 by Ronald L. Moy, MD, and Corey Levenson, PhD
by Ronald L. Moy, MD, and Corey Levenson, PhD
Dr. Moy is from Facial Cosmetic Surgery in Beverly Hills, California, and Dr. Levenson is an employee of Santalis Pharmaceuticals, San Antonio, Texas.
Funding: No funding was provided.
Disclosures: Dr. Moy has no financial conflicts relevant to the content of this article. Dr. Levenson is an employee of
Santalis Pharmaceuticals, which holds several Investigational New Drug Applications (INDs) for clinical investigations on sandalwood oil, the topic of this review.
Abstract: Many skin conditions and diseases are characterized by inflammation, infection, and hyperplasia. Safe and effective topical treatment options that can be used long-term are needed. Traditional botanical medicines, which are often complex mixtures that exert their biological activities via multiple mechanisms of action, are being studied as potential new active ingredients in dermatology. Sandalwood album oil (SAO), also known as East Indian sandalwood oil (EISO), is an essential oil distilled from the Santalum album tree and has demonstrated biological activity as an anti-inflammatory, anti-microbial, and anti-proliferative agent. Sandalwood album oil has also shown promise in clinical trials for treatment of acne, psoriasis, eczema, common warts, and molluscum contagiosum. The favorable safety profile, ease of topical use, and recent availability of pharmaceutical-grade sandalwood album oil support its broader use as the basis of novel therapies in dermatology.
Keywords: Santalum album oil, East Indian sandalwood oil, sandalwood oil, botanical drugs, anti-inflammatory, antimicrobial, antiproliferative, acne, psoriasis, Molluscum contagiosum, atopic dermatitis, eczema
J Clin Aesthet Dermatol. 2017;10(10):34–39
Sandalwood album oil (SAO) has been utilized topically for centuries in both Ayurvedic and traditional Chinese medicine. The oil is distilled from the heartwood of the Santalum album tree and contains over 125 structurally related compounds, with fewer than a dozen components present in concentrations greater than 1% by weight.1 Because SAO is a significant item of commerce, used in many personal care products and perfumes, there is an international specification for the oil (ISO 3518:2002). SAO is listed in the United States Food and Drug Administration (FDA) Food Chemicals Codex as a natural flavoring ingredient, and the Australian Therapeutic Goods Administration (TGA) has classified the oil as a Listed Medicine, available as an active ingredient in many non-prescription products.
Worldwide, there are more than a dozen species of sandalwood, most of which have served as sources of essential oil. However, the International Organization for Standardization (ISO) has issued standards for only two species: Santalum album and Santalum spicatum (West Australian sandalwood). Of the two species, S. album produces oil with much higher concentrations of alpha- and beta-santalol. SAO was previously produced from wild-grown trees in India, but over-harvesting and poaching has led to Santalum album trees being pushed to the brink of extinction in their native habitats. Since 1998, the trees have been listed as Vulnerable by the International Union for the Conservation of Nature, and the harvesting and export of wild-grown Indian trees is highly restricted. The trees that are currently being used to produce sandalwood album oil for pharmaceutical applications are sustainably cultivated by Quintis, Ltd. (formerly TFS Corp. Ltd.) on Australian plantations.
The FDA has issued guidelines for the development of traditional medicines derived from plants.2 Such botanicals are often mixtures of numerous active compounds acting via multiple mechanisms of action. In general, if the mixture’s composition is under tight control and there are no known safety issues, botanical drugs can be studied in clinical trials as mixtures and can receive marketing approval as long as they are shown to be safe and effective. Veragen®, a green tea (Camellia senensis) extract for treatment of genital warts, and Fulyzac®, an anti-diarrheal extract from Croton lechleri, were the first two botanical drugs approved for sale in the United States under the FDA guidelines.
Anti-inflammatory, anti-oxidant, and related properties of sandalwood album oil. SAO is known to mediate its anti-inflammatory properties in vitro through multiple mechanisms. The oil inhibits the oxidative enzyme 5-lipoxygenase and has DPPH radical scavenging activity and, in vivo, SAO was able to protect mouse livers from damage resulting from oxidative stress and the formation of reactive oxygen species.3-7
In co-cultures of dermal fibroblasts and keratinocytes, the oil suppressed the production of numerous pro-inflammatory chemokines and cytokines produced in response to stimulation by lipopolysaccharide (LPS). Production of PGE2 was also suppressed, suggesting that SAO might be acting, at least in part, through inhibition of cyclooxygenase.8 Additional anti-inflammatory activity in skin was reported to rely on the activation of the enzyme 11b-HSD1, which plays a role in cortisol synthesis by keratinocytes. The oil also suppressed the expression of the pro-inflammatory cytokine, IL-1b, in keratinocytes and reduced irritant dermatitis in mouse skin stimulated with haptens.9
Recent interest in inflammatory-specific targets for the treatment of skin conditions such as psoriasis and atopic dermatitis has led to the development of a number of drugs and drug candidates that reduce levels of IL-17 and the activity of PDE4.10-13 SAO has been shown to specifically inhibit both of these targets in various in-vitro models,14-16 suggesting a mechanism for the activity seen in clinical studies of the oil in the treatment of these skin conditions.
Alpha-santalol was found to be an inhibitor of tyrosinase, a key enzyme in the biosynthetic pathway for the skin pigment melanin.17 This intriguing finding suggests that SAO may potentially act as an inhibitor of abnormal pigmentation associated with aging and exposure to ultraviolet light.
Anti-microbial properties of album oil. The use of essential oils fell out of favor in the 1900s with the advent of sulfa drugs and other antibiotics, but interest in essential oils, such as SAO, has been re-vitalized in recent years due to their activity against antibiotic-resistant strains of bacteria, such as methicillin-resistant Staphylococcus aureus (MRSA).18,19
Album oil has been found to be broadly active against many gram-positive strains of bacteria, including Staphylococcus (including antibiotic-resistant strains MRSA and VRSA), Streptococcus, and some gram-negative bacteria.20–23
SAO has demonstrated potent activity against many fungal dermatophytes and yeasts including Trichophyton, Microsporum and Candida.4, 24–29 Album oil is active against Herpes simplex viruses-1 and -2.30,31 Beta-santalol, one of the principal components of SAO, was found to inhibit the replication of influenza virus A/HK (H3N2) in vitro at 100µg/mL.32
The anti-microbial mechanism(s) of action of album oil have not been thoroughly elucidated but the effects seen might be partly due to the disruption of membrane integrity, a phenomenon that has been demonstrated for other essential oils.33-35
Anti-proliferative and anti-cancer properties of sandalwood album oil.
The ability of SAO to prevent the formation of tumors in mouse skin as a result of exposure to chemical carcinogens and ultraviolet light has been extensively studied in vitro and in vivo by the Dwivedi group.36 The anti-cancer effects of album oil and its major components have also been demonstrated in bladder cancer cells and oral cancer cells.37,38 In two papers published in 2013, Saraswati reported that alpha-santalol, the primary component of SAO, is anti-angiogenic and inhibits the growth of hepatocellular carcinoma and prostate tumors in vitro and in vivo.39,40 A common mechanistic feature in these studies seems to be the ability of the oil to cause cell cycle arrest at G2/M and to induce apoptosis and subsequent cell death. SAO was also shown to induce autophagy and cell death in proliferating keratinocytes, suggesting that album oil may be able to prevent the progression of pre-cancerous conditions, such as actinic keratosis, to skin cancers.41
When SAO was screened against the National Cancer Institute’s NCI-60 panel of 60 human tumor cell lines, the oil inhibited the growth of all cells lines with IC50s ranging from 7 to 126?m (unpublished data, 2010, Southern Research Institute, Birmingham Alabama). Interestingly, this cytotoxicity was generally not seen when album oil was applied to non-cancerous cells.
A recently published study examined the effects of essential oil from Santalum austrocalidonicum trees on human breast cancer cell lines (MCF-7) and non-tumorigenic epithelial breast cells (MCF-10A).42 The authors demonstrated that the oil induced deoxyribonucleic acid (DNA) strand breaks in both cell lines. Unlike the MCF-10A cells, the MCF-7 cells were not able to repair the damage, and therefore, the essential oil showed a selective cytotoxicity toward the MCF-7 breast cancer cell line. It should be noted that the spectrum of components in Santalum austrocalidonicum oil differs from that seen in SAO but the general phenomenon observed might explain, in part, why sandalwood oil seems to be preferentially cytotoxic towards cancerous cell lines.
A recent publication presented data showing that, in cultured keratinocytes, SAO enhanced expression of transcription factors (snail, twist) and mesenchymal factor (vimentin), all of which are related to the epithelial-mesenchymal transition (EMT). Album oil also promoted epidermal wound healing in vivo compared to vehicle control.43
The olfactory receptor, OR2AT4, is expressed in keratinocytes and has been shown to bind to sandalwood odorants resulting in elevation of intracellular calcium levels and phosphorylation of extracellular kinases (Erk1/2) and p38 mitogen-activated kinases, promoting keratinocyte proliferation and wound healing in human skin ex vivo.44 The relationship between OR2AT4 (or other sandalwood component receptors) and chemopreventive mechanisms in skin is speculative at this time, but these initial, intriguing findings are likely to stimulate further studies exploring the role of dermal sandalwood receptors in cellular proliferation.45
Safety profile of sandalwood album oil. SAO has a long history of topical use as a traditional medicine and in personal care products. The dermal LD50 in rats is in excess of 5gm/kg of body weight.46 When applied to human subjects in patch testing, neither neat sandalwood oil, nor a 10% SAO ointment, produced irritation or sensitization (unpublished data, 2013, Santalis Healthcare Corporation, San Antonio, Texas). In routine testing, a small percentage (0.1–2.4%) of people have been found to be allergic to album oil.1 However, in many of these studies, the provenance, purity, and source of the sandalwood is not clear. For example, other species of sandalwood, such as Western Australian (Santalum spicatum) or Hawaiian sandalwood (Santalum paniculatum), contain significant percentages of farnesol, an irritant, that is not found in oil from S. album.
SAO contains no known carcinogens and was not genotoxic in the Bacillus subtilis rec-assay.47 The safety profile of album oil was reviewed by Burdock in 2008 and, more recently, by Tisserand and Young.48,49
Little is known about the metabolism of SAO and its components by humans. However, in rabbits, alpha-santalol is oxidized to various diols; in dogs, it is oxidized to a carboxylic acid.50
 Human clinical trials of SAO. Pediatric clinical studies looking at SAO in the treatment of human papillomavirus (HPV) warts or Molluscum contagiosum led to the issuance of three United States patents related to the use of sandalwood album oil for the treatment of these skin conditions.51–53 Since those early studies, a number of additional studies in the field of dermatology have been initiated in the United States and Australia.
Human clinical trials of SAO. Pediatric clinical studies looking at SAO in the treatment of human papillomavirus (HPV) warts or Molluscum contagiosum led to the issuance of three United States patents related to the use of sandalwood album oil for the treatment of these skin conditions.51–53 Since those early studies, a number of additional studies in the field of dermatology have been initiated in the United States and Australia.
Several on-going and completed clinical trials studying SAO are listed on clinicaltrials.gov in a variety of indications, including Verruca vulgaris (common warts), Molluscum contagiosum, genital warts, psoriasis, oral mucositis, and atopic dermatitis.54
In an open-label study of 50 patients undergoing high-dose radiation therapy for head and neck cancer, a cream containing SAO and turmeric was compared to baby oil for its ability to reduce the severity of radiodermatitis.55 The SAO/turmeric group had reduced incidence of Grade 3 radiodermatitis, and a comparison between the two groups for the degree of radiodermatitis showed that the cohorts using the SAO/turmeric cream had delayed appearance and reduced levels of dermatitis at all time points. The drawbacks of the study included lack of blinding and the fact that the beneficial effects could not be attributed to the album oil, the turmeric, or a combination of the two.
A randomized, double-blind, placebo-controlled dose range finding trial of SAO ointment (10%, 20%, and 30% strengths) was studied in subjects with common warts (Verruca vulgaris) caused by HPV (NCT01286441). The primary endpoints of the trial were efficacy, safety, and tolerability. All three treatment arms were deemed to be safe and well tolerated. There were no serious adverse events considered to be related to the study medication, and only four adverse events (3 in the 30% arm and 1 in the 10% arm) were deemed to be related to the study medication; notably, all were mild, reversible irritation at the site of application. All three treatment arms showed greater rates of wart clearance and reduction in wart area than did those in the placebo arm (unpublished results, 2016, Santalis Healthcare Corporation, San Antonio, Texas).
In addition to the on-going and completed studies looking at SAO being conducted under multiple investigational new drug (IND) applications in the United States, several small proof-of-concept studies have been conducted examining SAO in combination with various over-the-counter monograph drugs for the treatment of acne, common warts, and atopic dermatitis.
A single-center, open-label pilot study of a novel over-the-counter topical blend of 0.5% salicylic acid and up to 2% SAO was conducted in adolescent and adult subjects with mild to moderate facial acne. Over the course of the eight-week treatment period, approximately 89 percent of participants experienced an improvement in their disease when compared with baseline. No adverse events were observed that would limit use of the regimen.56 The four-component treatment regimen employed in the Moy acne study56 subsequently became commercially available in the United States as an over-the-counter acne product line sold by Galderma (Fort Worth, Texas) under the Benzac® name.
Atopic dermatitis was treated in a pediatric population with a novel three-product regimen containing 0.1% colloidal oatmeal and SAO in a single-center, open-label confirmatory study. Overall, the treatment regimen was very well-tolerated, safe, and seemed to be effective at reducing the severity of atopic dermatitis in the target patient population.57
For the treatment of common warts, a proprietary topical blend of 17% salicylic acid and approximately 2% alpha-santalol was used in two open-label studies in children and adolescents with common warts. For the two studies, 44 percent (11/25) and 30 percent (10/33) of patients who completed treatment and follow-up met the primary endpoint. A total of 16 percent (4/25) and 21 percent (7/33) of patients experienced complete resolution of treated warts. The treatment was well tolerated with 10 to 30 percent of patients experiencing mild-to-moderate itching, burning, dryness, and stinging.58
Discussion
Botanical therapies have been used for centuries by many cultures as traditional medicines to treat a wide variety of skin and other conditions. The use of botanical remedies, such as green tea and tea tree oil, in modern dermatology clinical trials has been reviewed.59–61 More recent reviews have focused on the use of botanical therapies for specific skin conditions such as rosacea and psoriasis.62,63 Botanicals and phytochemicals have shown promise in the treatment of skin disorders, and this class of drugs is generally well-received by patients who tend to perceive botanical remedies as being “natural.”
SAO has been, and continues to be, extensively used as an herbal medicine, particularly in Asia. It is also widely used as an ingredient in many personal care products and fragrances, but at very low concentrations. Its safety profile has been well-characterized and, although a small percentage of the general population is allergic to SAO, it has been shown through extensive Human Repeat Insult Patch Test (HRPIT) testing to be non-irritating and non-sensitizing as a pure oil or when formulated for topical use. In preclinical models, the oil has been shown to have broad anti-inflammatory, anti-infective, and anti-proliferative properties. These properties are likely due to multiple mechanisms of action resulting from the interaction of the many components in the oil with multiple biological targets. The various mechanisms of action of SAO are depicted in Figure 1.
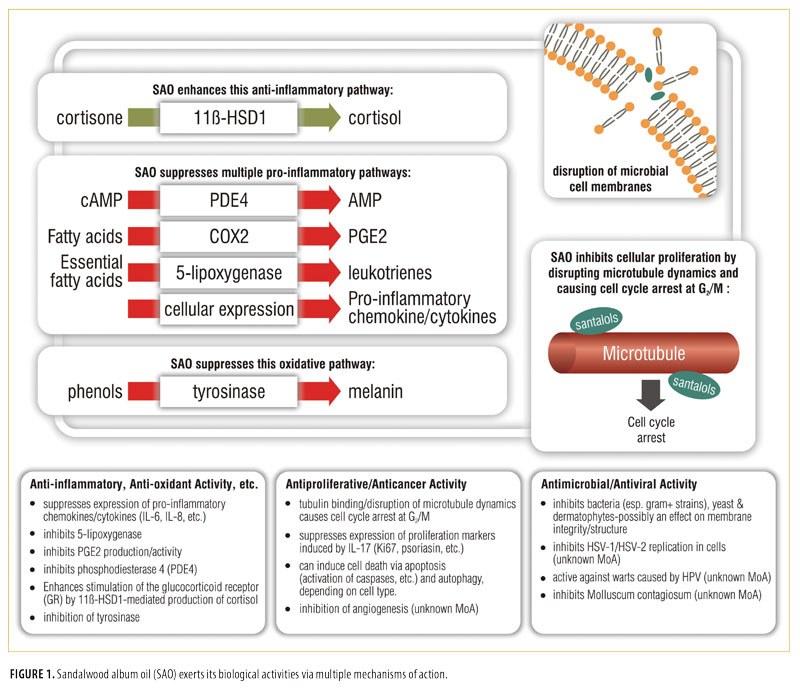
Data from initial proof-of-concept clinical studies in acne, warts, Molluscum contagiosum, atopic dermatitis, and psoriasis indicate that SAO is safe, well-tolerated, and has potential for broader use as a novel botanical therapeutic. SAO is now being produced in compliance with Current Good Manufacturing Practices (cGMPs) from sustainably grown cultivated trees and is available for use in commercial drug development programs. Larger, confirmatory clinical trials are underway and, if successful, might pave the way for the introduction of SAO-containing topical drugs for treatment of a variety of skin conditions.
References
- De Groot AC, Schmidt E. Sandalwood oil. In: De Groot AC, Schmidt E (eds). Essential Oils, Contact Allergy and Chemical Composition. Boca Raton, FL: CRC Press; 2016:751–764.
- Botanical drug development—guidance for industry. United States Food and Drug Administration site. Available at: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidanc es/ucm458484.pdf. Accessed May 12, 2017.
- Baylac S, Racine P. Inhibition of 5-lipoxygenase by essential oils and other natural fragrant extracts. Int J Aromatherapy. 2003;13(2-3):138–142.
- Inouye S, Takahashi M, Abe S. Composition, antifungal and radical scavenging activities of 15 rare essential oils. International Journal of Essential Oil Therapeutics. 2010;4:1–10.
- Kamal R, Yadav S, Mathur M, Katariya P. Antiradical efficiency of 20 selected medicinal plants. Nat Prod Res. 2012;26(11):1054–1062.
- Banerjee S, Ecavade A, Rao AR. Modulatory influence of sandalwood oil on mouse hepatic glutathione S-transferase activity and acid soluble sulphydryl level. Cancer Lett. 1993;68(2):105–109.
- Misra B, Satyahari D. Evaluation of in-vivo anti-hyperglycemic and antioxidant potentials of a-santalol and sandalwood oil. Phytomedicine. 2013;20(5):409–416.
- Sharma M, Levenson C, Bell RH, et al. Suppression of lipopolysaccharide-stimulated cytokine/chemokine production in skin cells by sandalwood oils and purified ?-santalol and ?-santalol. Phytother Res. 2014;28(6):925–932.
- Itoi-Ochi S, Matsumura S, Terao T, et al. Sandalwood downregulates skin inflammation through 11b-HSD1 activation in keratinocytes. J Dermatological Sci. 2016;84:e134.
- Golden JB, McCormick TS, Ward NL. IL-17 in psoriasis: implications for therapy and cardiovascular co-morbidities. Cytokine. 2013;6(2)2:195–201.
- Wasilewska A, Winiarska M, Olszewska M, Rudnicka L. Interleukin-17 inhibitors: a new era in treatment of psoriasis and other skin diseases. Postepy Dermatol Alergol. 2016;33(4):247–252.
- Page CP, Spina D. Phosphodiesterase inhibitors in the treatment of inflammatory diseases. In: Francis SH, Conti M, Houslay MD (eds). Phosphodiesterases as Drug Targets. Berlin: Springer-Verlag; 2011:391–414.
- Wittmann M, Helliwell PS. Phosphodiesterase 4 inhibition in the treatment of psoriasis, psoriatic arthritis and other chronic inflammatory diseases. Dermatol Ther (Heidelb).
2013;3(1):1–15. - Sharma M, Levenson C, Clements I, et al. East Indian sandalwood oil (EISO) alleviates inflammatory and proliferative pathologies of psoriasis. Front Pharmacol. 2017;8:125.
- Sharma M, Levenson C, Clements I, et al. Anti-inflammatory and anti-proliferative effects of East Indian sandalwood oil (EISO): mitigation of the effects of IL-17 on a psoriasis tissue model. Manuscript in preparation.
- Sharma M, Levenson C, Clements I, et al. East Indian sandalwood oil (EISO) is an inhibitor of phosphodiesterase 4 (PDE 4): a new therapeutic option in the treatment of inflammatory skin disease. Manuscript in preparation.
- Misra BB, Dey S. TLC-bioautographic evaluation of in-vitro anti-tyrosinase andanti-cholinesterase potentials of sandalwood oil. Nat Prod Commun. 2013;8(2):253–256.
- Chao S, Young G, Oberg C, Nakaoka K. Inhibition of methicillin-resistant Staphylococcus aureus (MRSA) by essential oils. Flavour Fragr J. 2008;23(6):444–449.
- Warnke PH, Becker ST, Podschun R, et al. The battle against multi-resistant strains: renaissance of antimicrobial essential oils as a promising force to fight hospital-acquired infections. J Craniomaxillofac Surg. 2009;37(7):392–397.
- Morris JA, Khettry A, Seitz EW. Antimicrobial activity of aroma chemicals and essential oils. J Am Oil Chem Soc. 1979;56(5):595–603.
- Chourasia OP, Nigam SS. Antibacterial activity of the essential oils of Santalum album & Glossogyne pinnatifidia. Indian Perfumer. 1978;3:205–206.
- Jirovetz L, Buchbauer G, Denkova Z, et al. Comparative study on the antimicrobial activities of different sandalwood essential oils of various origin. Flavour Fragr J. 2006;21(3):465–468.
- Misra BB, Dey S. Comparative phytochemical analysis and antibacterial efficacy of in vitro and in vivo extracts from East Indian sandalwood tree (Santalum album L.). Lett Appl Microbiol. 2012;55(6):476–486.
- Okazaki K, Oshima S. Antimicrobial effect of essential oils. Yakugaku Zasshi. 1953;7:344–347.
- Dikshit A, Husain A. Antifungal action of some essential oils against animal pathogens. Fitoterapia. 1984;55:171–176.
- Hammer KA, Carson CF, Riley TV. Antimicrobial activity of essential oils and other plant extracts. J Applied Microbiol. 1999;86(6):985–990.
- Hammer KA, Carson CF, Riley TV. In-vitro activity of essential oils, in particular Melaleuca alternifolia (tea tree) oil and tea tree oil products, against Candida spp. J Antimicrob Chemother. 1998;42(5):591–595.
- Inouye S, Uchida K, Abe S. Vaporactivity of 72 essential oils against a Trichophyton mentagrophytes. J Infect Chemother. 2006;12(4):210–216.
- Nardoni S, Giovanelli S, Pistelli L, et al. In vitro activity of twenty commercially available, plant-derived essential oils against selected dermatophyte species. Nat Prod Commun. 2015;10(8):1473–1478.
- Benencia F, Courrèges MC. Antiviral activity of sandalwood oil against herpes simplex viruses-1 and -2. Phytomedicine. 1999;6(2):119–123.
- Koch C, Reichling J, Schneele J, Schnitzler P. Inhibitory effect of essential oils against herpes simplex virus type 2. Phytomedicine. 2008;15(1-2):71–78.
- Paulpandi M, Kannan S, Thangam R, et al. In-vitro anti-viral effect of ?-santalol against influenza viral replication. Phytomedicine. 2012;19(3-4):231–235.
- Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils—a review. Food Chem Toxicol. 2008;46(2):446–475.
- Carson CF, Hammer KA, Riley TV. Melaleuca alternifolia (tea tree) oil: a review of antimicrobial and other medicinal properties. Clin Microbiol Rev. 2006;19(1):50–62.
- Sikkema J, de Bont JA, Poolman B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev. 1995;59:201–222.
- Santha S, Dwivedi C. Anticancer effects of sandalwood (Santalum album). Anticancer Res. 2015;35(6):3137–3145.
- Dozmorov MG, Yang Q, Wu W, et al. Differential effects of selective frankincense (Ru Xiang) essential oil versus non-selective sandalwood (Tan Xiang) essential oil on cultured bladder cancer cells: a microarray and bioinformatics study. Chin Med. 2014;9:18.
- Lee B, Bohmann J, Reeves T, et al. ?- and ?-santalols directly interact with tubulin and cause mitotic arrest and cytotoxicity in oral cancer cells. J Nat Prod. 2015;78(6):1357–1362.
- Saraswati S, Kanaujia PK, Agrawal SS. ?-Santalol demonstrates antitumor and antiantiangiogenic activities in models of hepatocellular carcinoma in vitro and in vivo. Abstracts of the 3rd ITLT Essen 2013 / Digestive and Liver Disease 45S (2013) S249.
- Saraswati S, Kumar S, Alhaider AA. ?-santalol inhibits the angiogenesis and growth of human prostate tumor growth by targeting vascular endothelial growth factor receptor 2- mediated AKT/mTOR/P70S6K signaling pathway. Mol Cancer. 2013;12:147.
- Dickinson SE, Olson ER, Levenson C, et al. A novel chemopreventive mechanism for a traditional medicine: East Indian sandalwood oil induces autophagy and cell death in proliferating keratinocytes. Arch Biochem Biophys. 2014;558:143–152.
- Ortiz C, Morales L, Sastre M, et al. Cytotoxicity and genotoxicity assessment of sandalwood essential oil in human breast cell lines MCF-7 and MCF-10A. Evid Based Complement Alternat Med. 2016;3696232.
- Matsumura S, Itoi-Ochi S, Terao M, et al. Sandalwood oil enhanced epithelial-mesenchymal transition and promoted wound healing. J Dermatological Sci. 2016;84:e137.
- Busse D, Kudella P, Grüning NM, et al. A synthetic sandalwood odorant induces wound- healing processes in human keratinocytes via the olfactory receptor OR2AT4. J Invest Dermatol. 2014;134(11):2823–2832.
- Denda M. Newly discovered olfactory receptors in epidermal keratinocytes are associated with proliferation, migration, and re-epithelialization of keratinocytes. J Invest Dermatol. 2014;134(11):2677–2679.
- Opdyke D. Reviews on fragrance raw materials. Sandalwood oil, East Indian. Food and Cosmetics Toxicology. 1974;12(Supp):989–990.
- Ishizaki M, Ueno S, Oyamada N, et al. The DNA-damaging activity of natural food additives (III). J Food Hygiene Society, Japan 1985;26:23–527.
- Burdock GA, Carabin IG. Safety assessment of sandalwood oil (Santalum album L.). Food Chem Toxicol. 2008;46(2):421–432.
- Tisserand R, Young R. Essential oil profiles. In: Tisserand R, Young R (eds). Essential Oil Safety. 2nd ed. Edinburgh, UK: Churchhill Livingstone Elsevier; 2014:418–419.
- Zundel JL. PhD Thesis. Université Louis Pasteur, Strasbourg France (1976).
- Haque, MH, Haque AU. Compositions for the prevention and treatment of warts, skin blemishes and other viral-induced tumors. US Patent. 1999;5,945,116.
- Haque, MH, Haque AU. Use of alpha- and beta-santalols, major constituents of sandalwood oil, in the treatment of warts, skin blemishes and other viral-induced tumors. US Patent. 2002;6,406,706.
- Haque, MH, Haque AU. Use of sandalwood oil for the prevention and treatment of warts, skin blemishes and other viral-induced tumors. US Patent. 2000;6,132,756.
- Clinicaltrials.gov. Searched for “East Indian sandalwood oil.” Available at: https://clinicaltrials.gov/ct2/results term=east+indian+sandalwood+oil &Search=Search. Accessed May 12, 2017.
- Palatty PL, Azmidah A, Rao S, et al. Topical application of a sandalwood oil and turmeric based cream prevents radiodermatitis in head and neck cancer patients undergoing external beam radiotherapy: a pilot study. Br J Radiol. 2014;87(1038):20130490.
- Moy RL, Levenson C, So JJ, Rock JA. Single-center, open-label study of a proprietary topical 0.5% salicylic acid-based treatment regimen containing sandalwood oil in adolescents and adults with mild to moderate acne. J Drugs Dermatol. 2012;11(12):1403–1408.
- Browning JC, Rock J, Levenson C, Becker EM. Safety, tolerability and efficacy of a novel regimen containing 0.1% colloidal oatmeal and East Indian sandalwood oil (EISO) for the treatment of mild, moderate and severe pediatric eczema (atopic dermatitis) – results of a single-center, open-label study. Presented as a poster at the Orlando Derm and Clinical Aesthetic Conference. Doral, FL. 13–16 January 2017.
- Browning JC, Rock J, Levenson C, Becker EM. Open-label marketing trials to evaluate an over-the-counter (OTC) 17% salicylic acid regimen containing highly purified sandalwood oil for the treatment of common warts (Verruca vulgaris) in pediatrics. Presented as a poster at the Orlando Derm and Clinical Aesthetic Conference. Doral, FL. 13–16 January 2017.
- Reuter J, Merfort I, Schempp CM. Botanicals in dermatology: an evidence-based review. Am J Clin Dermatol. 2010;11(4):247–267.
- Thandar Y, Gray A, Botha J, Mosam A. Topical herbal medicines for atopic eczema: a systematic review of randomized controlled trials. Br J Dermatol. 2017;176(2):330–343.
- Shenefelt PD. Herbal Treatment for Dermatologic Disorders. In: Benzie IFF, Wachtel-Galor S, eds. In: Herbal Medicine: Biomolecular and Clinical Aspects. 2nd edition. Boca Raton, FL: CRC Press/Taylor & Francis; 2011.
- Fisk WA, Lev-Tov HA, Clark AK, Sivamani RK. Phytochemical and botanical therapies for rosacea: a systematic review. Phytother Res. 2015;29(10):1439–1451.
- Herman A, Herman AP. Topically used herbal products for the treatment of psoriasis: mechanism of action, drug delivery, clinical studies. Planta Med. 2016. [Epub ahead of print].
- RM Aldens Labs, Culver City California; unpublished results, 2010.
- University of Texas Health Science Center at San Antonio Fungal Testing Laboratory, San Antonio Texas; unpublished results, 2010.

