 J Clin Aesthet Dermatol. 2022;15(5):47–58.
J Clin Aesthet Dermatol. 2022;15(5):47–58.
by Lauren Hoffman, MD and Sabrina Fabi, MD
Dr. Hoffman is with the Albert Einstein College of Medicine and Montefiore Medical Center, Department of Medicine and the Division of Dermatology in Bronx, New York. Dr. Fabi is with the University of California, San Diego and Cosmetic Laser Dermatology in San Diego, California.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. Patients pursue cosmetic procedures to improve physical appearance as well as self-esteem and confidence, which translates into better quality of life. As aesthetic providers, it is important to understand the improvement in quality of life that can be achieved from various aesthetic procedures, best measured by patient reported outcomes (PROs), such as the validated FACE-Q.
Objective. This review summarizes FACE-Q outcomes after nonsurgical dermatological facial cosmetic procedures.
Methods: A review of relevant clinical terms was performed on the PUBMED database. All abstracts were reviewed; articles were included based on relevancy; bibliographies of selected articles were reviewed; and supplemental articles were added accordingly.
Results: The current literature has 31 articles using the FACE-Q to measure quality of life, focusing on satisfaction with appearance, psychological wellbeing, social wellbeing, age appraisal, and recovery early life impact (i.e. disruption of the procedure on daily life activities) after minimally invasive aesthetic facial procedures. Clinical studies focused on treatment with dermal fillers for mid-face rejuvenation, chin/lower-face enhancement, lip enhancement, botulinum toxin-A for glabellar lines, combined filler and botulinum toxin-A, and other. Nearly all FACE-Q domains improved following minimally invasive cosmetic procedures. Absolute changes in FACE-Q psychological wellbeing and age appraisal were greatest with combined treatments compared to single treatments.
Limitations. Limitations included varying follow-up times, lack of control groups, and publication bias for positive findings.
Conclusion. To maximize patient satisfaction and retention, providers should consider a combined treatment approach, to improve patient psychological wellbeing and age appraisal, and ultimately quality of life.
Keywords: Aesthetic dermatology, cosmetic dermatology, minimally invasive, FACE-Q, quality of life, patient reported outcomes, psychosocial impact
The demand for minimally invasive facial aesthetic procedures is dramatically rising. In 2020, despite the COVID-19 pandemic, numbers of minimally invasive cosmetic procedures were still very high. The top five minimally invasive cosmetic procedures were botulinum toxin type A (n= 4.4 million), soft tissue fillers (n=3.4 million), laser skin resurfacing (n=997,245), chemical peels (n= 931,473), and intense pulsed light (n=827,409).1
Several factors contribute to this rising trend; patients are not only looking to improve their physical appearance, but also the desire to improve their mental and emotional health along with social wellbeing. In an interview study on patient reported motivations of patients considering cosmetic dermatologic procedures, concerns about mental and emotional health were mentioned more times than any of the other identified themes, citing the appeal for improved confidence, feeling depressed and anxious with current appearance, and feeling consumed by efforts to conceal undesirable physical features as motivations to pursue cosmetic procedures.2 Furthermore, Maisel et al3, found that increasing self-confidence was the top motivation for patients seeking a variety of cosmetic procedures including injectables, laser treatment, and liposuction or noninvasive fat reduction.
Despite several studies confirming that patients seek cosmetic procedures for improved psychological and emotional wellbeing, there is little direct evidence showing exactly how much cosmetic treatments improve these aspects. To adequately assess if these patient desires have been met, it is essential to measure patient satisfaction with tools such as patient-reported outcome (PRO) measures. Several PROs have been developed to measure a range of outcomes (i.e., symptoms, satisfaction with appearance, satisfaction with procedure, and quality of life (QoL)) in which responses are collected directly from the patient. Results from PROs that assess patient satisfaction with outcome and quality of life following the aesthetic procedure can be used to help compare techniques or quantify positive effects of treatment on social and psychological wellbeing. Providers can also use these results to analyze and provide tangible feedback on the entire patient treatment experience.
The FACE-Q is a multimodule, validated patient reported outcome instrument that consists of more than 40 independently functioning scales. It was developed to measure relevant themes and symptoms for patients undergoing elective aesthetic procedures involving the face.4 The scales are distributed to facial aesthetic patients to measure their perspective on appearance, patient experience, adverse effects, and quality of life. Raw scores are further analyzed to provide a score from 0 (worst) to 100 (best). Separate scales have been developed for different parts of the face (i.e. forehead, nose, cheek, chin, lips, and eyes) and providers can choose to administer scales that are relevant to a patient or procedure.5-9 FACE-Q scales that address quality of life ask about psychological wellbeing, social wellbeing, age appraisal, expectations and motivations, psychological distress, and recovery early life impact (i.e. disruption of the procedure on daily life activities).10 The purpose of this article is to review FACE-Q scores from clinical trials of minimally invasive (non-surgical) aesthetic facial procedures to determine the impact of treatment on satisfaction with appearance, psychological wellbeing, age appraisal, and satisfaction with outcome measures, to gain further insight on how much looking aesthetically better translates to feeling good and living better.
Methods
The PubMed database was searched on July 30, 2021, using the following Boolean search terms: (“FACE-Q”) AND (“cosmetic dermatology” OR “aesthetic dermatology”) with limits to English language articles and human subjects. Bibliographies of selected articles were also reviewed, and supplemental articles were added accordingly. Original research studies and case series were included. Inclusion criteria included prospective studies using the FACE-Q instrument to directly evaluate the impact of non-surgical facial aesthetic procedural intervention. Exclusion criteria included studies not utilizing the FACE-Q instrument, studies reporting on surgical facial cosmetic procedures only, anecdotal reports, studies of skin cancer, studies about cosmetic correction of medical problems (i.e., scleroderma), studies with sample sizes less than 10 subjects, and conference abstracts without full-text publications. Because of the study design heterogeneity, the authors were unable to complete a quantitative review and thus present their findings qualitatively.
Results
The search yielded 46 articles, of which 31 articles, describing 27 different patient populations, used FACE-Q to measure patient reported outcomes after minimally invasive aesthetic facial procedures. Following validation of the FACE-Q scales, Klassen and colleagues pooled a sample of almost 1,000 facial aesthetic patients and found that single treatment with neuromodulators, facial filler, and other minimally invasive skin treatments resulted in higher post-procedure Satisfaction with Appearance.7 Specific products and treatment volumes were not stated.7 The remaining articles were subdivided into the following categories based on type of treatment received: facial filler (upper, mid, and lower face), lip filler, botulinum toxin-A, combined treatment (facial filler and botulinum toxin-A), and other.
FACE-Q Scores with Minimally Invasive Aesthetic Facial Procedures. Nasolabial folds.
Four studies examined FACE-Q after corrective treatment of the nasolabial folds, mid-face, and skin quality (Table 1). These four studies utilized the following FACE-Q scales: Satisfaction with Skin; Satisfaction with Nasolabial fold; and Satisfaction with Outcome. None of the four studies utilized Age Appraisal or Psychological Wellbeing scales.
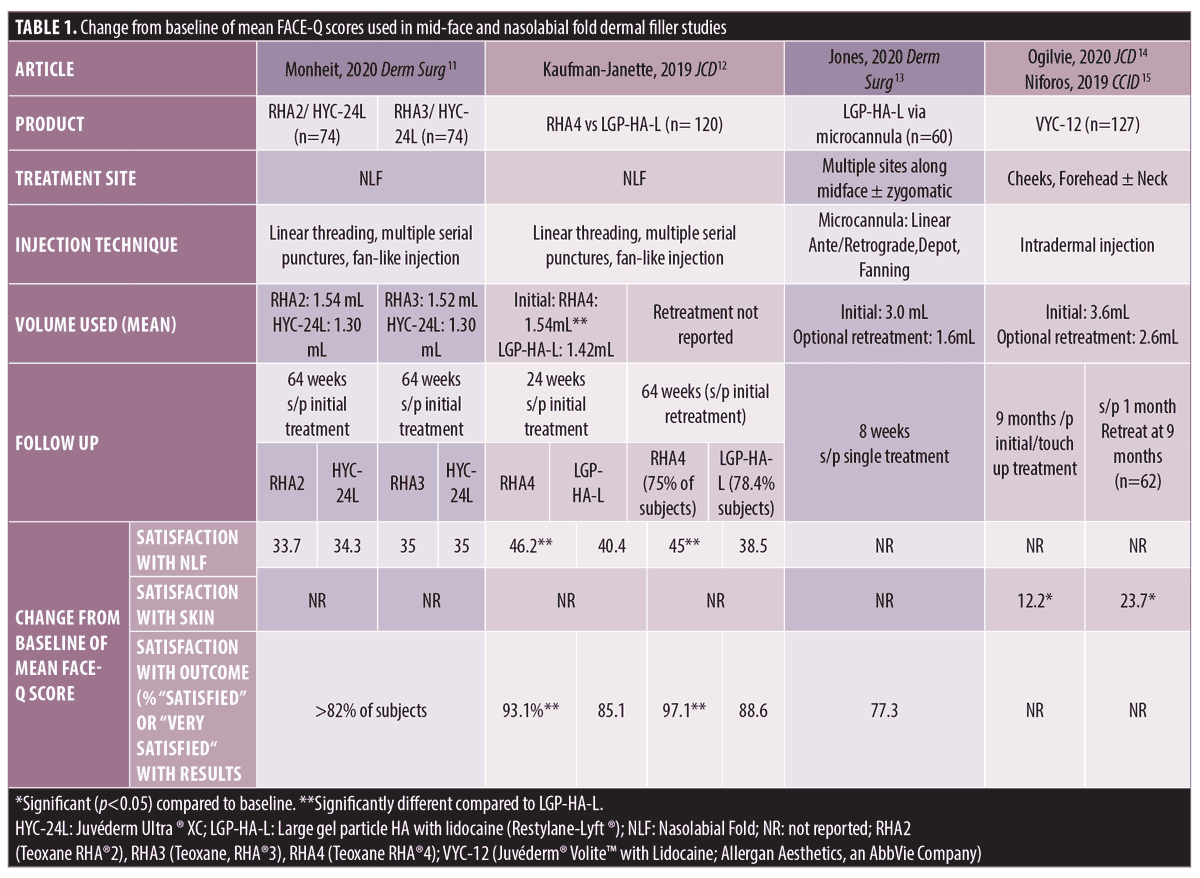
In one split-face study comparing the use of the resilient hyaluronic acid (RHA) range of fillers to a hylacross hyaluronic acid for correction of NLFs in diverse skin-phototypes, 174 subjects with moderate-to-severe bilateral NLFs were randomized to Group 1) RHA2 (Teoxane RHA®2; TEOXANE Laboratories; Geneva, Switzerland) versus HYC-24L (Juvéderm Ultra ® XC, Allergan Aesthetics, an AbbVie Company), Group 2) RHA3 (Teoxane, RHA®3; TEOXANE Laboratories; Geneva, Switzerland) vs HYC-24L, or Group 3) untreated control group.11 Subjects in Group 1 received mean treatment (initial + touch up) volumes of 1.54mL RHA2 and 1.30 mL HYC-24L and subjects in Group 2 received mean treatment (initial + touch up) volumes of 1.52 mL RHA3 and 1.30 mL HYC-24L.11 Subjects received treatment at baseline with optional touch-up at Week 2 and optional retreatment was offered starting at Week 24, 36, 52 or 64 if NLF scores went back to pretreatment levels or if Wrinkle Severity Rating Scale (WSRS) was graded greater than or equal to two.11 Hemifaces were retreated in equal proportions at each possible time point.11 After 64 weeks from initial treatment, both treatment groups experienced > 2-fold improvement in NLF specific FACE-Q scores; however, there was no difference in FACE-Q scores between filler types.11 More than 82 percent of subjects rated their Satisfaction with Outcome as either “satisfied” or “very satisfied”.11
Another split face trial evaluated RHA4 (Teoxane RHA®4; TEOXANE Laboratories; Geneva, Switzerland) vs a large gel particle hyaluronic acid dermal filler with lidocaine (LGP-HA-L; Restylane® Lyft, Galderma SA, Lausanne, Switzerland).12 Initially, subjects received higher mean initial volumes of RHA4 (1.54 mL) compared to LGP-HA-L (1.42mL); however, at the 2 week follow up visit a significantly lower proportion of hemifaces treated with RHA4 received a touch up compared to the LGP-HA-L group (31.8% vs. 43.2%, respectively).12 Mean volumes used for touch-up treatments were not different between products used on hemifaces.12 Optional retreatment was offered at Weeks 24, 36, 52, or 64 if NLF went back to pretreatment scores or if WSRS was graded greater than or equal to two.12 A similar percentage of hemifaces randomized to RHA4 and LGP-HA-L were retreated (75% and 78.4%, respectively; volumes not listed).12 Overall, the RHA4 group showed consistently higher satisfaction of FACE-Q NLF scores than with LGP-HA-L, with significant differences at all time points starting at Week 2 after initial treatment until 64 weeks with optional touch up and/or retreatment as outlined above.12
Midface. Jones and colleagues found that after single treatment with a LGP-HA-L (Restylane® Lyft, Galderma Laboratories, Fort Worth, Texas) via microcannula into the midface using an initial treatment volume of 3.0 mL, mean Satisfaction with Outcome score at Week 8 was 77.3.13 At Week 8 after a single treatment, more than 91.5 percent of subjects agreed with statements such as “I am pleased with results”, “The results turned out great’, “The result was just as I expected”, and “The result is fantastic”, indicating the subjects were generally pleased with their aesthetic results.13 Retreatment was offered at 16 weeks from baseline treatment, but FACE-Q scores were only measured at Week 8 after initial treatment.
Skin Quality. In one study examining skin quality after treatment with VYC-12 (Juvéderm ®Volite with Lidocaine; Allergan Aesthetics, an AbbVie Company) to cheeks, forehead, and neck, authors found improved mean Satisfaction with Skin scores starting at one month after a single treatment.14 The median initial injection volume was 3.6 mL, 24% (31/131) received an optional touch up treatment at 30 days (median volume 1.0 mL), and 47% (62/131) received an optional retreatment at nine months (median volume 2.6 mL).15 Improvement with Satisfaction with Skin scores lasted up to nine months following initial/touch -up treatment.14 The FACE-Q Satisfaction with Skin scale assessed skin quality, with questions asking about radiance, hydration, smoothness, health of skin, how refreshed skin looks, how clear skin looks, pores, even color, tone, attractiveness, appearance of skin at first wake up and at the end of day. Subject outcome satisfaction with VYC-12 was rated as high as 90.8% at Month 1 after initial treatment, fell to 76.4% by Month 9 after last treatment, but increased to about 91.9 percent one month after repeat treatment.14
Chin/ Lower face. Three studies measured FACE-Q after treatment of volume deficit in the lower face and chin (Table 2). FACE-Q scales used were Satisfaction with Outcome, Satisfaction with Chin augmentation, Satisfaction with Lower Face/Jawline, and Psychological Wellbeing. None of the three studies utilized Age Appraisal scales.
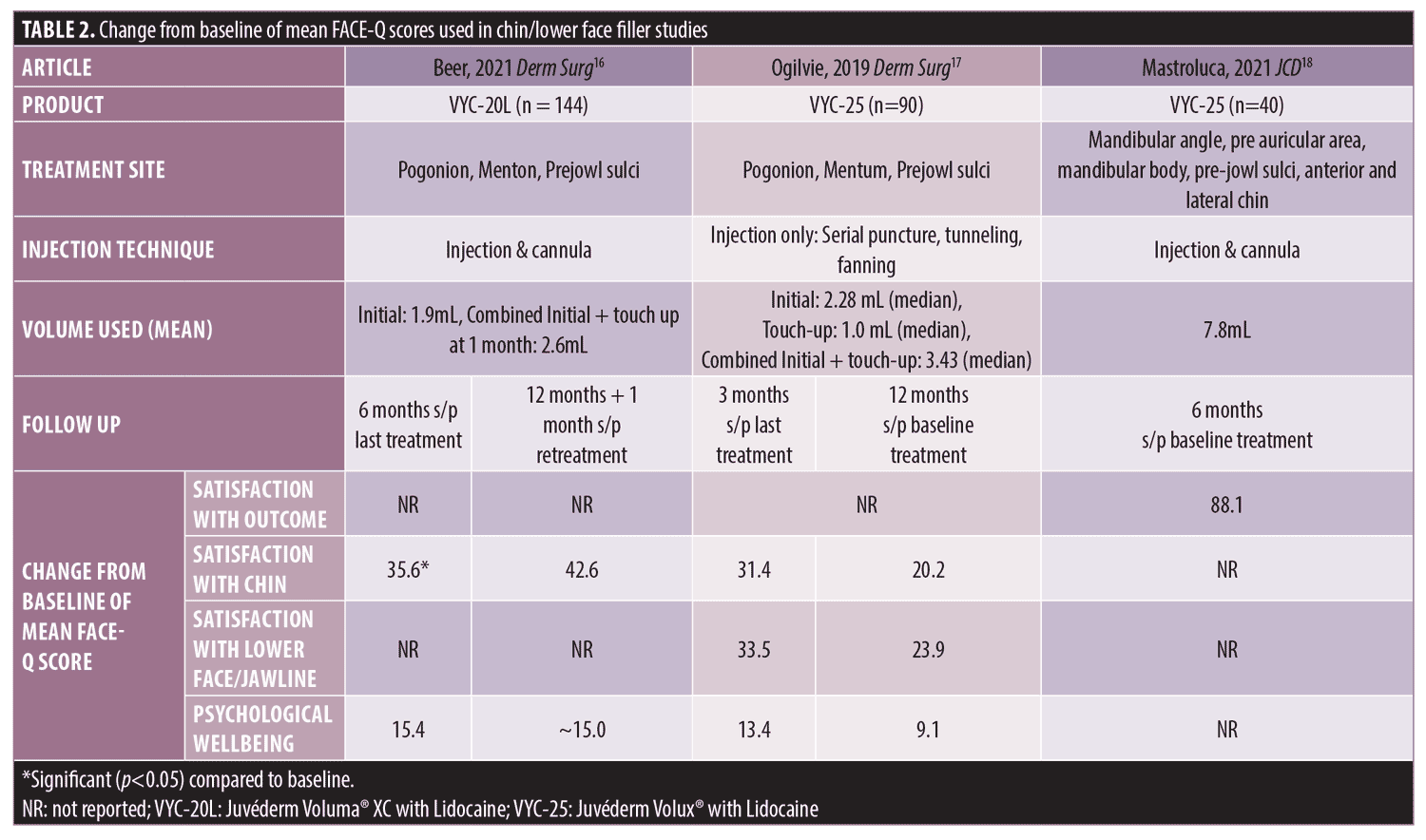
In a study using VYC-20L (Juvéderm Voluma® XC with Lidocaine; Allergan Aesthetics, an AbbVie Company), 56.3 percent of treated subjects showed at least a one point improvement in the Allergan Chin Retrusion Scale (ACRS) at six months after initial plus optional touch up treatment.16 The mean initial injection volume was 1.9 mL; 61.1% (88/144) of participants received a touch up treatment at 30 days (mean initial + touch up volume 2.6 mL) and 51.4% (74/144) received retreatment at Month 12 after last visit (mean injection volume 1.4 mL).16 Psychological Wellbeing and Satisfaction with Chin augmentation showed improvements from baseline starting at one month after initial/touch-up treatment and lasting up to 12 months after last treatment. Improvements in Satisfaction with Chin augmentation were only significant at six months. There was a trend in improvement in psychological wellbeing , but it was not statistically significant at any time point.16 One month after repeat treatment at one year, Satisfaction with Chin and Psychological Wellbeing remained higher than baseline, however significance compared to baseline or other time points were not reported. No metrics were obtained to capture a correlation with improvement in PROs to ACRS improvement.
Treatment of the chin and jawline with VYC-25L (Juvéderm Volux® with Lidocaine; Allergan Aesthetics, an AbbVie Company) showed improved Satisfaction with Chin augmentation, Satisfaction with Face and Jawline, and Psychological Wellbeing scores at three months after the last treatment and persisting up to 12 months after the last treatment.17 The median initial volume injected was 2.28mL and 77% (69/90) of subjects received a touch up at 30 days (median volume of 1.0 mL).17 Treated subjects had a significantly greater mean change in the glabella-subnasale-pogonion facial angle at three months compared to baseline indicating an improvement in chin retrusion; however, no correlation or association metrics were done compared to improvement in facial angle with FACE-Q scores.17
Another study using VYC-25 focused on lower face rejuvenation (both chin and jawline) in a cohort of men, particularly tailoring the consultation to help male patients overcome barriers to proceeding with treatment. Much higher injection volumes were used compared to the previous two studies (mean 7.8 mL; range 5-10 mL), resulting in a mean Satisfaction with Outcome scores of 88.1 at 18-22 months post- initial treatment, indicating that patients were satisfied with their treatment.18
Lip and Perioral Filler. Four studies examined improvement in FACE-Q questionnaires after treatment with lip filler (Table 3). The FACE-Q scales used were Satisfaction with Lips, Lip Line Appraisal Scales, Recovery Early Life and Recovery Early Symptoms.
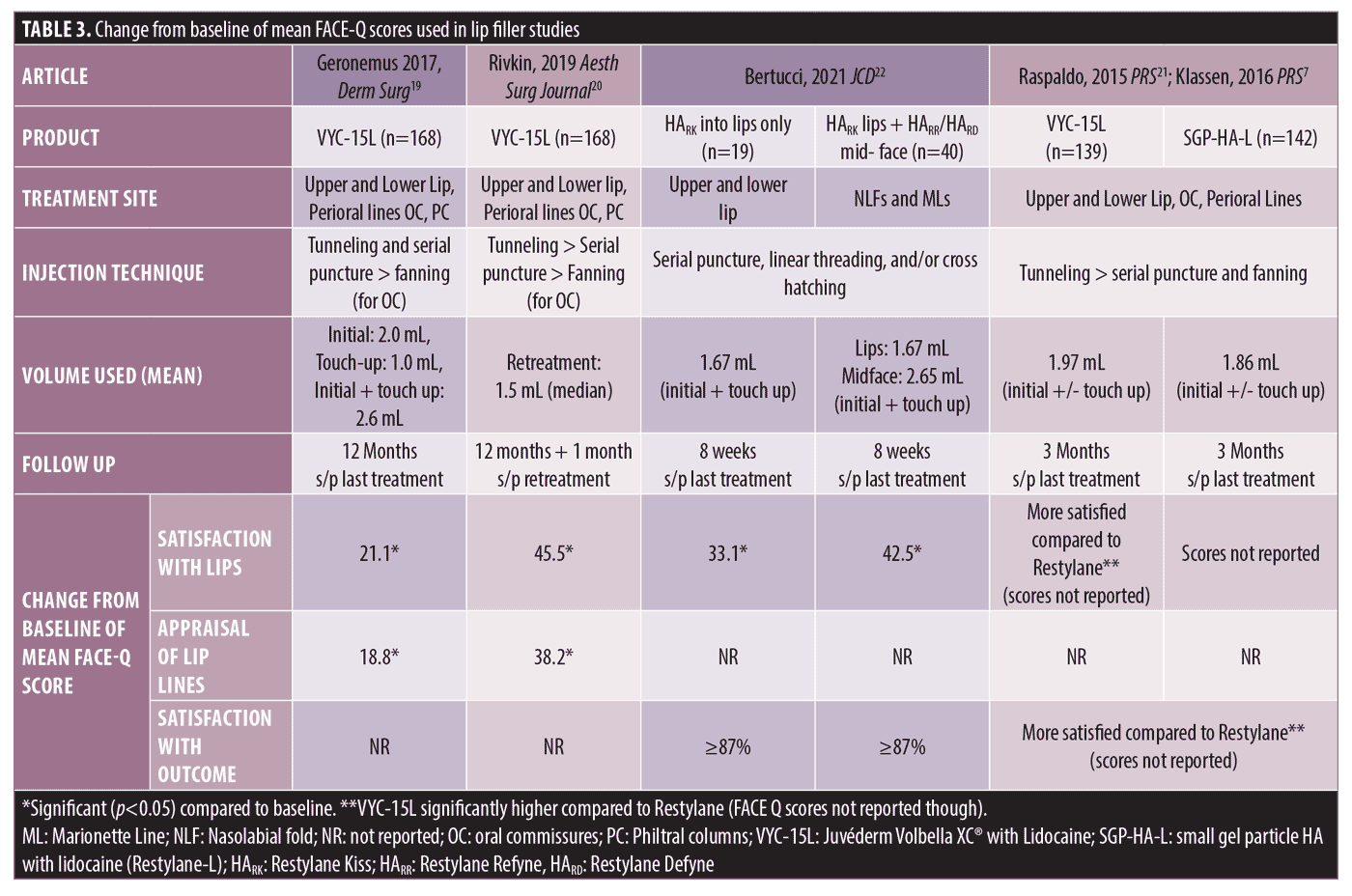
In a comparison study of VYC-15L (Juvéderm Volbella XC® with Lidocaine; Allergan Aesthetics, an AbbVie Company) to a small gel particle HA with lidocaine (SGP-HA-L; Restylane-L, Medicis, Inc., Scottsdale, Arizona) for lip and perioral enhancement, it was found that VYC-15L had higher Recovery Early Life Impact Scores at Day 3 after initial treatment compared to SGP-HA-L, indicating less disruption after treatment to return to daily activities.19 FACE-Q Satisfaction with Lips and Satisfaction with Lip Line Scores were only reported in the VYC-15L treatment group. The mean initial injection volume was 2.0 mL and 62 percent (104/168) of participants received a touch up treatment at 30 days (median volume 1.0 mL).19 Mean Satisfaction with Lip Scores and Satisfaction with Lip Lines were significantly improved from baseline starting at Month 1 following last treatment and remained high at the one year follow up from the last treatment.19
Another study evaluated the effectiveness of single repeat treatment with VYC-15L at 1 year after initial treatment for lip and perioral enhancement. Mean combined initial and touch-up volume was 2.6 mL and 73.8 percent of subjects received repeat treatment at one year with median volume of 1.5 mL injected.20 Improvement in Satisfaction with Lips and Appraisal of Lip Lines scores compared to baseline were significant at one month after initial/touch up treatment and scores remained significantly higher than baseline after last treatment through one year.20 One month after single repeat treatment at one year, Satisfaction with Lips and Appraisal of Lip Lines scores increased again to values similar to those observed at one month after initial/touch up treatment.20
Raspaldo and colleagues found similar results; patients treated with VYC-15L (mean injection volume 1.97 mL for combined initial and optional two week touch up) had improved Satisfaction with Lips and Satisfaction with Outcome at three months after last treatment compared to a SGP-HA-L (mean injection volume 1.86 mL for combined initial plus optional two week touch up).21 There was no difference in injection volume of upper lip, lower lip, and oral commissure between the two products, but there was a significantly greater volume injected into perioral lines with VYC-15L (0.38 mL) compared to SGP-HA-L (0.29 mL).21 At two weeks from the baseline treatment, subjects treated with VYC-15L reported significant improvement in individual Satisfaction with Lip scores including lip fullness, natural look of lips, softness, attractiveness of lip, and overall look of lips.7 Recovery Early Life and Recovery Early Symptoms scores were higher on the first day after initial treatment with VYC-15L compared to SGP-HA-L indicating less disruption to normal daily activities with VYC-15L.21
One study using HA products with XpresHAn Technology™ compared Satisfaction with Lips with combined lip and mid-face rejuvenation compared to lip enhancement alone. HARK (Restylane Kiss; Medicis, Inc., Scottsdale, Arizona) was used for lip enhancement and either HARR (Restylane Refyne; Medicis, Inc., Scottsdale, Arizona) or HARD (Restylane Defyne; Medicis, Inc., Scottsdale, Arizona) was injected into NLF and marionette lines for mid-face rejuvenation.22 Subjects were treated at baseline and offered optional touch-up treatment after four weeks. At eight weeks after last treatment, Satisfaction with Lips overall was significantly improved in both groups compared to baseline, but no difference was found when two areas were treated instead of one.22 Additionally, at Week 8 after last treatment, more than 87 percent of all subjects from both treatment groups reported satisfaction in individual Satisfaction with Lip questions, specifically with high satisfaction ratings for shape of lip, lip fullness, lip style, lip size, and satisfaction with smiling.22 No other FACE-Q questionnaires were evaluated in this trial.
All studies found that treatment with lip filler improved Satisfaction with Lips from baseline. The greatest change from baseline in Satisfaction with Lip score was seen with VYC-15L at one month after single retreatment at one year (change in mean FACE-Q score was 45.5) compared to change in satisfaction one year after initial/touch-up treatment (change in mean FACE-Q score was 21.1).19, 20 Slightly lower volumes were used for retreatment (1.5mL) compared to initial treatment (2.0 mL).19 Treatment of two areas (lips with HARK and NLF and marionette lines with HARR or HARD) also showed greater change in satisfaction with lips scores at eight weeks after last treatment (change in mean FACE-Q score was 42.5) as compared to treatment with lips alone (change in mean FACE-Q score was 33.1), but the difference was not significant.22 Studies using VYC-15L included more perioral treatment sites (upper and lower up, perioral lines, oral commissures and philtral columns) as compared to HARK which reported upper and lower lip alone.19-22 Injection technique did not differ between filler types, however, tunneling was reported to be more common in studies using VYC-15L.
Botulinum Toxin Type A. Four studies assessed FACE-Q for neuromodulator treatment of glabellar lines (Table 4). FACE-Q scales used were Satisfaction with Appearance, Psychological Functioning, Appraisal of Lines, Age Appraisal, and Satisfaction with Age.
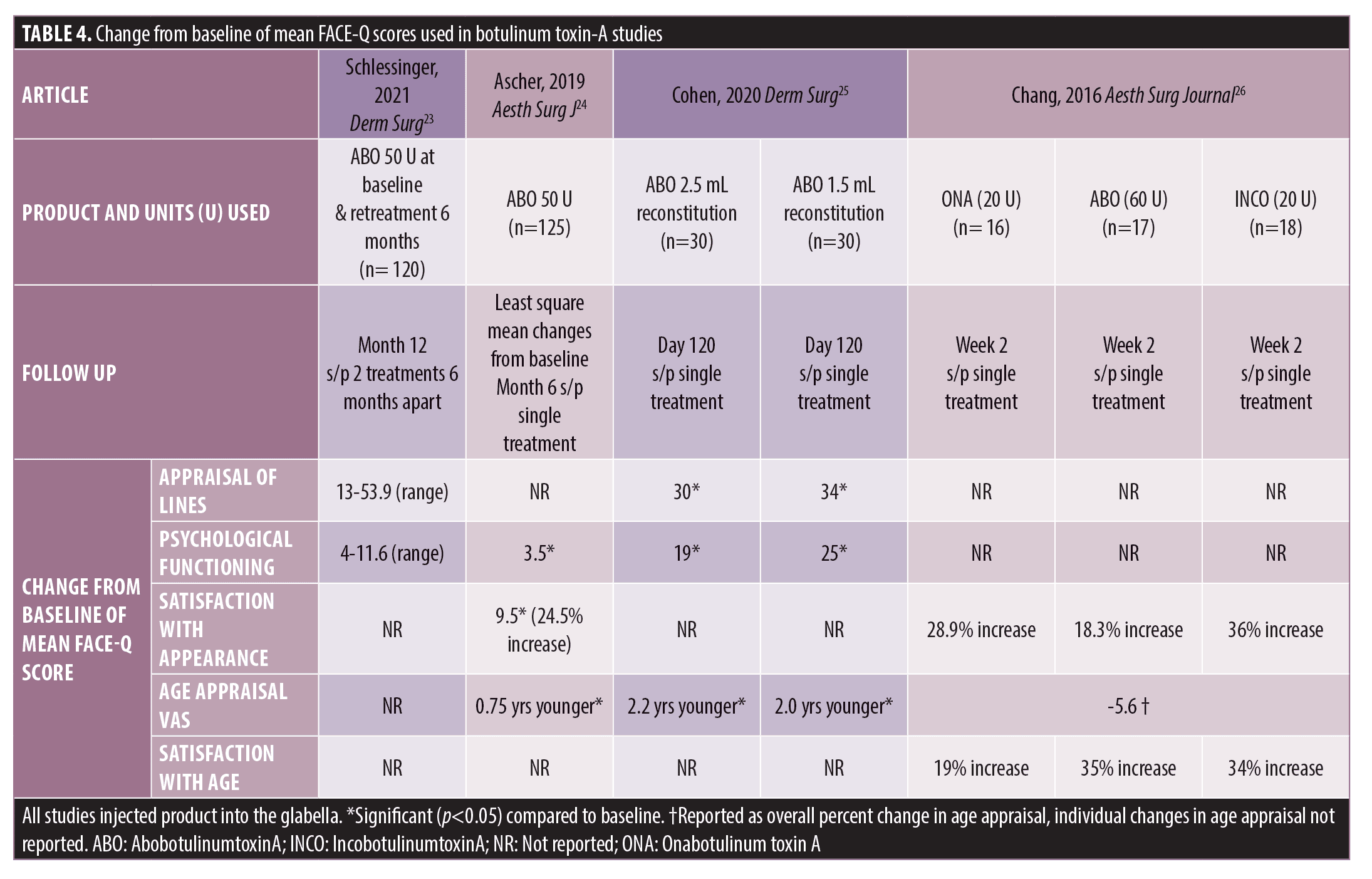
One study assessed patient satisfaction following 50 units of AbobotulinumtoxinA (ABO) (Dysport® ; Galderma)injected into glabellar lines at baseline and with repeat treatment at six months.23 The majority of subjects (95%) rated their Satisfaction with Outcome as “highly satisfied” or “satisfied” at one month after initial treatment, with similar satisfaction scores lasting until six months after initial treatment.23 After retreatment with 50 U at six months, again, the majority (96%) of subjects were “highly satisfied” or “satisfied” at one month follow up and at six months after repeat treatment, 85 percent were still “highly satisfied” or “satisfied” with their treatment.23 Increased mean scores in Psychological Function and Appraisal of Lines at 12 months from baseline suggested that subjects were less bothered by glabellar lines and felt better about their appearance.23
Ascher and colleagues showed that patients had significant improvements in Satisfaction with Aging, Facial Appearance and Psychological Wellbeing for up to six months after single treatment with 50 units of a liquid formulation ABO.24 Within the Satisfaction with Facial Appearance domain, there were significant improvements up to Day 113 after single treatment for the following individual items assessed: “how balanced your face looks”, “how fresh your face looks”, and “how your face looks at the end of the day”.24 Similarly, in assessment of Psychological Wellbeing, the individual items “I feel okay about myself” and “I feel attractive” were significantly improved at each study visit after an initial visit until Day 183, and the items “I feel great about myself” and “I like myself” showed improvement at six or more non-sequential follow up visits, with notable significant differences compared to placebo at Day 183.24 Improvement in subject Satisfaction with Facial Appearance and Psychological Wellbeing correlated with improvements in investigator assessments of glabellar lines at maximum frown.24
In a four months comparison trial of subject satisfaction with a single treatment of 1.5mL vs 2.5 mL reconstituted ABO, overall scores of Appraisal of Lines between eyebrows, Psychological Wellbeing, and Age Appraisal Visual Analogue Scale (VAS) showed significant improvement at Day 30 post single injection and was sustained through Day 120, with no differences between treatment groups, despite a decrease in investigator efficacy assessments of glabellar line maximal frown in both treatment groups by Day 120.25
One study compared Satisfaction with Facial Appearance after receiving single treatment of either 20 units of OnabotulinumtoxinA (ONA) (Botox Cosmetic®; Allergan Aesthetics, an AbbVie Company), 20 units of IncobotulinumtoxinA (INCO) (Xeomin®; Merz Aesthetics), or 60 units ABO.26 At two weeks after initial injection, total patient Satisfaction with Facial Appearance demonstrated a significant increase of 28 percent compared to baseline, with higher individual treatment satisfaction ratings, although not significant, with INCO, followed by ONA, then ABO.26 Furthermore, all treatment groups showed statistically significant improvement in all individual questions of Satisfaction with Facial Appearance including symmetry, balance, proportion, appearance at the end of the day, freshness, restfulness, profile appearance, appearance in photos, and appearance under bright lights.26 Improved patient satisfaction scores showed an inverse relationship with degree of glabellar strain.26
All prospective neuromodulator studies that used the FACE-Q instrument to directly evaluate the impact of non-surgical facial aesthetic showed improved Age Appraisal and either improved Satisfaction with Appearance or Satisfaction with Facial Lines after neuromodulator treatment. The highest reported change in age appraisal was improvement (i.e. younger than stated age) by 5.6 years in subjects treated with a single treatment of either ONA, ABO, or INCO, however, age appraisal scores broken down by treatment were not provided.25 In three studies evaluating treatment with ABO, the range of improvement in age appraisal was 0.75-2.2 years younger; with the highest improvement seen in ABO with 2.5mL reconstitution (2.2 years younger) at four months after single treatment.24-26 Satisfaction with Appearance had the highest improvement with INCO (36% increase), followed by treatment with ONA (28.9% increase), and finally ABO (range 18.3-24.5% increase).24, 26 Psychological wellbeing was only reported in studies evaluating treatment with ABO. The highest improvements in psychological wellbeing were seen with single treatments of ABO 1.5 and ABO 2.5mL reconstitution at four months, followed by treatment with ABO at one year follow up after two treatment sessions six months apart, and finally lowest scores seen with a single treatment of a liquid formulation of ABO at six-month follow up.23-25
Combination Neuromodulator/Filler Treatment for Panfacial Rejuvenation. Seven studies assessed FACE-Q after combination treatment with modalities including botulinum toxin type A, soft-tissue filler, and laser (Table 5). The FACE-Q scales used included Satisfaction with Facial Appearance, Psychological Wellbeing, Age Appearance Appraisal and Age Appraisal VAS, Social Confidence, and Social Functioning, and site-specific appraisal scales.
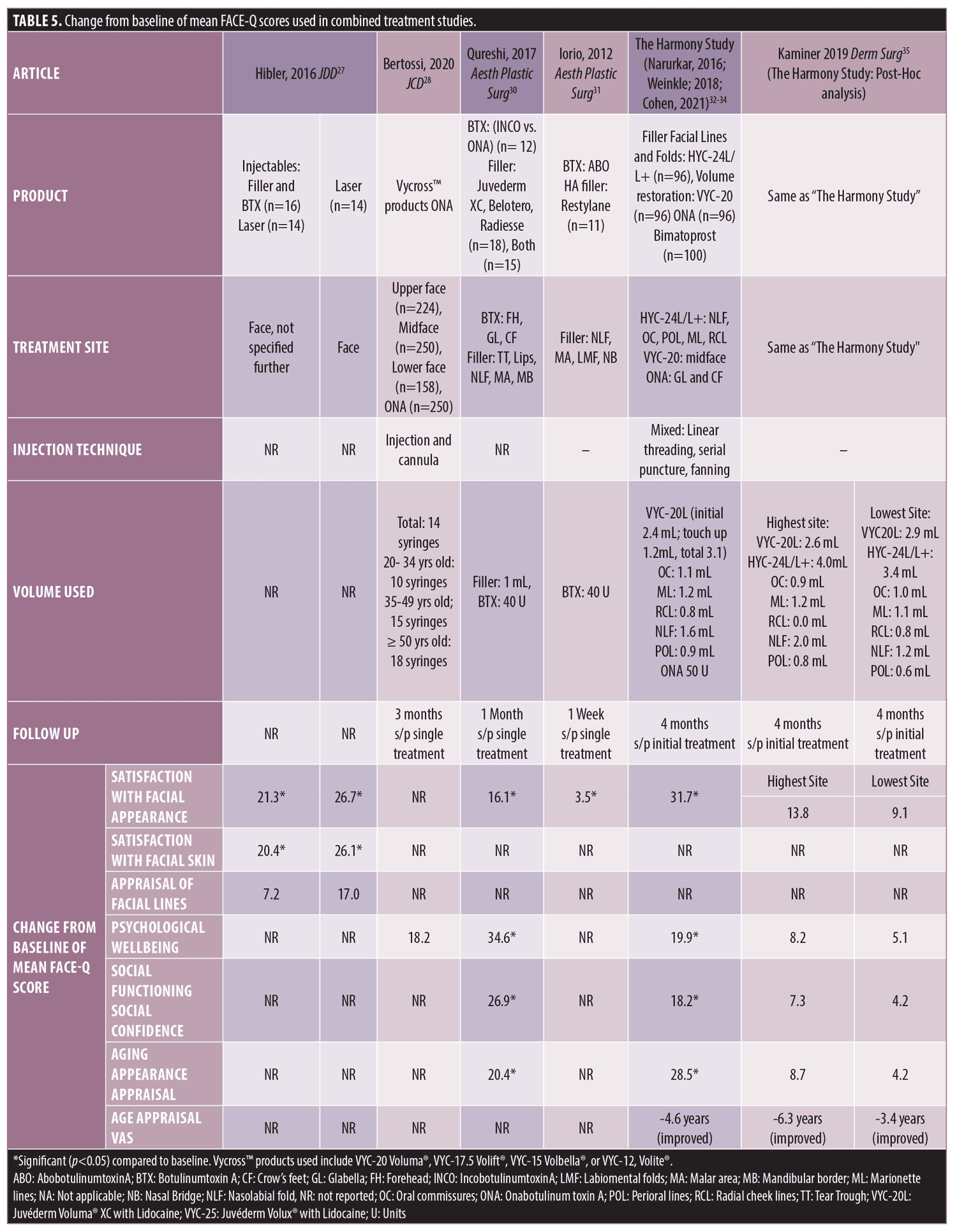
Hibler and colleagues assessed Satisfaction with Facial Appearance, Satisfaction with Facial Skin, and Appraisal of Facial Lines after either injectable treatment with dermal filler and neuromodulators (specific products or volumes not reported) vs laser resurfacing alone vs one patient who received both laser and injectable treatment. In subgroup analyses, post-treatment with either injectables or laser resurfacing demonstrated significant improvement in Satisfaction with Facial Appearance and Satisfaction with Facial Skin.27 There was also post-treatment improvement in Appraisal of Facial Lines in both groups, but the improvements did not meet significance.27 There was no significant difference in mean change of pre vs. post treatment scores between the injectables and laser treatment groups.27
Bertossi and colleagues rejuvenated the face using the MD Codes™ approach with ONA and dermal fillers VYC-20, VYC-17.5 (Volift®, Allergan Aesthetics, an AbbVie Company), VYC-15, or VYC-12.28 The MD Codes™ approach provides a structured method to facilitate treatment choice by standardizing injection device, product volume and technique according to treatment area.29 Indications for treatment of the upper face were temple voids, reduced nasofrontal angle, reduced eyebrow projection, forehead lines and ptosis of eyebrow. Indications for treatment of the midface included midfacial deficit resulting in reduced malar projection, tear trough defect and more pronounced nasolabial folds. The lower face was treated for marionette lines, jawline deficit, chin defects, and lip enhancement. Filler type was used as indicated by the MD Codes™ approach, with VYC-20 recommended for temple, mid-face and jawline/chin rejuvenation, VYC17.5 for lips, forehead, eyebrow and periorbital areas, and VYC-15 recommended for infraorbital areas and pyriform aperture.28 Twenty units of ONA was used to treat glabellar lines and crow’s feet lines were treated with 12-20 U per side.28 Volume of filler used was reported by mean number of syringes injected: overall, a mean of 14 syringes were used per patient. This was further broken down by age bracket as follows: 20-34 years, 10 syringes; 35-49 years, 15 syringes; >50 years, 18 syringes.28 The authors found a significant improvement in psychosocial distress three months post single treatment.28
Two groups used FACE-Q scales to analyze treatment outcomes in a plastic surgery resident clinic to evaluate PROs in procedures performed by residents. Qureshi and colleagues used both neuromodulator (ONA or INCO, median of 40 units, range 25-50 units) to forehead, glabella, and crow’s feet and dermal HA fillers (Juvederm range, technology not specified) and a cohesive polydensified HA matrix gel (Belotero®, Merz Aesthetics) and calcium hydroxylapatite (Radiesse®, Merz Aesthetics); median total volume of 1.00 mL (range 0.4 – 3.4 mL) to mid-face and/or lips and found significant improvement in Aging Appearance, Psychological Wellbeing, Social Functioning, and Satisfaction with Facial Appearance at one month after single treatment.30
Similarly, from another plastic surgery resident clinic, rejuvenation with ABO (units and target treatment areas not reported) and SGP-HA-L (Restylane®, Galderma Laboratories, Fort Worth, Texas) (volume not reported) targeted to nasolabial folds, malar prominences and labiomental folds, resulted in improved Satisfaction with Facial Appearance and improved subgroup ratings of Satisfaction of cheekbones, marionette line, and nasal bridge at one week after initial treatment.31
In a study designed to assess the aesthetic impact of a multimodal facial treatment approach, using a combination of treatment modalities, HYC-24L/L+ (Juvederm ® Ultra XC and Juvederm ® Ultra XC Plus; Allergan Aesthetics, an AbbVie Company) were used for facial lines and folds in areas including nasolabial folds, oral commissures, perioral lines, marionette lines, and radial cheek lines, VYC-20L was used for mid-face volume restoration, ONA for glabella and crow’s feet lines, and 0.03% bimatoprost ophthalmic solution for hypotrichosis.32 Filler treatments were administered at baseline with an optional touch-up treatment at Day 14. ONA was administered at three months. It was found that four months after initial treatment, subjects reported significant improvement in Satisfaction with Facial Appearance, Psychological Wellbeing, the Social Confidence scale, Aging Appearance Appraisal scale, and Age Appraisal VAS.33, 34 In a post-hoc analysis of the two sites with the highest and two sites with the lowest improvement scores, higher scoring sites favored lateral malar augmentation and used less volume medially compared to the lower scoring sites and in the lower face, the highest scoring sites used greater volumes.35 Initial treatment volumes were more conservative at the highest sites compared to the lowest sites, and greater volumes were used by the highest sites in touch-up treatments on Day 14.35
Nearly all studies showed improvement in FACE-Q modules measured. Changes in Satisfaction with Facial Appearance scores were highest from “The Harmony Study” where injection volumes were higher (VYC-20L, mean 3.1mL and HYC-24L/L+, range 0.8-1.2 mL in each region treated) and treatment areas were greater (most subjects with 4-5 treated areas).32, 34 However, Qureshi and colleagues reported the greatest score change in Psychological Wellbeing of 34.6 at one month after single treatment with neuromodulator and notably less filler (median total volume of 1.00 mL; range 0.4 – 3.4 mL), followed by “The Harmony Study” which reported change from baseline to be 19.9 at four month follow up after initial/touch-up treatment, and then Bertossi and colleagues showing a score change of 18.2 at three months after single treatment, despite using more syringes of filler compared to the first two studies.28, 30, 33 Finally, of the two studies evaluating Age Appearance Appraisal, “The Harmony Study”, using more volume of filler and more treated areas, had greater change in scores at four months after initial/touch-up treatment compared to Qureshi and colleagues at one month after single treatment.30, 33
Other. Seven additional studies were found that did not fit into the previously defined categories (Table 6). Four of these studies utilized Platelet Rich Fibrin (PRF) or Platelet Rich Plasma (PRP) for facial rejuvenation and acne scarring. The two studies utilizing PRF for facial rejuvenation found that subjects reported improvement in Satisfaction with Facial Appearance and Facial Skin at three month follow up after at least three treatment sessions over three months.36, 37 Nacopoulos and colleagues found that longer telomere length, measured using a quantitative PCR based technique, was related to baseline higher FACE-Q results, but scores did not significantly change after treatment with PRF; however, genetic variations affecting telomere length were related to the increased changes in FACE-Q following treatment.37 The authors propose that telomere length and genetic variations affecting telomere length could potentially serve as biomarkers to predict patient satisfaction with PRF facial regeneration.37
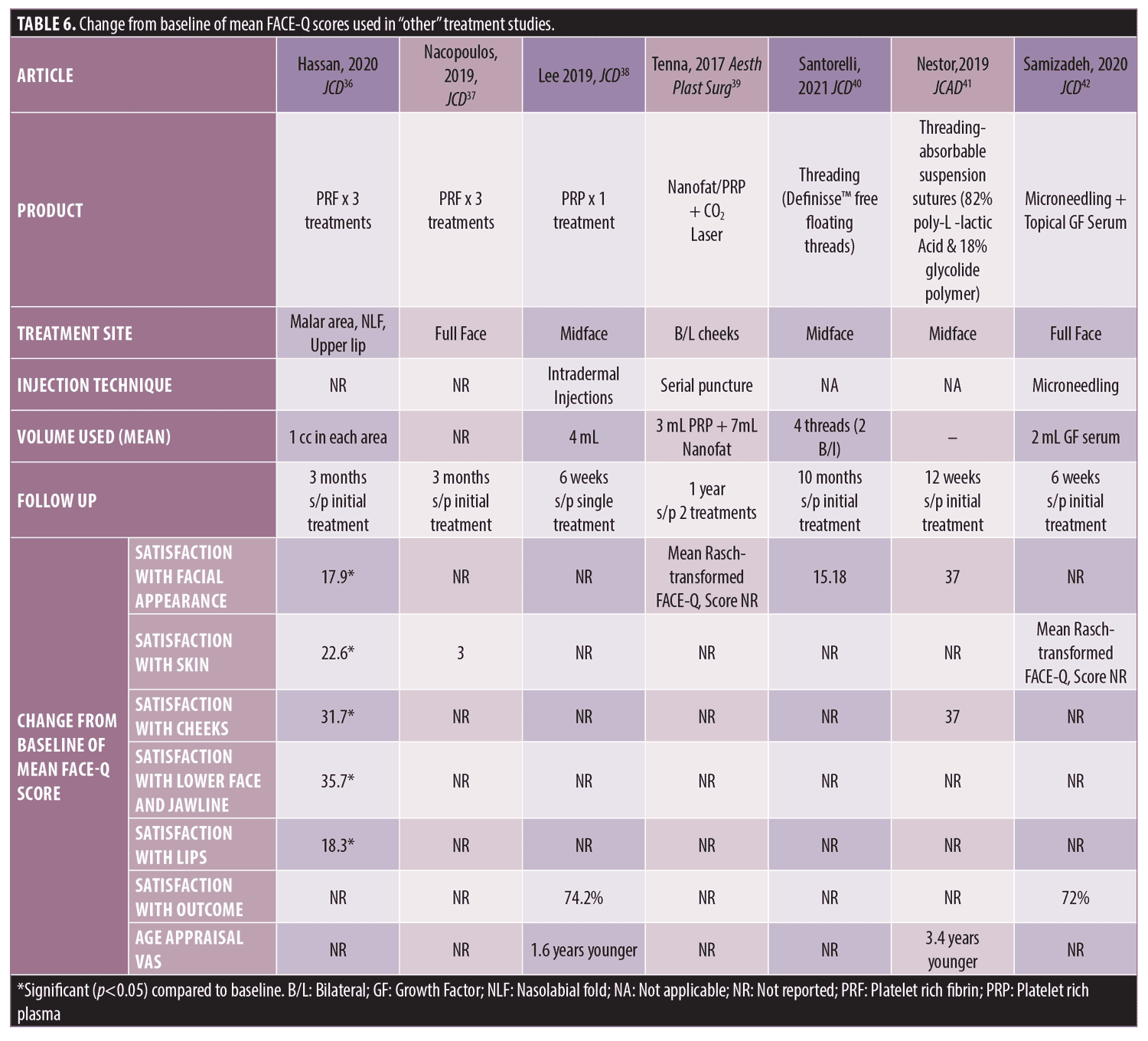
A single treatment of PRP infiltration into the midface for treatment of photodamaged skin resulted in improved Satisfaction with Facial Appearance and Satisfaction with Cheek scores at median follow up time of 5.7 weeks.38 Tenna and colleagues found improvement in facial acne scars and Satisfaction with Appearance at one year follow-up after two treatment sessions (mean interval of 6 months) with both nanofat and PRP infiltration combined with fractional CO2 laser resurfacing and with Nanofat/PRP infiltration alone.39
One study examining placement of barbed free floating threads composed of L-lactide and ε-caprolactone (Definisse™; Relife Menarini Group, France) in 60 patients demonstrated significant improvement in FACE-Q Satisfaction with Facial Appearance scores at six months after baseline treatment where two threads were placed on each side of the face.40 A second study examined placement of absorbable suspension sutures composed of poly-L-lactic Acid and glycolide polymer (Silhouette InstaLift™; Sinclair Pharma, Irvine, California) in 20 patients where 10 patients received six total sutures (3 on each side) and 10 received eight total sutures (4 on each side).41 Satisfaction with Cheek scores and Age Appraisal scores were significantly improved at one week following baseline treatment and remained significant up to 12 week follow up.41 At 12 weeks after baseline treatment, mean patient reported age appraisal score was 3.4 years younger than actual age.41 Eleven of the initial 20 patients were followed up through 12 months. At 12 month follow up from baseline treatment, Satisfaction with Cheek scores continued to be significantly improved from baseline and the Age Appraisal scores continued to demonstrate an overall trend of younger appearance.41
Samizadeh and colleagues assessed improvement of FACE-Q scores after two treatments of microneedling followed by application of a topical growth factor serum for facial rejuvenation, spaced two weeks apart.42 At two weeks after the second treatment, subjects scored satisfaction with treatment and improvement with overall skin appearance very highly, specifically with improvements in brightness, skin texture and tightness.42
Discussion
With the increase in demand for facial cosmetic procedures, understanding why patients seek these treatments and assessing treatment outcomes based on patient reported measures is essential for providing patient-centered care. Since its inception in 2010, followed by years of validation studies, the FACE-Q has been increasingly utilized in clinical trials of both surgical and non-surgical aesthetic facial procedures to capture the patient’s perspective on satisfaction of appearance, quality of life, adverse effects, and process of care.43 The authors sought to determine if FACE-Q patient reported outcomes improved after non-surgical aesthetic facial procedures, and if some procedures rendered greater improvement than others.
The top four FACE-Q domains used amongst nonsurgical aesthetic treatments were the Satisfaction with (overall) Facial Appearance, Satisfaction with site-specific areas (i.e., lips, cheek, chin, forehead), Psychological Wellbeing, and Age Appraisal scales. Based on this review of the literature, the evidence suggests that these four FACE-Q domains did improve following minimally invasive cometic procedures. Combined treatment with neuromodulators and fillers showed a tendency for greater improvement of psychological wellbeing and age appraisal than individual treatments alone, as demonstrated with higher absolute changes in score with combined treatments.
Within each treatment category, the greatest change in scores for satisfaction with either site- specific areas or overall facial appearance were seen with the following products: Satisfaction with NLF was highest with RHA4 (mean volume 1.54 mL) at 64 weeks, Satisfaction with Chin highest with VYC-20L (mean initial + touch up volume 2.6 mL) at 6 months, Satisfaction with Lips with VYC-15L (mean initial volume 2.0 mL + retreatment at one year with median 1.5mL) at one month s/p retreatment at one year, Satisfaction with Appearance with 20 U ONA to glabella at two weeks, and Satisfaction with Appearance with combined HYC-24-L/L+ to facial lines and folds, VYC-20 to midface, and ONA to glabella and crow’s feet at four months. Substantial changes in each individual treatment category reinforces the notion that if combined, multiple treatment modalities have the potential to improve overall satisfaction with multiple aspects of facial appearance, as seen with the combined treatment studies.
With the landscape of non-surgical cosmetic interventions continuously evolving, the medical community must determine if these interventions have a positive impact beyond improvement in physical appearance. Patient-centered outcomes and effectiveness are now required for FDA approval of new pharmaceuticals but quantifying and understanding improvement in wellbeing goes beyond government regulation. Understanding the value of psychosocial impact of treatment gives insight into reasons why individuals seek aesthetic treatments in the first place. With this information, providers may be able to screen and identify patients most likely to benefit from these nonsurgical aesthetic procedures. Physicians can also provide patients with sound scientific evidence confirming the significant positive emotional impact these procedures can have on individuals during the counseling process.
The FACE-Q is distinguished from other patient reported outcomes instruments not only because it is validated but also comprehensive; it can capture patient satisfaction with overall facial appearance, as well as individual facial regions, psychological wellbeing, age appraisal, and adverse effects. With multiple domains included in one survey, it eliminates the need to combine multiple surveys. However, for PROs to gain popularity in clinical practice, ideally, they should be short for patients to complete all aspects of the survey. The FACE-Q, with more than 40-questionnaires, was created so it can be pared down to fit areas of interest as seen in many of the articles reviewed.
There are notable limitations to many of the studies in this review. Primarily, studies suffered from poorly defined sample vs. control groups, many of them lacking control groups at all. Follow-up times and FACE-Q scales varied between studies, making it difficult to compare to each other. Finally, we were unable to control for publication bias, as there may be a tendency to publish studies that report positive findings. Future studies should compare different procedures with the same FACE-Q scales to better determine the impact of these treatments on satisfaction with appearance, psychological functioning, quality of life, and adverse events. Of note, many of the studies enrolled white woman, so additional future consideration would be to include a more diverse subject population, including men and subjects with diverse skin of color as their motivations for and outcomes with aesthetic procedures may differ.
Conclusion
The FACE-Q is a validated patient reported outcome measurement that can be used to assess facial aesthetic procedures. We sought to determine if FACE-Q outcomes improved with nonsurgical cosmetic facial procedures. Based on this review, the evidence suggests that Satisfaction with Facial Appearance, Psychological Wellbeing, and Age Appraisal did improve following nonsurgical facial aesthetic procedures. Combined treatment with neuromodulators and fillers showed a tendency for greater improvement of psychological wellbeing and age appraisal than individual treatments alone, as demonstrated with higher absolute changes in score with combined treatments. To maximize patient satisfaction and retention, providers should consider a combined treatment approach with both filler and neuromodulators, compared to either alone to ultimately improve patient quality of life. More rigorous studies comparing different procedures in a more inclusive study population will generate stronger recommendations regarding impact of these procedures on satisfaction with appearance and psychological and social functioning.
References
- American Society of Plastic Surgeons. 2020 Plastic Surgery Statistics 2020; Available from: https://www.plasticsurgery.org/documents/News/Statistics/2020/top-five-cosmetic-plastic-surgery-procedures-2020.pdf.
- Waldman, A., et al., Patients believe that cosmetic procedures affect their quality of life: An interview study of patient-reported motivations. J Am Acad Dermatol. 2019. 80(6): p. 1671–1681.
- Maisel, A., et al., Self-reported Patient Motivations for Seeking Cosmetic Procedures. JAMA Dermatol. 2018. 154(10): p. 1167–1174.
- Klassen, A.F., et al., Measuring patient-reported outcomes in facial aesthetic patients: development of the FACE-Q. Facial Plast Surg. 2010. 26(4): p. 303–309.
- Klassen, A.F., et al., Self-Report Scales to Measure Expectations and Appearance-Related Psychosocial Distress in Patients Seeking Cosmetic Treatments. Aesthet Surg J. 2016. 36(9): p. 1068-78.
- Klassen, A.F., et al., FACE-Q Eye Module for Measuring Patient-Reported Outcomes Following Cosmetic Eye Treatments. JAMA Facial Plast Surg, 2017. 19(1): p. 7–14.
- Klassen, A.F., S.J. Cano, and A.L. Pusic, FACE-Q Satisfaction with Appearance Scores from Close to 1000 Facial Aesthetic Patients. Plast Reconstr Surg. 2016. 137(3): p. 651e–652e.
- Klassen, A.F., et al., Development and Psychometric Validation of the FACE-Q Skin, Lips, and Facial Rhytids Appearance Scales and Adverse Effects Checklists for Cosmetic Procedures. JAMA Dermatol, 2016. 152(4): p. 443–451.
- Klassen, A.F., et al., FACE-Q scales for health-related quality of life, early life impact, satisfaction with outcomes, and decision to have treatment: development and validation. Plast Reconstr Surg. 2015. 135(2): p. 375–386.
- Pusic, A.L., et al., Development and psychometric evaluation of the FACE-Q satisfaction with appearance scale: a new patient-reported outcome instrument for facial aesthetics patients. Clin Plast Surg. 2013. 40(2): p. 249–260.
- Monheit, G., et al., Efficacy and Safety of Two Resilient Hyaluronic Acid Fillers in the Treatment of Moderate-to-Severe Nasolabial Folds: A 64-Week, Prospective, Multicenter, Controlled, Randomized, Double-Blinded, and Within-Subject Study. Dermatol Surg, 2020. 46(12): p. 1521–1529.
- Kaufman-Janette, J., et al., Efficacy and safety of a new resilient hyaluronic acid dermal filler, in the correction of moderate-to-severe nasolabial folds: A 64-week, prospective, multicenter, controlled, randomized, double-blind and within-subject study. J Cosmet Dermatol. 2019.
- Jones, D.H., et al., Microcannula Injection of Large Gel Particle Hyaluronic Acid for Cheek Augmentation and the Correction of Age-Related Midface Contour Deficiencies. Dermatol Surg. 2020. 46(4): p. 465–472.
- Ogilvie, P., et al., Improvements in satisfaction with skin after treatment of facial fine lines with VYC-12 injectable gel: Patient-reported outcomes from a prospective study. J Cosmet Dermatol, 2020. 19(5): p. 1065–1070.
- Niforos, F., et al., VYC-12 Injectable Gel Is Safe And Effective For Improvement Of Facial Skin Topography: A Prospective Study. Clin Cosmet Investig Dermatol. 2019. 12: p. 791–798.
- Beer, K., et al., Safe and Effective Chin Augmentation With the Hyaluronic Acid Injectable Filler, VYC-20L. Dermatol Surg, 2021. 47(1): p. 80–85.
- Ogilvie, P., et al., Safe, Effective Chin and Jaw Restoration With VYC-25L Hyaluronic Acid Injectable Gel. Dermatol Surg, 2019. 45(10): p. 1294–1303.
- Mastroluca, E., M. Patalano, and D. Bertossi, Minimally invasive aesthetic treatment of male patients: The importance of consultation and the lower third of the face. J Cosmet Dermatol, 2021. 20(7): p. 2086–2092.
- Geronemus, R.G., et al., Safety and Effectiveness of VYC-15L, a Hyaluronic Acid Filler for Lip and Perioral Enhancement: One-Year Results From a Randomized, Controlled Study. Dermatol Surg. 2017. 43(3): p. 396–404.
- Rivkin, A., et al., Safety and Effectiveness of Repeat Treatment With VYC-15L for Lip and Perioral Enhancement: Results From a Prospective Multicenter Study. Aesthet Surg J, 2019. 39(4): p. 413–422.
- Raspaldo, H., et al., Juvederm volbella with lidocaine for lip and perioral enhancement: a prospective, randomized, controlled trial. Plast Reconstr Surg Glob Open, 2015. 3(3): p. e321.
- Bertucci, V., et al., Subject and partner satisfaction with lip and perioral enhancement using flexible hyaluronic acid fillers. J Cosmet Dermatol, 2021. 20(5): p. 1499–1504.
- Schlessinger, J., et al., A Multicenter Study to Evaluate Subject Satisfaction With Two Treatments of AbobotulinumtoxinA a Year in the Glabellar Lines. Dermatol Surg. 2021. 47(4): p. 504–509.
- Ascher, B., et al., Significantly Increased Patient Satisfaction Following Liquid Formulation AbobotulinumtoxinA Treatment in Glabellar Lines: FACE-Q Outcomes From a Phase 3 Clinical Trial. Aesthet Surg J. 2020. 40(9): p. 1000–1008.
- Cohen, J.L., et al., Assessment of Psychological Well-being After AbobotulinumtoxinA Treatment: A Comparison of 2 Reconstitution Volumes. Dermatol Surg, 2020. 46(2): p. 289–292.
- Chang, B.L., et al., Patient Perceived Benefit in Facial Aesthetic Procedures: FACE-Q as a Tool to Study Botulinum Toxin Injection Outcomes. Aesthet Surg J, 2016. 36(7): p. 810–820.
- Hibler, B.P., J. Schwitzer, and A.M. Rossi, Assessing Improvement of Facial Appearance and Quality of Life after Minimally-Invasive Cosmetic Dermatology Procedures Using the FACE-Q Scales. J Drugs Dermatol, 2016. 15(1): p. 62–67.
- Bertossi, D., et al., Non surgical facial reshaping using MD Codes. J Cosmet Dermatol, 2020. 19(9): p. 2219–2228.
- de Maio, M., MD Codes: A Methodological Approach to Facial Aesthetic Treatment with Injectable Hyaluronic Acid Fillers. Aesthetic Plast Surg, 2021. 45(2): p. 690–709.
- Qureshi, A.A., et al., Nonsurgical Facial Rejuvenation: Outcomes and Safety of Neuromodulator and Soft-Tissue Filler Procedures Performed in a Resident Cosmetic Clinic. Aesthetic Plast Surg, 2017. 41(5): p. 1177–1183.
- Iorio, M.L., et al., Plastic surgery training: evaluating patient satisfaction with facial fillers in a resident clinic. Aesthetic Plast Surg. 2012. 36(6): p. 1361–1366.
- Narurkar, V.A., et al., A Comprehensive Approach to Multimodal Facial Aesthetic Treatment: Injection Techniques and Treatment Characteristics From the HARMONY Study. Dermatol Surg. 2016. 42 Suppl 2: p. S177–91.
- Cohen, J.L., et al., Multimodal Facial Aesthetic Treatment on the Appearance of Aging, Social Confidence, and Psychological Wellbeing: HARMONY Study. Aesthet Surg J. 2021.
- Weinkle, S.H., et al., Impact of Comprehensive, Minimally Invasive, Multimodal Aesthetic Treatment on Satisfaction With Facial Appearance: The HARMONY Study. Aesthet Surg J, 2018. 38(5): p. 540–556.
- Kaminer, M.S., et al., Maximizing Panfacial Aesthetic Outcomes: Findings and Recommendations From the HARMONY Study. Dermatol Surg. 2020. 46(6): p. 810–817.
- Hassan, H., D.J. Quinlan, and A. Ghanem, Injectable platelet-rich fibrin for facial rejuvenation: A prospective, single-center study. J Cosmet Dermatol. 2020. 19(12): p. 3213–3221.
- Nacopoulos, C., et al., Telomere length and genetic variations affecting telomere length as biomarkers for facial regeneration with platelet-rich fibrin based on the low-speed centrifugation concept. J Cosmet Dermatol,.2019. 18(1): p. 408–413.
- Lee, Z.H., et al., Platelet rich plasma for photodamaged skin: A pilot study. J Cosmet Dermatol. 2019. 18(1): p. 77–83.
- Tenna, S., et al., Comparative Study Using Autologous Fat Grafts Plus Platelet-Rich Plasma With or Without Fractional CO2 Laser Resurfacing in Treatment of Acne Scars: Analysis of Outcomes and Satisfaction With FACE-Q. Aesthetic Plast Sur., 2017. 41(3): p. 661–666.
- Santorelli, A., et al., Mid-face reshaping using threads with bidirectional convergent barbs: A retrospective study. J Cosmet Dermatol, 2021. 20(6): p. 1591–1597.
- Nestor, M.S., Facial Lift and Patient Satisfaction Following Treatment with Absorbable Suspension Sutures: 12-Month Data from a Prospective, Masked, Controlled Clinical Study. J Clin Aesthet Dermatol. 2019. 12(3): p.18–26.
- Samizadeh, S. and L. Belhaouari, Effectiveness of growth factor-induced therapy for skin rejuvenation: A case series. J Cosmet Dermatol. 2021. 20(6): p. 1867–1874.
- Mori, S. and E.H. Lee, Beyond the physician’s perspective: A review of patient-reported outcomes in dermatologic surgery and cosmetic dermatology. Int J Womens Dermatol, 2019. 5(1): p. 21–26.

