 J Clin Aesthet Dermatol. 2021;14(3):46–49.
J Clin Aesthet Dermatol. 2021;14(3):46–49.
by Izabella Picinin Safe, PhD; Viviane Macedo, MD; Wuelton Marcelo, MD; Djane Baia-Da-Silva, PhD; Monique Freire, MD; Renata Spener, MD;Victor Oliveira, MD; Jaquelane De Jesus, MD; Marcus Lacerda, PhdD And Marcelo Cordeiro-Santos, PhD
Drs. Safe, Macedo, Baia-da-Silva, Santana, Spener, Moraes, de Jesus, Lacerda, and Cordeiro-Santos are with the Fundação de Medicina Tropical Doutor Heitor Vieira Dourado in Manaus, Amazonas, Brazil. Drs. Safe, Monteiro, Santana, Spener, Moraes, Lacerda, and Cordeiro-Santos are with the Universidade do Estado do Amazonas in Manaus, Amazonas, Brazil. Drs. Safe, Moraes, Lacerda, and Cordeiro-Santos are with Clínica de Infectologia de Manaus in Amazonas, Brazil.
FUNDING: No funding was provided for this study.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: Nontuberculous mycobacteria (NTM) have been increasingly identified as causative of numerous cosmetic procedure related infections worldwide. This manuscript reports clinical aspects and antimicrobial resistance profiles of NTM infections associated with aesthetic procedures diagnosed in a private infectious disease clinic in the Brazilian Amazon. Four patients developed skin and soft tissue infections between August 2015 and August 2019. Clinical, microbiological, and epidemiological data were collected. M. conceptionense, M. abscessus and M. fortuitum were isolated. The histopathology showed dermal granulomatous inflammation. All patients were treated with a combination of antimycobacterial regimens, mainly with moxifloxacin and clarithromycin.
Keywords: Nontuberculous mycobacteria, aesthetic procedures, treatment
Interest in aesthetic and body-modifying procedures has increased significantly over the last few decades. The expanding cosmetic procedure market has opened a door to unskilled aesthetic practitioners. Currently, there exist practioners that perform a wide variety of cosmetic procedures without minimal aseptic requirements and quality control. Although the associated risk is generally low, nontuberculous mycobacteria (NTM) have been identified as the cause of cosmetic procedure-related infections worldwide.1
Generally, NTM are free-living organisms that are ubiquitous. Water, soil, and dust are important reservoirs that can be colonizers of medical equipment, such as endoscopes and surgical tools.2 Therefore, NTM infection usually results from inadequate sterilization techniques and contamination from nonsterile water.3 Because these organisms require laboratory techniques that are more sophisticated than the standard gram staining, such as aerobic and anaerobic culture and sensitivity tests, delay in diagnosis and appropriate antimicrobial management may occur. NTM usually do not respond to gram-positives first-line empirical antibiotics. This manuscript reports clinical aspects and antimicrobial resistance profile of four cases of NTM infections associated with cosmetic procedures diagnosed in a private infectious diseases clinic in the Brazilian Amazon.
Case Reports
Patients with a history of aesthetic procedures presenting to our clinic with skin and subcutaneous infections persisting for longer than two weeks and resistant to standard management were regarded as highly suspect of having NTM infection. The four patients presented here developed skin and soft tissue infections between August 2015 and August 2019.
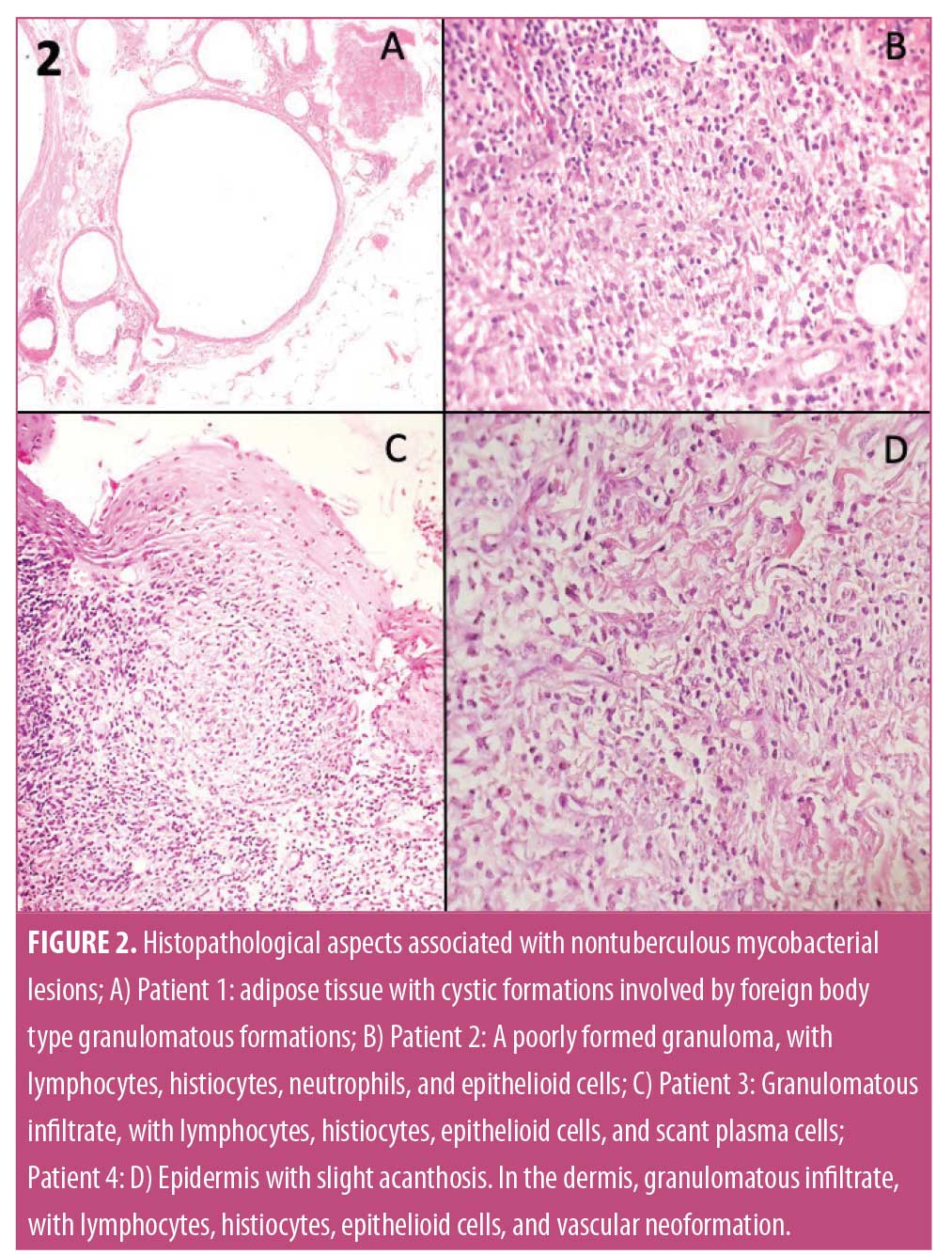
Clinical, microbiological, and epidemiological data were collected (Table 1). Bacterial isolation from excised skin tissue specimen was achieved using standard methods and direct inoculation into BACTEC 960 BD™. Bacterioscopy using Gram, culture for aerobics and GenXpert™ for Mycobacterium tuberculosis were performed. Magnetic resonance imaging (MRI) and histopathology were used to aid the diagnosis. Hematoxylin-eosin and Ziehl-Neelsen stains were used for histopathological examination. Antimicrobial resistance test were also performed (Table 2). Patients were followed up with for evaluation of relapses during different time intervals.
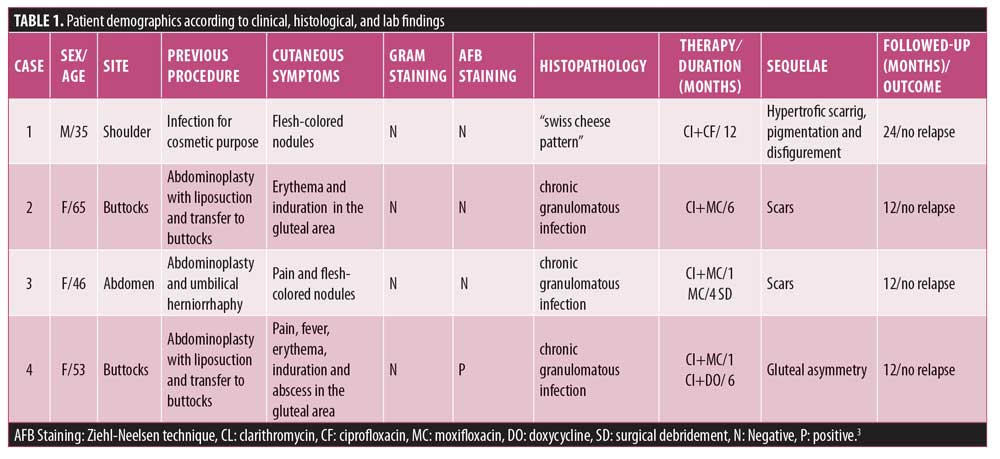
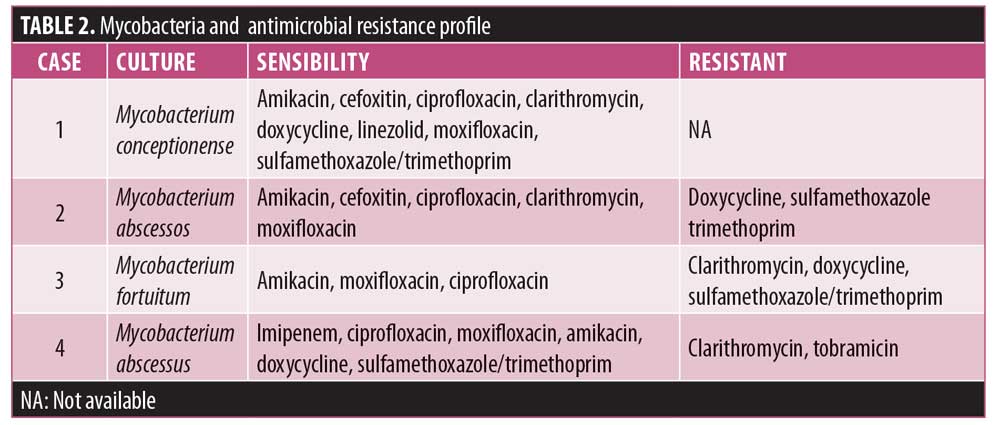
Case 1. A 35-year-old, human immunodeficiency virus (HIV)-positive male patient, who used muscular injection of oily steroids, presented to our clinic with erythematous to violaceous plaques and nodules on the shoulder (Figure 1A). MRI showed edema and thickening of the subcutaneous tissue of the upper lateral region of the shoulder with an abscess measuring 1.2cm. CD4+TL count was 604 cells/mm3 with undetectable viral load (using dolutegravir, tenofovir, and lamivudine). Biopsied skin showed a “swiss cheese” pattern (Figure 2A), consistent with an oleoma result of a chronic foreign body reaction. Mycobacterium conceptionense was identified by NTM culture. Empiric regimens of oral moxifloxacin 400mg daily and oral clarithromycin 500mg BID were initiated while awaiting species identification and sensitivities. After the confirmation of the NTM infection and sensitivity testing, the patient was kept on treatment for 12 months (Figure 1B). The patient was followed up with for an additional 24 months without relapse.
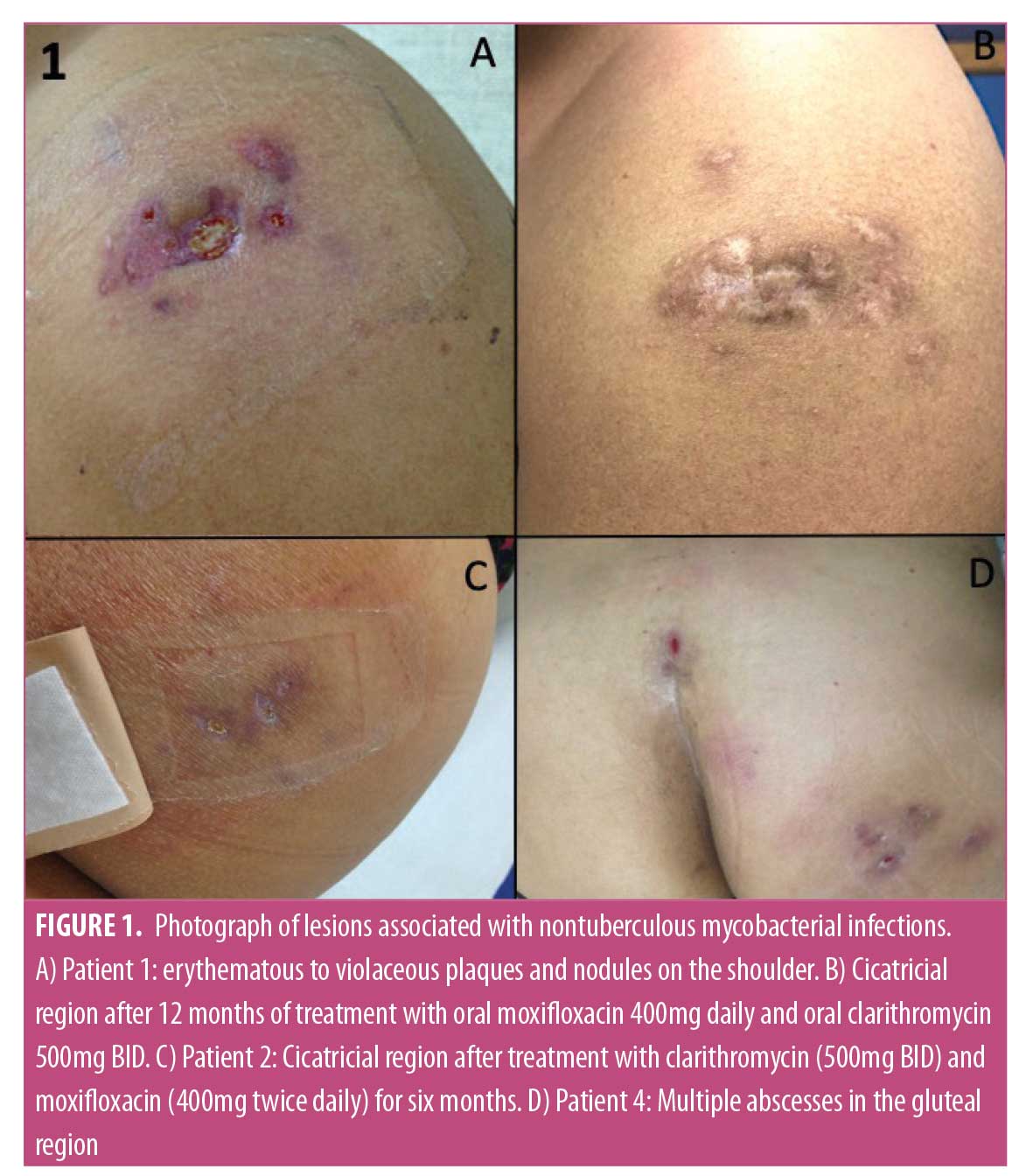
Case 2. A 65-year-old otherwise healthy female patient with a history of abdominoplasty with liposuction and fat transfer to her buttocks presented with redness and swelling in the gluteal area. Physical examination revealed an abscess in the gluteal area of approximately 6cm in diameter. The patient was treated with cephalexin 500mg BID for seven days without improvement. An MRI showed thickening of the subcutaneous tissue and gluteal musculature with the presence of isolated and confluent abscess. Biopsied skin showed dermal granulomatous inflammation (Figure 2B). Mycobacterium abscessus was identified. Resistance to antibiotics doxycycline, sulfamethoxazole-trimethoprim was evidenced. The patient was treated with empirical use of clarithromycin (500mg BID) and moxifloxacin (400mg BID) for six months. The patient was followed up with for an additional 12 months without relapse (Figure 1C).
Case 3. A 46-year-old otherwise healthy women with a history of abdominoplasty presented with redness and swelling in the right iliac fossa and epigastric region, which progressed slowly over three months following plastic surgery. Physical examination revealed an erythematous, hardened, painful area approximately 2cm in diameter. The patient was treated with empirical use of cefadroxil (500mg BID) for 14 days without improvement. An MRI revealed irregularities in the subcutaneous tissue of the abdominal wall and two small subcutaneous cystic formations. Biopsied skin showed dermal granulomatous inflammation (Figure 2C). Mycobacterium fortuitum was identified. The patient was treated with empirical use of clarithromycin (500mg BID) and moxifloxacin (400mg once daily [QD]) for one month. Clarithromycin resistance was evidenced, and the patient was kept on moxifloxacin (400mg QD) for five months. The patient was followed up with for additional 12 months without relapse.
Case 4. A 53-year-old otherwise healthy woman with a history of abdominoplasty with liposuction and fat transfer to buttocks presented with fever, pain, redness, and swelling in the gluteal area that progressed over four weeks after plastic surgery. A physical examination revealed multiple abscesses in the gluteal region (Figure 1D). The patient reported using amoxicillin, oxacillin, gentamicin, and metronidazole without improvement, but was unclear for how long these were used. An MRI showed superficial gluteal collection joining large deep subcutaneous collections in contact with the muscular fascia, associated with diffuse infiltration of the neighboring subcutaneous tissue and the gluteus maximus muscles. She underwent incision and drainage and surgical debridement of the gluteal area abscesses. Biopsied skin showed dermal granulomatous inflammation (Figure 2D). In this case, Ziehl-Neelsen staining was positive (++). Mycobacterium fortuitum was identified. The patient was treated with empirical use of clarithromycin (500mg BID) and moxifloxacin (400mg QD). After one month, clarithromycin was switched to doxycycline (200mg QD), and the patient was kept on moxifloxacin and doxycycline for six months. After six months, small hyperpigmented scars were observed in the right gluteal region. Some of the scars were not hidden in a conventional swimsuit. The patient was followed up with for an additional 12 months without any relapse.
Discussion
The incidence of NTM infections has increased in the last few years due to the greater prevalence of immunosuppression and skin surgical procedures in the general population. Most mycobacterial skin and soft tissue infection outbreaks have involved the rapidly growing species of the M. fortuitum complex, which is mainly composed of M. fortuitum, M. abscessus, M. chelonae, and M. conceptionense.4,5 These bacteria are increasingly implicated in cosmetic procedure-related infections.3,6 Possible sources of surgical-site infections may occur due to the use of nonsterile water for cleaning surgical instruments followed by inadequate disinfectants and sterilization procedures. NTM are relatively resistant to standard disinfectants, such as chlorine, organomercurials, and alkaline glutaraldehydes.7 M. conceptionense, M. abscessus, and M. fortuitum were isolated in the patients comprising this case series and records associated with these bacteria were reported in two cases associated with infection after mesotherapy in Brazil.8
The diagnosis was performed by biopsies of the skin lesions and abscess followed by cultures and histopathology in all cases. Isolation and identification of the isolates were performed in all cases of our study. However, PCR for NTM is advised for patients presenting with cutaneous lesions after aesthetic procedures, as it is already known to improve NTM detection in false negative cultures, which allows the rapid treatment and consequent clinical outcome of the patient.9 Cutaneous NTM infections occurred with variable clinical and histopathological features. The histopathology in immunocompetent patients was characterized by pseudoepitheliomatous epidermal hyperplasia, intraepithelial abscesses, transepidermal elimination, and dermal granulomatous inflammation accompanied by necrosis and suppuration.10 In this case series, the histopathology showed dermal granulomatous inflammation, associated with flesh-colored nodules, erythema, induration, pain, fever, erythema, induration, and abscesses. Induration, furuncle, cutaneous abscess, or deep draining tract with history of aesthetic procedures not responding to routine oral antibiotics reinforces the suspicion. Gram staining and regular culture usually do not yield any results and specific stains and cultures for mycobacteria are required.11–13 To minimize diagnostic delays, the patients should have appropriate testing performed, including tissue biopsy, Ziehl-Neelsen stain, culture and PCR.
The optimal treatment regimens for skin and soft tissue infection with NTM are not well established. All the patients were treated with a combination of antimycobacterial regimens, preferably with moxifloxacin and clarithromycin. Antimycobacterial drugs were started on an empirical basis, and were later modified according to results of sensitivity testing. Frustratingly, the culture of NTM requires time, and thus, information on the available spectrum of antimicrobial susceptibility is delayed. Therefore, an empiric choice of antibiotic is made and therapy might fail initially. In this case series, the species, profile, and permanence were defined. Three patients had isolates resistant to clarithromycin, one of the antibiotics used in therapeutics. Resistance to clarithromycin is reported in the literature for M. abscessus subspecies by many researchers.14,15
Surgical debridement was performed in a single patient who had had silicone implants. In some NTM infections, such as those with M. abscessus, surgical debridement is useful in addition to antimicrobial treatments, since during surgical re-examination, foreign bodies are usually removed and the presence of lipid coating in the mycobacterial cell walls is proven to be the culprit in biofilm formation on the surface of implants.16
Conclusion
NTM should be considered in the differential diagnosis of an unusual skin and soft-tissue infection. A low index of suspicion of NTM often delays the correct diagnosis. The confirmation takes too much time, and, hence, appropriate treatments cannot be started on time. Moreover, the misdiagnosis and the delay of applying appropriate antibiotics obscures the treatment process. This results in patients suffering severe aesthetic sequelae, such as scarring, pigmentation, and disfigurement. We propose an empirical regimen of clarithromycin and moxifloxacin as an efficient treatment option for an NTM infection, and surgical debridement with collection of material for analysis should be mandatory.
References
- Hypolite T, Grant-Kels JM, Chirch LM. Nontuberculous mycobacterial infections: a potential complication of cosmetic procedures. Int J Womens Dermatol. 2015;1(1): 51–54.
- Johnson MM, Odell JA. Nontuberculous mycobacterial pulmonary infections. J Thorac Dis. 2014;6(3):210–220.
- Schnabel D, Esposito DH, Gaines J, et al. Multisite US Outbreak of Rapidly Growing Mycobacterial Infections Associated with Medical Tourism to the Dominican Republic, 2013–2014[1]. Emerg Infect Dis. 2016;22(8):1340–1347.
- O’Neill MB, Mortimer TD, Pepperell CS. Diversity of Mycobacterium tuberculosis across evolutionary scales. PLoS Pathog. 2015;11(11):e1005257.
- Prevots DR, Shaw PA, Strickland D, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated healthcare delivery systems. Am J Respir Crit Care Med. 2010;182(7):970–976.
- Zhang X, Liu W, Liu W, et al. Cutaneous infections caused by rapidly growing mycobacteria: case reports and review of clinical and laboratory aspects. Acta Derm Venereol. 2015;95(8):985–989.
- Selvaraju SB, Khan IU, Yadav JS. Biocidal activity of formaldehyde and nonformaldehyde biocides toward Mycobacterium immunogenum and Pseudomonas fluorescens in pure and mixed suspensions in synthetic metalworking fluid and saline. Appl Environ Microbiol. 2005;71(1):542-546.
- Reis CMS, Wen L, Barreto AMW, et al. Complicaçöes cutâneas da mesoterapia: infecçäo por Mycobacterium fortuitum (duas observaçöes) / Cutaneous complications of mesotherapy: mycobacterium fortuitum infections (2 cases). An Bras Dermatol. 1993;68:277–280
- Perng CL, Chen HY, Chiueh TS,et al. Identification of non-tuberculous mycobacteria by real-time PCR coupled with a high-resolution melting system. J Med Microbiol. 2012;61(7):944–51.
- Li JJ, Beresford R, Fyfe J, Henderson C. Clinical and histopathological features of cutaneous nontuberculous mycobacterial infection: A review of 13 cases. J Cutan Pathol. 2017;44(5):433–443.
- Gonzalez-Santiago TM, Drage LA. Nontuberculous Mycobacteria: Skin and Soft Tissue Infections. Dermatol Clin. 2015;33(3):563–577.
- Lamb RC, Dawn G. Cutaneous non-tuberculous mycobacterial infections. Int J Dermatol. 2014;53(10):1197–1204.
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367–416.
- Mougari F, Bouziane F, Crockett F, et al. Selection of Resistance to Clarithromycin in Mycobacterium abscessus Subspecies. Antimicrob Agents Chemother. 2016;61(1): e00943–16.
- Brown-Elliott BA, Vasireddy S, Vasireddy R, et al. Utility of sequencing the erm(41) gene in isolates of Mycobacterium abscessus subsp. abscessus with low and intermediate clarithromycin MICs. J Clin Microbiol. 2015;53:1211–1215.
- Thomas M, D’Silva JA, Borole AJ, et al. Periprosthetic atypical mycobaterial infection in breast implants: A new kid on the block! J Plast Reconstr Aesthet Surg. 2013;66:e16–e19.

