks, significantly improved the GBSG. While all patients were in Grade 3 or 4 initially, 38.9 percent reached Grade 1 in 24 weeks. This result is similar to ours, but in our study, the time was shorter. On the other hand, Afra et al33 applied micro-needling and 0.1% topical tazarotene separately for 12 weeks to two groups with atrophic acne scars. They did not observe a significant GBSG improvement in 24 weeks in both groups.
We observed a bilateral decrease in dermal thickness after OI treatment, with more significance on the right side. Yiğit et al34 also reported a substantial decrease in nasal skin dermal thickness in the eighth week of OI (0.5mg/kg/day) treatment. The reduction in dermal thickness with OI may be attributed to apoptosis in sebocytes and decreased sebum levels.35,36 Additionally, the anti-inflammatory effects of OI might reduce dermal edema.37 Dryness, reduced skin moisture, and dermal hydration could also contribute to decreased dermal thickness.38,39 Despite the decrease in dermal thickness, we observed an increase in the levels of MEM, suggesting preserved or even increased collagen and elastin, along with literature.40,41 Differences in dermal thickness between sides may be due to various factors, such as varying initial seborrhea, acne, acne scarring severity, or environmental influences. Additionally, the small sample size and/or short treatment duration may have influenced the results.
We observed a significant bilateral increase in subcutaneous tissue thickness. Although retinoids typically inhibit adipocyte hypertrophy and adipogenesis, our findings may be due to several reasons.42,43 One reason could be the inclusion of patients with inflammatory acne, as retinoic acid exposure in such cases may stimulate reactive adipogenesis, contributing to tissue thickening.44 Specific markers or probes that can distinguish superficial and deep adipocytes have not yet been developed. Thus, the measured subcutaneous tissue thickness in our study may include dermal white adipose tissue (dWAT), known for its dynamic nature and involvement in wound healing and immunity.45 The effect of OI treatment on subcutaneous tissue thickness may vary depending on inflammation and dWAT’s plasticity. measured subcutaneous tissue thickness in our study may include both dWAT and adipose tissue because they appear in the same fat density by ultrasound. Therefore, its increase could be explained by a change in dWAT, which is highly labile. It is known that dWAT-derived adipocytes have proliferated for wound healing in the scar region. They also activate the mature adipocytes to refill the scar and decrease the depth of depression following the fibroblast migration.46 It seems OI treatment mimics an intrinsic version of the autologous fat injection for atrophic acne scars. This could be another explanation for our clinical improvement in scars. Another potential reason for the increase could be the positive effect of OI on collagen synthesis, enhancing the extracellular matrix content in the subcutaneous tissue.47,48
Some dWAT-derived adipocytes transform into fibroblasts during remodeling.49 All-trans retinoic acid increases Type 1 and 3 collagen,50 and OI stimulates the release of adiponectin, increasing hyaluronic acid and collagen production.51,52 OI also inhibits collagenases, reduces MMP-9 and 13, and prevents scar formation.53,54 These factors may explain the reduction in bilateral scar size and the increase in scar and subcutaneous tissue MEMs observed in our study.
The effects of OI on subcutaneous adipose tissue and MMPs may have prevented scar formation and positively supported scar repair by altering the collagen and elastin production/destruction balance. Our clinical and sonoelastographic measurements showed these effects. However, the measured improvement in scar size and MEMs of the scar and subcutaneous tissue were statistically insignificant. Similar studies also reported insignificant changes in skin elasticity after OI treatment.38 While our study suggests a positive relationship between OI and skin MEM, longer treatment durations may yield more remarkable tissue-level improvements. The short study period might be the reason for the lack of statistically significant MEM increases in our results.
Our results should be interpreted considering certain limitations. Firstly, the scar scale used in this study does not assess atrophic acne scars individually and lacks quantitative measurement. Nonetheless, it is a commonly employed scar grading system in scientific research, and its reliability has been tested repeatedly. Secondly, our measurement area was determined based on the most prominent scar on each cheek, leading to standardized results for individual scars but making subcutaneous adipose tissue comparison challenging. Selecting precise anatomical locations on both sides of the face for each patient could have provided a more appropriate evaluation of adipose tissue and result reliability. Thirdly, we measured the effect of OI on scars in patients with acne. Unfortunately, we couldn’t evaluate the OI effect on only atrophic acne scars due to the OI is not on-label used for scar treatment. Fourthly, the study lacked a control group for comparison, which could have provided a better understanding of OI’s specific effects on scar improvement. To account for the lack of a control group, we utilized each subject’s baseline measurements as a point of reference for comparison. Lastly, we used the self-report system to follow up on the subjects’ weight during the study instead of measuring them by a scale which could have provided more accurate information regarding any potential influence of weight changes on the observed outcomes. Additionally, our probes could not provide detailed epidermal visualization. Future studies are needed to investigate subcutaneous adipose tissue and dermal white adipose tissue (dWAT) separately.
Conclusion
Our study demonstrates that OI therapy significantly improved the clinical presentation and tissue characteristics of atrophic acne scars and increased the elasticity of the affected scars. These findings suggest OI therapy as a practical option for treating atrophic acne scars. SWE, as the primary measurement modality, provided valid and objective results for tissue and scar elasticity and skin layer thickness, enabling comprehensive treatment follow-up. Overall, this work highlights the potential benefits of OI therapy and the significance of utilizing SWE in scar management, offering valuable insights for improving patient outcomes.
References
- Cunliffe WJ, Shuster S. Pathogenesis of acne. Lancet. 1969 Apr 5;1(7597):685-7.
- Goodman GJ. Postacne scarring: a review of its pathophysiology and treatment. Dermatol Surg. 2000 Sep;26(9):857-71.
- Layton A, Henderson C, Cunliffe W. A clinical evaluation of acne scarring and its incidence. Clinical and experimental dermatology. 1994;19(4):303-8.
- Fabbrocini G, Annunziata MC, D’Arco V, De Vita V, Lodi G, Mauriello MC, et al. Acne scars: pathogenesis, classification and treatment. Dermatol Res Pract. 2010;2010:893080.
- Picardo M, Eichenfield LF, Tan J. Acne and Rosacea. Dermatol Ther (Heidelb). 2017 Jan;7(Suppl 1):43-52.
- Nast A, Dréno B, Bettoli V, Degitz K, Erdmann R, Finlay AY, et al. European evidence-based (S3) guidelines for the treatment of acne. J Eur Acad Dermatol Venereol. 2012 Feb;26 Suppl 1:1-29.
- Zaenglein AL, Pathy AL, Schlosser BJ, Alikhan A, Baldwin HE, Berson DS, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016 May;74(5):945-73.e33.
- Bagatin E, dos Santos Guadanhim LR, Yarak S, Kamamoto CS, de Almeida FA. Dermabrasion for acne scars during treatment with oral isotretinoin. Dermatol Surg. 2010 Apr;36(4):483-9.
- Xue H, Ye D, Huang SL, He SJ, Liu J, Mu SZ, et al. Early acne scar intervention with 1064 nm picosecond laser in patients receiving oral isotretinoin: a randomized split-face controlled pilot study. Lasers Med Sci. 2023 Jan 12;38(1):40.
- Kalil CLPV, Fachinello FZ, Lamb FM, Comunello LN. Use of oral isotretinoin in photoaging therapy. SKINmed: Dermatology for the Clinician. 2008;7(1):10-4.
- Rabello Fonseca R, Azulay D, Luiz R, Mandarim de Lacerda C, Cuzzi T, Manela-Azulay M. Oral isotretinoin in photoaging: clinical and histopathological evidence of efficacy of an off-label indication. Journal of the European Academy of Dermatology and Venereology. 2009;23(2):115-23.
- Rivera AE. Acne scarring: a review and current treatment modalities. J Am Acad Dermatol. 2008 Oct;59(4):659-76.
- Davis LC, Baumer TG, Bey MJ, Holsbeeck MV. Clinical utilization of shear wave elastography in the musculoskeletal system. Ultrasonography. 2019 Jan;38(1):2-12.
- Sigrist RMS, Liau J, Kaffas AE, Chammas MC, Willmann JK. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics. 2017;7(5):1303-29.
- Ambroziak M, Noszczyk B, Pietruski P, Guz W, Paluch Ł. Elastography reference values of facial skin elasticity. Postepy Dermatol Alergol. 2019 Oct;36(5):626-34.
- Doshi A, Zaheer A, Stiller MJ. A comparison of current acne grading systems and proposal of a novel system. International journal of dermatology. 1997;36(6):416-8.
- Goodman GJ, Baron JA. Postacne scarring: a qualitative global scarring grading system. Dermatologic Surgery. 2006;32(12):1458-66.
- Patel L, McGrouther D, Chakrabarty K. Evaluating evidence for atrophic scarring treatment modalities. JRSM Open. 2014 Sep;5(9):2054270414540139.
- Goodman GJ, Baron JA. The management of postacne scarring. Dermatol Surg. 2007 Oct;33(10):1175-88.
- Fife D. Practical evaluation and management of atrophic acne scars: tips for the general dermatologist. J Clin Aesthet Dermatol. 2011;4(8):50.
- Tsai WY, Hsueh YY, Chen PY, Hung KS, Huang CC. High-Frequency Ultrasound Elastography for Assessing Elastic Properties of Skin and Scars. IEEE Trans Ultrason Ferroelectr Freq Control. 2022 Jun;69(6):1871-80.
- Gonzalez MJ, Sturgill WH, Ross EV, Uebelhoer NS. Treatment of acne scars using the plasma skin regeneration (PSR) system. Lasers Surg Med. 2008 Feb;40(2):124-7.
- Abou Eitta RS, Ismail AA, Abdelmaksoud RA, Ghezlan NA, Mehanna RA. Evaluation of autologous adipose-derived stem cells vs. fractional carbon dioxide laser in the treatment of post acne scars: a split-face study. International journal of dermatology. 2019;58(10):1212-22.
- Connolly D, Vu HL, Mariwalla K, Saedi N. Acne Scarring-Pathogenesis, Evaluation, and Treatment Options. J Clin Aesthet Dermatol. 2017 Sep;10(9):12-23.
- Kang S, Fisher GJ, Voorhees JJ. Photoaging and topical tretinoin: therapy, pathogenesis, and prevention. Archives of dermatology. 1997;133(10):1280-4.
- Loss MJ, Leung S, Chien A, Kerrouche N, Fischer AH, Kang S. Adapalene 0.3% Gel Shows Efficacy for the Treatment of Atrophic Acne Scars. Dermatol Ther (Heidelb). 2018 Jun;8(2):245-57.
- Fisher GJ, Datta S, Wang Z, Li X-Y, Quan T, Chung JH, et al. c-Jun–dependent inhibition of cutaneous procollagen transcription following ultraviolet irradiation is reversed by all-trans retinoic acid. J Clin Invest. 2000;106(5):663-70.
- Chandrashekar BS, Sriram R, Mysore R, Bhaskar S, Shetty A. Evaluation of microneedling fractional radiofrequency device for treatment of acne scars. J Cutan Aesthet Surg. 2014 Apr;7(2):93-7.
- Gadkari R, Nayak C. A split-face comparative study to evaluate efficacy of combined subcision and dermaroller against combined subcision and cryoroller in treatment of acne scars. J Cosmet Derm. 2014;13(1):38-43.
- Deshmukh NS, Belgaumkar VA. Platelet-Rich Plasma Augments Subcision in Atrophic Acne Scars: A Split-Face Comparative Study. Derm Surg. 2019;45(1):90-8.
- Long T, Gupta A, Ma S, Hsu S. Platelet-rich plasma in noninvasive procedures for atrophic acne scars: A systematic review and meta-analysis. J Cosmet Derm. 2020;19(4):836-44.
- Saadawi AN, Esawy AM, Kandeel AH, El-Sayed W. Microneedling by dermapen and glycolic acid peel for the treatment of acne scars: Comparative study. J Cosmet Derm. 2019;18(1):107-14.
- Afra T, Razmi M, Narang T, Dogra S, Kumar A. Topical tazarotene gel, 0.1%, as a novel treatment approach for atrophic postacne scars: a randomized active-controlled clinical trial. JAMA facial plastic surgery. 2019;21(2):125-32.
- Yigit E, Rakici IT, Seden N, Manav V, Kaygisiz I, Yigit O. The Impact of Isotretinoin Therapy on the Nasal Skin Thickness and Elasticity: An ultrasonography and elastography based assessment in relation to dose and duration of therapy. Aesthetic Plastic Surgery. 2021:1-11.
- Strauss JS, Stranieri AM. Changes in long-term sebum production from isotretinoin therapy. J Am Acad Dermatol. 1982 Apr;6(4 Pt 2 Suppl):751-6.
- Nelson AM, Gilliland KL, Cong Z, Thiboutot DM. 13-cis Retinoic acid induces apoptosis and cell cycle arrest in human SEB-1 sebocytes. J Invest Dermatol. 2006 Oct;126(10):2178-89.
- Dispenza MC, Wolpert EB, Gilliland KL, Dai JP, Cong Z, Nelson AM, et al. Systemic isotretinoin therapy normalizes exaggerated TLR-2-mediated innate immune responses in acne patients. J Invest Dermatol. 2012 Sep;132(9):2198-205.
- Gencebay G, Aşkın Ö, Serdaroğlu S. Evaluation of the changes in sebum, moisturization and elasticity in acne vulgaris patients receiving systemic isotretinoin treatment. Cutaneous and Ocular Toxicology. 2021;40(2):140-4.
- Mlosek RK, Malinowska S, Sikora M, Dębowska R, Stępień A, Czekaj K, et al. The use of high frequency ultrasound imaging in skin moisturization measurement. Skin Research and Technology. 2013;19(2):169-75.
- Melnik B. Isotretinoin and FoxO1: A scientific hypothesis. Dermato-endocrinology. 2011;3(3):141-65.
- Nelson AM, Zhao W, Gilliland KL, Zaenglein AL, Liu W, Thiboutot DM. Isotretinoin temporally regulates distinct sets of genes in patient skin. J Invest Dermatol. 2009 Apr;129(4):1038-42.
- Schwarz EJ, Reginato MJ, Shao D, Krakow SL, Lazar MA. Retinoic acid blocks adipogenesis by inhibiting C/EBPbeta-mediated transcription. Mol Cell Biol. 1997 Mar;17(3):1552-61.
- Berry DC, DeSantis D, Soltanian H, Croniger CM, Noy N. Retinoic acid upregulates preadipocyte genes to block adipogenesis and suppress diet-induced obesity. Diabetes. 2012 May;61(5):1112-21.
- Liggins MC, Li F, Zhang LJ, Dokoshi T, Gallo RL. Retinoids Enhance the Expression of Cathelicidin Antimicrobial Peptide during Reactive Dermal Adipogenesis. J Immunol. 2019 Sep 15;203(6):1589-97.
- Kruglikov I, Trujillo O, Kristen Q, Isac K, Zorko J, Fam M, et al. The Facial Adipose Tissue: A Revision. Facial Plast Surg. 2016 Dec;32(6):671-82.
- Schmidt BA, Horsley V. Intradermal adipocytes mediate fibroblast recruitment during skin wound healing. Development. 2013 Apr;140(7):1517-27.
- Ghassemi A, Prescher A, Riediger D, Axer H. Anatomy of the SMAS revisited. Aesthetic Plast Surg. 2003 Jul-Aug;27(4):258-64.
- Kruglikov I, Trujillo O, Kristen Q, Isac K, Zorko J, Fam M, et al. The facial adipose tissue: a revision. Facial Plastic Surgery. 2016;32(06):671-82.
- Marangoni RG, Korman BD, Wei J, Wood TA, Graham LV, Whitfield ML, et al. Myofibroblasts in murine cutaneous fibrosis originate from adiponectin-positive intradermal progenitors. Arthritis Rheumatol. 2015 Apr;67(4):1062-73.
- Schwartz E, Cruickshank FA, Mezick JA, Kligman LH. Topical all-trans retinoic acid stimulates collagen synthesis in vivo. J Invest Dermatol. 1991 Jun;96(6):975-8.
- Ezure T, Amano S. Influence of subcutaneous adipose tissue mass on dermal elasticity and sagging severity in lower cheek. Skin Res Technol. 2010 Aug;16(3):332-338.
- Karadag AS, Ertugrul DT, Takci Z, Bilgili SG, Namuslu M, Ata N, et al. The effect of isotretinoin on retinol-binding protein 4, leptin, adiponectin and insulin resistance in acne vulgaris patients. Dermatology. 2015;230(1):70-4.
- Gulsum Gencoglan M, tosun PhD M. Isotretinoin-induced effects of mast cells on wound healing. Journal of Drugs in Dermatology. 2010;9(10).
- Papakonstantinou E, Aletras AJ, Glass E, Tsogas P, Dionyssopoulos A, Adjaye J, et al. Matrix metalloproteinases of epithelial origin in facial sebum of patients with acne and their regulation by isotretinoin. J Invest Dermatol. 2005 Oct;125(4):673-84.